Abstract
Inflammatory bowel diseases are chronic inflammatory disorders of the gastrointestinal tract. Vasoactive intestinal peptide (VIP) is a neuropeptide with known anti-inflammatory activity. We have demonstrated previously that administration of VIP inhibits leucocyte migration in a murine model of delayed-type hypersensitivity, and anti-inflammatory efficacy is supported by other studies. The aim of this study was to investigate the VIP effects in a murine model of intestinal inflammation. Colitis was induced in BALB/c mice by a 2·5 mg enema of 2,4,6-trinitrobenzenesulphonic acid (TNBS) and the mice were killed on day 7. Mice were administered either a 3-day (therapeutic) or 7-day (prophylactic) constant infusion of VIP by subcutaneously implanted mini-osmotic pumps, or intraperitoneal (i.p.) injection of VIP on alternate days over 7 days. Clinical disease scores, weight changes, histopathology of colon tissues, plasma VIP levels, cytokine levels and chemotaxis of peripheral blood mononuclear cells were evaluated. After administration of TNBS, mice quickly developed severe colitis accompanied by dramatic body weight loss (20% by day 6) and high mortality (30%). Prophylactic treatment using high-dose VIP abrogated leucocyte chemotaxis; however, it failed to ameliorate the weight loss and mortality. Moreover, VIP delivered either by constant infusion or i.p. failed to modify the clinical, histological or cytokine markers of disease. Our studies show that, despite an ability to inhibit chemokine-induced chemotaxis of mononuclear cells, VIP was unable to modulate TNBS-induced colitis. This contrasts with the efficacy of VIP in models of mild inflammatory disease and suggests that VIP is unlikely to provide a useful model for novel anti-IBD therapy.
Keywords: chemotaxis, inflammatory bowel disease, murine TNBS-colitis, vasoactive intestinal peptide
Introduction
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders of the gastrointestinal tract of unknown aetiology. Crohn's disease is characterized by a transmural leucocytic infiltrate affecting any part of the gastrointestinal tract, while ulcerative colitis affects mainly the mucosal layer of the large intestine [1,2]. Much of our understanding of the pathogenesis of IBD comes from studies in animal models of intestinal inflammation [1,3,4]. One well-established model is the hapten-induced model of colitis in which 2,4,6-trinitrobenzenesulphonic acid (TNBS) is administered intrarectally to induce a T helper cell type 1 (Th1) response [5–7]. This model is similar immunologically to human Crohn's disease, which is also associated with a Th1 response [8–10].
Such animal models provide the opportunity to evaluate novel therapeutic approaches for the treatment of IBD. A number of agents have been examined, including the use of protein/peptide-[anti-tumour necrosis factor (TNF)-α, anti-interleukin (IL)-12 and anti-IL-6 antibodies, rIL-10] and gene therapy-based treatments [11–13], with anti-TNF-α treatment now established in clinical practice [14]. One such peptide that has emerged as a potential candidate in the treatment of IBD is the neuropeptide, vasoactive intestinal peptide (VIP) [15,16]. VIP is a 28 amino acid protein belonging to the glucagon/secretin family [17,18]. Localized in the central and peripheral nervous system, VIP has been shown to exert a wide range of biological actions affecting a number of systems, including the cardiovascular, respiratory and gastrointestinal systems [19]. VIP is expressed constitutively in the lymphoid microenvironment, and has also been reported to modulate a variety of functions affecting both innate and acquired immunity [20]. In particular, VIP alters co-stimulatory activity of antigen-presenting cells [21], inhibits innate immune responses [22] and polarizes Th2 cells [23]. VIP has been reported to treat inflammatory disorders including endotoxic shock [24] and immune-mediated arthritis [25] in animal models, following its identification as a potent anti-inflammatory factor [15,16].
Given that VIP has emerged as a potential anti-inflammatory agent, and based on our previous demonstrations that VIP is able to inhibit inflammation in a murine model of delayed-type hypersensitivity (DTH) by phosphorylating and desensitizing chemokine receptors, thus impairing leucocyte migration [26], we chose to investigate the potential therapeutic effects of VIP administered in a murine model of TNBS-induced colitis. We demonstrated that while VIP was able to inhibit the chemotactic potential of circulating leucocytes in the model, it failed to ameliorate or prevent the severe colonic inflammation and systemic manifestations of TNBS-induced colitis. Our findings stand in contradistinction to the observations from a recent study that reported the therapeutic effects of VIP administered in a similar model of IBD [27].
Materials and methods
Animals
Specific pathogen-free (SPF) 6-week-old male BALB/c mice were purchased from the Animal Resources Centre (Perth, Australia), and maintained in a SPF environment in the Biological Resource Facility at the University of New South Wales. Mice were housed in groups of five in filter top cages and allowed to adapt to the facility for 5–7 days before commencement of the experiments. All experiments were approved by the Animal Care and Ethics Committee of the University of New South Wales, Australia.
Induction of TNBS colitis
To induce colitis, each mouse was first anaesthetized by intraperitoneal (i.p.) injection of a 100 µl solution containing 3·4 mg/kg xylazine (Ilium, Troy Laboratories Pty, Ltd, Smithfield, Australia) and 100 mg/kg ketamine (Parnell Laboratories, Sydney, Australia), followed by intrarectal administration of 2·5 mg of TNBS (Fluka, Buchs, Switzerland) dissolved in 45% ethanol. A total volume of 100 µl of TNBS solution was instilled slowly into the colon using a 20-gauge cannula fitted to a 1 ml syringe. In control experiments, mice received 45% ethanol alone using the same technique. After instillation, mice were held in a head-down position for 30 s to ensure distribution of TNBS or ethanol within the bowel and to prevent any leakage. To maintain hydration, mice were given a daily 1 ml injection of subcutaneous normal saline (0·9% NaCl for injection). Mice were killed on day 7 by CO2 asphyxiation.
Treatment protocols
To investigate the prophylactic and/or therapeutic effects of VIP, TNBS colitis mice were administered VIP (Sigma Chemicals, St Louis, MO, USA) or normal saline (control) by constant infusion or i.p. For constant infusion experiments, anaesthetized mice had subcutaneous implantation of mini-osmotic pumps (Alzet, Palo Alto, CA, USA) filled with 100 µl of VIP or saline from day 0 (VIP 1 × 10−4m or 5 × 10−4m) or day 4 (VIP 5 × 10−5m) after induction of colitis for continuous infusion of 7 or 3 days, respectively, before sacrifice on day 7 (these experimental groups are referred to below as ‘prophylactic’ and ‘therapeutic’ treatments, respectively). For i.p. experiments, 1 nmol or 5 nmol of VIP in 100 µl or 100 µl saline was administered to each mouse 12 h post-colitis induction, and on alternate days over 7 days. One h before sacrifice mice were given a final VIP i.p. injection for measurement of peak VIP levels in the blood (see below). Mice were monitored daily for mortality, weight change and clinical score prior to being killed on day 7.
Plasma VIP levels and leucocyte chemotaxis
Peripheral blood from mice was collected by performing direct cardiac puncture. Blood was added to either tubes containing 1·2 mg/ml EDTA and 500 KIU/ml Trasylol (Bayer Australia Limited, Sydney, Australia) or anticoagulant citrate dextrose (ACD) solution (Baxter Healthcare, Sydney, Australia). For determination of VIP levels, plasma was collected from blood samples containing EDTA and Trasylol following centrifugation at 9000 g for 5 min at 4°C and measured using a standard radioimmunoassay. The blood pellet or blood samples containing ACD were directly lysed with ammonium chloride solution (155 m m NH4Cl, 10 m m KHCO3, 1 m m EDTA, pH 7·3). Leucocytes were counted and assayed for chemokine responsiveness using chemotaxis assays as described previously [28]. Briefly, cells were assayed in 48-well microchemotaxis chambers using murine fibronectin (10 µg/ml) (Invitrogen, Carlsbad, CA, USA)-coated polyvinylpyrrolidone-free 5 mm pore size membranes (Nucleopore, Neuroprobe, Cabin John, MD, USA). Migration towards the chemokines, CCL2 (MCP-1), CCL3 (MIP-1α) and CXCL10 (IP-10) (R&D Systems, Minneapolis, MN, USA) was allowed for 90 min at 37°C in a humidified incubator before membrane removal and staining. The results were determined as chemotaxis index (mean number of cells per high-power field for chemokine diluent/mean number of cells per high power field for medium).
Th1/Th2 cytokine analysis
Plasma from the above preparations was collected and concentrations of circulating IL-2, IL-4, IL-5, IL-10, IL-12 (p70), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon (IFN)-γ and TNF-α were measured using the mouse Bio-Plex cytokine assay system according to the manufacturer's specifications (Bio-Rad, Hercules, CA, USA). Fluorescence was measured using the Luminex 100 system (Bio-Rad, Hercules, CA, USA) and results were analysed using Bio-plex Manager™ software (Bio-Rad, Hercules, CA, USA). The sensitivity of the assay is 1·95 pg/ml.
Clinical score
Animals were monitored daily for diarrhoea, mobility and coat quality. To obtain a clinical score, the above features were assessed individually to obtain a score between 0 and 2. Scores from each clinical feature were pooled, and a clinical score (0–6) was assigned. The scoring system was validated by two independent observers with an inter-rater correlation of r = 1 and P < 0·0 1 (assessed by Spearman's rank sum test).
Grading of histopathological changes
The colon of each mouse was removed carefully and divided into three segments: left, transverse and right. Within each segment the tissue was divided further into one longitudinal and two-cross sectional parts. Tissue samples were fixed in 10% buffered formalin, processed for paraffin blocks, and sections were stained with haematoxylin and eosin using standard techniques. Analysis of histopathology was performed in a blinded fashion using a previously validated objective scale [29]. Briefly, histology was scored on a scale of 0–6: 0, no leucocyte infiltration; 1, mild leucocyte infiltration; 3, prominent leucocyte infiltration; 5, extensive inflammatory infiltrate with ulceration and tissue necrosis, while scores of 2, 4 and 6 indicated preceding levels of inflammation that occupied more than 50% of the tissue section. Analysis of histomorphometry was also performed by enumerating mononuclear cells and neutrophils per square millimetre of tissue from randomly selected fields.
Statistical analysis
Values are expressed as mean ± s.d. or s.e.m. of n mice per group. One-way analysis of variance (anova) with post-hoc Bonferroni test was used to compare sequential data from two or more experimental groups. Comparison of cross-sectional data from two experimental groups was made using a two-tailed Student's t-test for unpaired data. Differences were considered significantly different if P was < 0·05. All tests were analysed by using graphpad prism for Windows (version 3·0).
Results
Induction of colitis
Following rectal administration of 2·5 mg TNBS, mice developed severe colitis rapidly, while mice receiving 45% ethanol alone (ethanol control) remained free of colonic inflammation. Systemic features associated with TNBS-induced colitis included poor clinical state, dramatic body weight loss and a mortality rate of approximately 30%. Typically, TNBS mice quickly developed signs including bloody diarrhoea, poor coat quality and reduced mobility.
Prophylactic treatment of high-dose VIP infusion inhibits leucocyte chemotaxis in TNBS-challenged mice
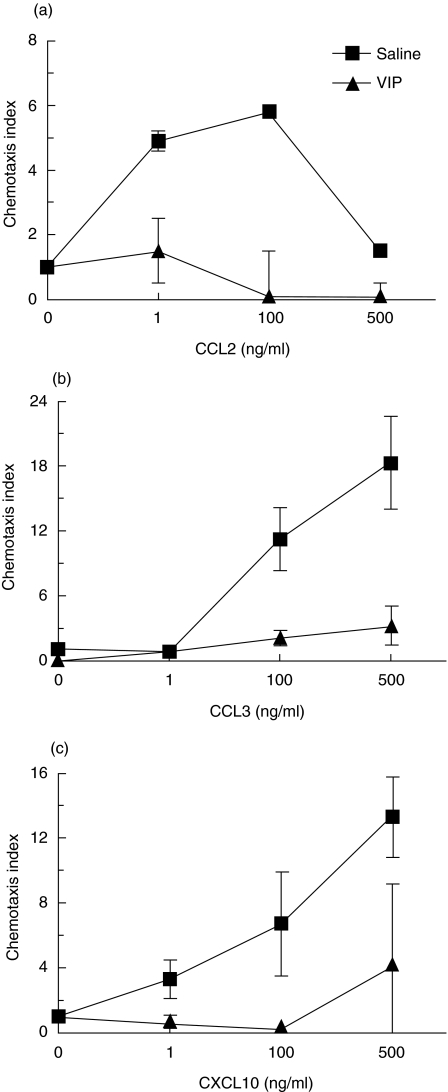
We have shown previously that various neuropeptides, including opioids [28] and VIP [26], inhibit leucocyte chemotaxis by cross-desensitization of chemokine receptors. Mononuclear cells were isolated from VIP-infused mice and subjected to in vitro chemotaxis assays. Prophylactic treatment of high-dose VIP (5 × 10−4m) infusion significantly reduced leucocyte chemotactic responses in TNBS-challenged animals. VIP infusion at high doses resulted in virtually complete abrogation of leucocyte migration in response to the chemokines CCL2, CCL3 and CXCL10 (Fig. 1). However, low-dose VIP (1 × 10−4m) infusion failed to exhibit the same inhibition of chemotaxis towards these chemokines (data not shown). Notably, VIP infusion at low and high doses in TNBS-challenged mice resulted in plasma levels of 2·7 × 10−9 ± 1·2 × 10−9m and 8·42 × 10−9 ± 1 × 10−9m, respectively (n = 3–5). These VIP levels were, respectively, two- and eightfold higher than saline-infused TNBS mice. These results show that while VIP administered at low doses does not impair chemokine responses, the inhibition of chemotaxis achieved at higher VIP plasma levels is biologically active, and comparable to a previous study which demonstrated human chemokine receptor desensitization in vitro[26].
Fig. 1.
VIP infusion impairs peripheral blood leucocyte migration to chemokines. BALB/c mice were given a 2·5 mg enema of TNBS and administered a constant infusion of VIP (5 × 10−4m) from day 0 over 7 days. Blood was collected at day 7 and chemotactic responses were measured to (a) CCL3, (b) CCL2 and (c) CXCL10. Each data point represent the mean (n = 3) and error bars represent the standard deviation.
Effects of low-dose VIP infusion − therapeutic and prophylactic treatments
Therapeutic mode
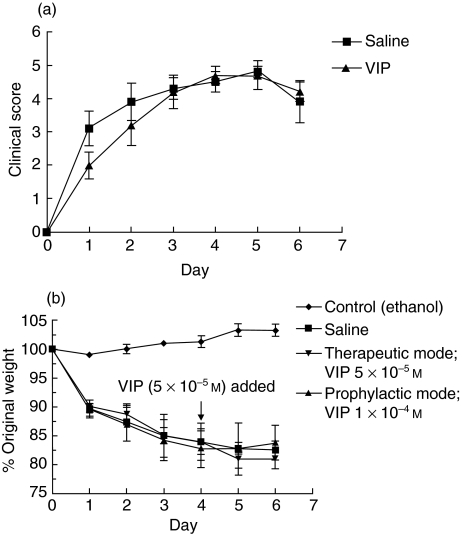
Therapeutic treatment of VIP (5 × 10−5m) administered by constant infusion from day 4 until the day of euthanasia (day 7) did not bring about any improvement in the clinical or histological features of TNBS-induced colitis (Figs 2 and 3, Table 1). As shown in Fig. 2a, no differences in clinical scores were observed between 3-day therapeutically treated VIP- and saline-infused TNBS mice. A dramatic and similar reduction in body weight was observed in both treatment groups of TNBS-challenged mice when compared to ethanol-challenged control animals (Fig. 2b). By day 2 there was more than 12% loss of original body weight, which continued to approximately 20% weight loss by day 6. The mortality rate by day 7 reached an average of 50% of animals in 3-day therapeutically treated VIP-infused mice compared to 30% in TNBS-challenged mice which received saline infusions only (Table 2).
Fig. 2.
Effect of low-dose VIP infusion in TNBS-induced mice. TNBS-challenged mice were administered a constant infusion of VIP from day 4 (5 × 10−5m) (therapeutic mode) or day 0 (1 × 10−4m) (prophylactic mode) post-induction of colitis with euthanasia at day 7. (a) Clinical score from 3-day VIP-infused mice and (b) weight changes of 3- and 7-day VIP-infused animals were assessed. Weight changes expressed as percentage of original weight from day 0. Each point represents mean ± s.d. from five to 10 mice.
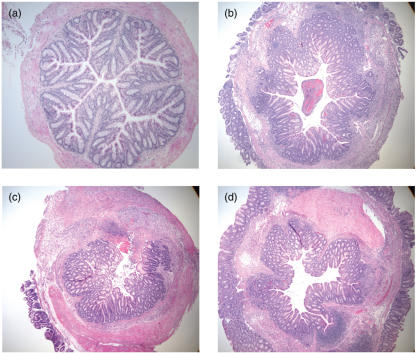
Fig. 3.
Histological evaluation (H&E staining) of the left colon from BALB/c mice killed 7 days after intrarectal administration of TNBS. (a) Normal appearance of colon taken from mice treated with 45% ethanol (control). (b) Loss of normal architecture with an extensive inflammatory infiltrate extending deeply into the muscular layers in saline-infused TNBS-treated mice. (c–d) No evidence in the improvement in histopathology of TNBS-challenged mice administered with a continuous infusion of (c) VIP (5 × 10−5m) for 3 days or (d) VIP (1 × 10−4m) for 7 days (original magnification ×4).
Table 1.
Histopathology score of colons taken at day 7 from therapeutic and prophylactic treated VIP-infused TNBS mice. Data represent mean ± s.d. from five to 10 mice. No statistical differences observed between saline- and VIP-infused mice.
| 3-day infusion | 7-day infusion | |||
|---|---|---|---|---|
| Colon section | Saline | VIP (5 × 10−5m) | Saline | VIP (1 × 10−4m) |
| Left | 5·0 ± 0·7 | 4·7 ± 0·5 | 4·1 ± 0·1 | 3·8 ± 0·6 |
| Transverse | 3·1 ± 1·0 | 3·4 ± 1·0 | 3·2 ± 0·5 | 2·7 ± 0·1 |
| Right | 1·8 ± 0·7 | 1·2 ± 0·3 | 2·2 ± 0·3 | 1·7 ± 0·3 |
Table 2.
Mortality rates of TNBS-challenged mice administered VIP or saline by constant infusion (therapeutic and prophylactic mode), or by intraperitoneal injection on alternate days over 7 days. Data represent percentage of deaths of animals at day 7 of VIP- or saline-treated TNBS-challenged mice. Statistical differences observed between VIP- (5 × 10−5m) and saline-infused mice (prophylactic mode).
| 3-day infusion (therapeutic mode) | 7-day infusion (prophylactic mode) | i.p. Injection | |||
|---|---|---|---|---|---|
| 5 × 10−5m | 1 × 10−4m | 5 × 10−4m | 1 nmol | 5 nmol | |
| VIP | 50% | 28·5 ± 12·0% | 65·0 ± 6·1%* | 40·0 ± 9·5% | 53·4 ± 18·8% |
| Saline | 30% | 33·5 ± 9·2% | 30·0 ± 5·6% | 26·6 ± 9·4% | |
P = 0·0056 (mean ± s.e.m.; n = 4 experiments). Remaining data: n = 1–2 experiments.
Microscopic analysis revealed that while no inflammation was detected in colons of ethanol control mice (Fig. 3a), the left colons from TNBS mice were affected most severely (Fig. 3b–d). No differences were observed in the microscopic assessment of colons from 3-day therapeutically treated VIP- or saline-infused TNBS-challenged mice (Fig. 3). Colonic histopathology was characterized by thickening of the wall and areas of prominent leucocytic infiltration extending through the mucosa, submucosa and muscularis propria. Ulceration and tissue necrosis extending into the muscular layer were also present. The inflammation was less severe in the right colon in all experimental groups (Table 1).
Prophylactic mode
As no therapeutic effect was observed from 3 days of VIP infusion, we determined the prophylactic effects of continuously infused VIP. Mice were administered a constant infusion of VIP (1 × 10−4 m) from day 0. By day 7, the number of deaths in these VIP-infused mice was similar to saline-infused TNBS-challenged mice (∼30%) (Table 2). VIP failed to reduce disease severity by any parameter when compared to TNBS mice (Figs 2b, 3, Table 1).
Effects of high-dose VIP infusion − prophylactic treatment
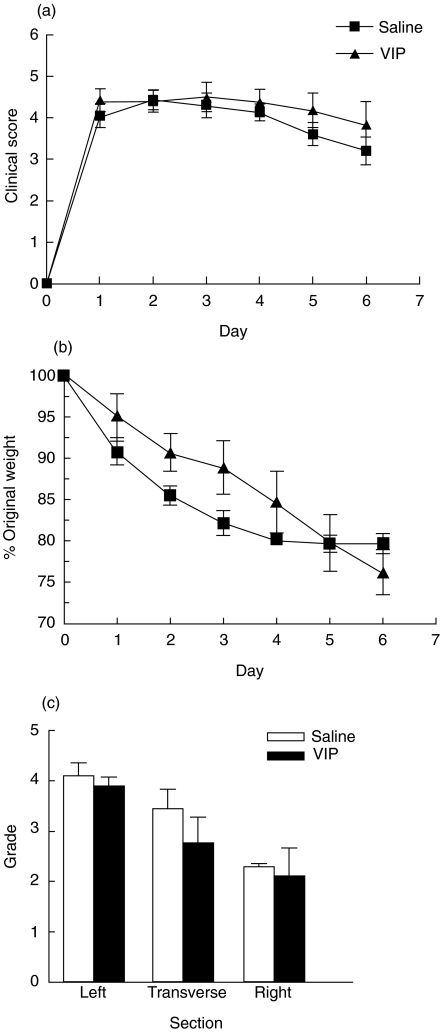
As we were unable to demonstrate either abrogation or amelioration of colitis using low-dose VIP infusions, we next examined the effects of high infused concentrations of VIP from the time of colitis induction. VIP (5 × 10−4 m) was administered by continuous infusion from day 0 over 7 days. As shown in Fig. 4a, no statistically significant differences were observed in the clinical scores between VIP- and saline-infused TNBS-challenged mice. Diarrhoea accompanied by severe weight loss was evident in both treated groups (Fig. 4b) and mortality was significantly higher in VIP-infused mice compared to saline-infused TNBS-challenged mice (VIP: 65·0 ± 6·1%; saline: 30·0 ± 5·6%; P = 0·0056) (Table 2). VIP infusion failed to abrogate the microscopic signs of colitis (Fig. 4c). Moreover, histomorphometry of the left colons demonstrated no statistical difference in the number of mononuclear cells or neutrophils in VIP-infused mice compared to saline-infused TNBS mice (mononuclear cells − VIP: 2891 ± 1073; saline: 1872 ± 1023, neutrophils −VIP: 184 ± 101; saline: 1491 ± 1179 − cells per square millimetre of tissue; n = 7–9 ± s.d.). These results demonstrate that administration of low-dose or high-dose VIP by infusion fails to either treat, or prevent, TNBS-induced colitis.
Fig. 4.
Infusion of high-dose VIP fails to modify the clinical and histological markers of disease severity. TNBS-challenged mice administered a constant infusion of VIP (5 × 10−4m) from day 0 over 7 days were monitored daily for (a) clinical score and (b) weight changes. Clinical score was a composite measure of diarrhoea, coat quality and mobility. Change in body weight was expressed as a percentage of the original body weight at the start of the experiment. (c) Histopathology score of the left, transverse and right segments of colon taken from VIP-infused TNBS mice at day 7. Data points from each result represent the mean ± s.e.m. of four independent experiments (5–13 mice/group/experiment). No significant differences in clinical score, weight and histology were observed between saline- and VIP-infused TNBS mice.
Intermittent intraperitoneal VIP in TNBS colitis
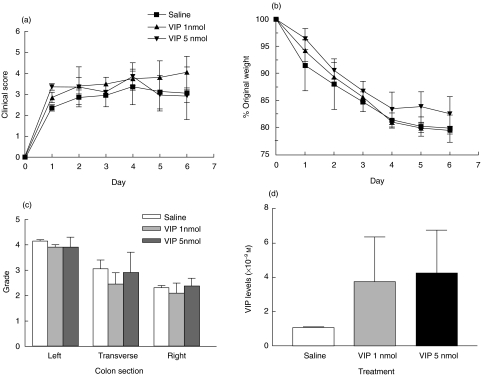
Several G protein-coupled receptors respond physiologically to pulsatile administration of their cognate ligands, rather than to steady-state levels [30]. In addition, a recent study by Abad et al. reported the therapeutic and prophylactic effects of VIP delivered intraperitoneally in mice with TNBS colitis [27]. For these reasons, VIP was administered intraperitoneally at 1 nmol or 5 nmol 12 h post-colitis induction and on alternate days over 7 days. As shown in Fig. 5a–c, and in contrast to the findings of Abad et al., VIP failed to prevent disease as assessed by clinical, weight and histological analysis. Moreover, we observed a dose-dependent increase in mortality. By day 7, mortality was 40% and 53% in 1 nmol and 5 nmol-injected TNBS mice, respectively, compared to approximately 30% in TNBS mice (Table 2). Peak VIP levels in the blood were three- or fourfold higher in 1 nmol- or 5 nmol-injected mice, respectively, when compared to saline-infused TNBS mice (Fig. 5d), again within the range in which we observe inhibition of chemokine receptors in vitro.
Fig. 5.
Intraperitoneal administration of VIP failed to prevent systemic features of colitis as assessed by (a) clinical score, (b) weight changes and (c) histopathology; 1 or 5 nmol doses of VIP were injected 12 h post-colitis induction and on alternate days over 7 days. (d) VIP levels determined by radioimmunoassay. Each data point represents the mean ± s.d. from five to 12 animals. No significant differences in clinical score, weight and histology were observed between saline- and VIP-infused TNBS mice.
VIP fails to modify Th1/Th2 cytokine profile in TNBS-challenged mice
TNBS-induced colitis is characterized by an excessive production of proinflammatory Th1 cytokines including IL-12, IFN-γ and TNF-α[3,31]. Both Th1 cytokines (TNF-α, IFN-γ, IL-2, IL-12) and anti-inflammatory Th2 cytokines (IL-4, IL-5, IL-10) were analysed in plasma samples from high-dose VIP (5 × 10−4m) or saline-infused (prophylactic mode), and i.p.-injected VIP (1 and 5 nmol) or i.p.-injected saline TNBS-challenged mice. While cytokine levels of IL-2, IL-4 and GM-CSF were below the threshold sensitivity of the assay, circulating levels of IL-5, IL-10, IL-12, IFN-γ and TNF-α were detected; however, no significant differences were observed between VIP- or saline-treated TNBS mice for both infused and i.p.-injected animals (data not shown). These results suggest that VIP failed to down-modulate Th1 responses and enhance Th2 cytokine production in TNBS-challenged mice.
Discussion
The administration of TNBS by rectal instillation in mice results in severe colitis. This model displays immunological and histological similarities to human Crohn's disease and is characterized by an excessive production of Th1 cytokines, including IL-12, INF-γ and TNF-α[3,31]. Previous studies have documented that TNBS-induced colitis responds well to a number of conventional therapies for IBD such as glucocorticoids [32], cyclosporin [33] and sulfasalazine or 5-aminosalicyclic acid [34]. Recently, however, attention has focused on using agents which target specific inflammatory molecules [12,13]. While TNBS colitis in BALB/c mice demonstrated initially a dominant Th1 response [7], subsequent studies also showed mixed Th1/Th2 responses [35]. Thus, in animal models of TNBS-induced colitis, blocking the production of Th1 cytokines by neutralizing antibodies to IL-12 or TNF-α was shown to be effective in the prevention or amelioration of gut inflammation [7,36]. Moreover, the administration of anti-adhesion molecules such as anti-CD44V7 and anti-CD11b/Cd18 which suppress recruitment and activation of circulating inflammatory cells to the gut has also been effective [37,38]. While therapeutic strategies have focused on attenuating Th1 responses, other studies have also demonstrated the use of Th2 cytokines to suppress the development of Th1 immune responses. This includes the gene-based delivery of IL-4 [39] and administration of recombinant human IL-11 in TNBS-treated rats [40].
VIP is a neuropeptide with potent anti-inflammatory activity [15,16,20]. Produced by Th2 cells following antigenic stimulation [23], and inhibiting Th1 immune responses [15,16] and chemokine-mediated mononuclear cell migration, VIP is documented to prevent or treat animal models of inflammation such as endotoxic shock [24], rheumatoid arthritis [25] and delayed-type hypersensitivity [26]. The broad anti-inflammatory properties of VIP have made it an attractive candidate for investigating the potential therapeutic and/or prophylactic effects in a model of intestinal inflammation. We demonstrated, however, that while high-dose VIP administered by infusion in an established model of IBD was able to impair chemotaxis of circulating leucocytes, it failed to ameliorate systemic features of colitis or to reduce the severity of colonic inflammation. Moreover, VIP delivered intraperitoneally also failed to improve the clinical and histological markers of disease severity. These findings are supported further by the inability of VIP to modify or regulate the Th1/Th2 cytokine profile in TNBS-challenged mice.
Chemokines and their receptors play a crucial role in regulation of the trafficking of leucocytes to sites of inflammation and immune responses [41]. In a previous study, we demonstrated that circulating mononuclear cells from VIP-infused mice subjected to a DTH challenge were unresponsive to chemokine signals as a result of cross-desensitization and phosphorylation of the chemokine receptors, CXCR4 and CCR5 [26]. The findings presented here suggest that the chemokine receptors, CCR1, CCR2, CCR5 and CXCR3, are also subject to cross-desensitization as a result of exposure to high levels of circulating VIP. However, desensitization of a number of chemokine receptors was not sufficient to control the florid inflammatory response, as reflected by the histological scores and the high numbers of mononuclear cells and neutrophils observed in VIP-treated TNBS colitis.
Given that a number of G protein-coupled hormone receptors respond physiologically to pulsatile administration of ligand rather than to steady state levels [30], we examined the effects of VIP (1 and 5 nmol) given intermittently by i.p. injection over 7 days. In contrast to the findings of Abad et al., who showed significant effects in both prevention and treatment of TNBS colitis using VIP (1 and 5 nmol) delivered intraperitoneally on alternate days [27], we observed no such effect. Several explanations for these divergent results can be postulated. First, and most importantly, the contrasting results may be explained by the relatively mild inflammation and limited systemic effects of TNBS produced in the model described by Abad et al. [27]. Comparable doses of TNBS were used in both studies and apparently identical mouse strains. The outcomes described in the present study, including a significant mortality and prominent weight loss, are very similar to previously published observations [7,42–46]. In contrast, the mice in the model reported by Abad et al. showed only a 5% weight loss. It is noteworthy that data from early dose-ranging studies in our laboratory indicated substantial animal-to-animal and experiment-to-experiment variability in the phenotype of TNBS colitis (data not shown).
Given the severity of the model, it was no surprise to observe that a significant proportion of TNBS mice did not survive the insult from TNBS (30% mortality). This was also true for other investigators, who documented mortality as high as 73% after 4–7 days post-induction of colitis [44–46]. We demonstrate that VIP administration by infusion or i.p. did not reduce mortality. Indeed, mortality was dose-dependent in both modes of VIP administration reaching as high as 65% and 53% in VIP-infused and i.p.-injected mice, respectively. Abad et al. do not report levels of mortality: if significant numbers of animals in that report are excluded via this outcome a substantially biased data set may remain, thus influencing strongly the reporting of outcomes. Moreover, it was interesting to note that no side effects of VIP were reported when used at 1 or 5 nmol, but when used at higher doses (10 nmol) VIP led to worsening of weight loss. Similarly, circulating VIP levels are not reported [27]. We observed worsened disease in all our treatment protocols as reflected by the high mortality rates, despite careful monitoring and hydration of animals. In addition, excess VIP production in humans is well recognized to cause profound watery diarrhoea [47]. Consistent with this anticipated adverse effect of VIP administration, we observed a significant increase in the frequency and severity of diarrhoea in VIP-treated animals.
In summary, our studies indicate that while VIP administered in an established model of IBD is able to impair leucocyte migration, it fails to ameliorate the clinical and histological markers of disease severity. While the Th2 effects of VIP have been well documented to inhibit cell migration and block Th1 immune responses, the excessive levels of proinflammatory cytokines produced and the range of chemokines elicited in TNBS colitis cannot be blocked by VIP. This contrasts with our previous findings with VIP administration in a model of DTH [26] and suggests that, despite demonstrable inhibition of a number of chemokine receptors, the antiphlogistic effects of VIP appear to be restricted to less severe inflammation. Combined with its evident toxicity, VIP is unlikely to provide a useful model for novel anti-IBD therapies.
Acknowledgments
The authors wish to acknowledge the assistance of Marilyn Brown (Department of Endocrinology, Royal Prince Alfred Hospital, Camperdown, NSW, Australia) who performed the radioimmunoassays for VIP on our samples. This project was supported by project grant 113853 from the National Health and Medical Research Council of Australia.
References
- 1.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–56. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan F. Crohn's disease. Lancet. 2002;359:62–9. doi: 10.1016/S0140-6736(02)07284-7. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 4.Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol Med. 2003;9:218–22. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 5.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 6.Fuss IJ, Marth T, Neurath MF, Pearlstein GR, Jain A, Strober W. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology. 1999;117:1078–88. doi: 10.1016/s0016-5085(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 7.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 9.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–73. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- 11.Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267–78. doi: 10.1002/jlb.67.3.267. [DOI] [PubMed] [Google Scholar]
- 12.Hibi T, Inoue N, Ogata H, Naganuma M. Introduction and overview: recent advances in the immunotherapy of inflammatory bowel disease. J Gastroenterol. 2003;38:36–42. [PubMed] [Google Scholar]
- 13.Wirtz S, Neurath MF. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis. 2000;15:144–60. doi: 10.1007/s003840000227. [DOI] [PubMed] [Google Scholar]
- 14.Kam LY, Targan SR. TNF-alpha antagonists for the treatment of Crohn's disease. Expert Opin Pharmacother. 2000;1:615–22. doi: 10.1517/14656566.1.4.615. [DOI] [PubMed] [Google Scholar]
- 15.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 16.Delgado M, Abad C, Martinez C, et al. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med. 2002;80:16–24. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- 17.Mutt V. Vasoactive intestinal polypeptide and related peptides. Isolation and chemistry. Ann NY Acad Sci. 1988;527:1–19. doi: 10.1111/j.1749-6632.1988.tb26968.x. [DOI] [PubMed] [Google Scholar]
- 18.Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors. neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 19.Said SI. Vasoactive intestinal polypepide − biological role in health and disease. Trends Endocrinol Metabolism. 1991;2:107–12. doi: 10.1016/s1043-2760(05)80006-2. [DOI] [PubMed] [Google Scholar]
- 20.Pozo D, Delgado M, Martínez C, et al. Immunobiology of vasoactive intestinal peptide (VIP) Immunol Today. 2000;21:7–11. doi: 10.1016/s0167-5699(99)01525-x. [DOI] [PubMed] [Google Scholar]
- 21.Delgado M, Sun W, Leceta J, Ganea D. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999;163:4213–23. [PubMed] [Google Scholar]
- 22.Delgado M, Leceta J, Abad C, Martinez C, Ganea D, Gomariz RP. Shedding of membrane-bound CD14 from lipopolysaccharide-stimulated macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide. J Neuroimmunol. 1999;99:61–71. doi: 10.1016/s0165-5728(99)00105-8. [DOI] [PubMed] [Google Scholar]
- 23.Delgado M, Ganea D. Is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001;166:2907–12. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- 24.Delgado M, Martinez C, Pozo D, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-α and IL-6. J Immunol. 1999;162:1200–5. [PubMed] [Google Scholar]
- 25.Williams RO. Therapeutic effect of vasoactive intestinal peptide in collagen-induced arthritis. Arthritis Rheum. 2002;46:271–3. doi: 10.1002/1529-0131(200201)46:1<271::AID-ART10039>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Grimm MC, Newman R, Hassim Z, et al. Cutting edge: vasoactive intestinal peptide acts as a potent suppressor of inflammation in vivo by trans-deactivating chemokine receptors. J Immunol. 2003;171:4990–4. doi: 10.4049/jimmunol.171.10.4990. [DOI] [PubMed] [Google Scholar]
- 27.Abad C, Martinez C, Juarranz MG, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–71. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 28.Grimm MC, Ben-Baruch A, Taub DD, et al. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998;188:317–25. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ameho CK, Adjei AA, Harrison EK, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–93. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason D, Hassan A, Chacko S, Thompson P. Acute and chronic regulation of pituitary receptors for vasopressin and corticotropin releasing hormone. Arch Physiol Biochem. 2002;110:74–89. doi: 10.1076/apab.110.1.74.905. [DOI] [PubMed] [Google Scholar]
- 31.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 32.Palmen MJ, Dieleman LA, Soesatyo M, Pena AS, Meuwissen SG, van Rees EP. Effects of local budesonide treatment on the cell-mediated immune response in acute and relapsing colitis in rats. Dig Dis Sci. 1998;43:2518–25. doi: 10.1023/a:1026606904531. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino H, Sugiyama S, Ohara A, Goto H, Tsukamoto Y, Ozawa T. Mechanism and prevention of chronic colonic inflammation with trinitrobenzene sulfonic acid in rats. Clin Exp Pharmacol Physiol. 1992;19:717–22. doi: 10.1111/j.1440-1681.1992.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 34.Selve N. Chronic intrajejunal TNBS application in TNBS-sensitized rats: a new model of chronic inflammatory bowel diseases. Agents Actions. 1992;Spec:C15–17. [PubMed] [Google Scholar]
- 35.Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H, McGhee JR. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999;189:1169–80. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neurath MF, Fuss I, Pasparakis M, et al. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27:1743–50. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 37.Wittig B, Schwarzler C, Fohr N, Gunthert U, Zoller M. Curative treatment of an experimentally induced colitis by a CD44 variant V7-specific antibody. J Immunol. 1998;161:1069–73. [PubMed] [Google Scholar]
- 38.Palmen MJ, Dijkstra CD, van der Ende MB, Pena AS, van Rees EP. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101:351–6. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogaboam CM, Vallance BA, Kumar A, et al. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J Clin Invest. 1997;100:2766–76. doi: 10.1172/JCI119823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu BS, Pfeiffer CJ, Keith JC., Jr Protection by recombinant human interleukin-11 against experimental TNB-induced colitis in rats. Dig Dis Sci. 1996;41:1625–30. doi: 10.1007/BF02087911. [DOI] [PubMed] [Google Scholar]
- 41.D’Ambrosio D, Panina-Bordignon P, Sinigaglia F. Chemokine receptors in inflammation: an overview. J Immunol Meth. 2003;273:3–13. doi: 10.1016/s0022-1759(02)00414-3. [DOI] [PubMed] [Google Scholar]
- 42.Stallmach A, Wittig B, Giese T, et al. Protection of trinitrobenzene sulfonic acid-induced colitis by an interleukin 2-IgG2b fusion protein in mice. Gastroenterology. 1999;117:866–76. doi: 10.1016/s0016-5085(99)70345-8. [DOI] [PubMed] [Google Scholar]
- 43.Kitani A, Fuss IJ, Nakamura K, Schwartz OM, Usui T, Strober W. Treatment of experimental (trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-beta1 plasmid. TGF-beta1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor beta2 chain downregulation. J Exp Med. 2000;192:41–52. doi: 10.1084/jem.192.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the micro opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111:1329–38. doi: 10.1172/JCI16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desreumaux P, Dubuquoy L, Nutten S, et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med. 2001;193:827–38. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugimoto K, Hanai H, Tozawa K, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–22. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 47.Bloom SR, Polak JM, Pearse AGE. Vasoactive intestinal polypeptide and watery-diarrhea syndrome. Lancet. 1973;2:14–6. doi: 10.1016/s0140-6736(73)91947-8. [DOI] [PubMed] [Google Scholar]