Abstract
Dendritic cell (DC)-based immunization represents a promising approach for the immunotherapy of cancer. The optimal conditions required to prepare DCs remain to be defined. Monocytes incubated in the presence of interferon (IFN)-β and interleukin (IL)-3 give rise to a distinct type of DCs (IFN-β/IL-3 DCs) that are particularly efficient at eliciting IFN-γ and IL-5 production by allogeneic helper T cells. We assessed the capacity of this new type of DCs to prime antigen-specific naive CD8+ T cells and compared them to the conventional DCs differentiated in the presence of granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-4 (GM-CSF/IL-4 DCs). We demonstrate that IFN-β/IL-3 DCs matured by TLR3 or CD40 ligation efficiently prime Melan-A26−35-specific CD8+ T cells in vitro, at a similar level as GM-CSF/IL-4 DCs. Activated antigen-specific CD8+ T cells produced IFN-γ and displayed potent cytotoxic activity against peptide-pulsed target cells. Expansion of CD8+ T cell numbers was generally higher following priming with CD40-L than with polyinosinic–polycytidylic acid (poly I:C) matured DCs. Cytolytic activity was induced by both maturing agents. These data indicate that IFN-β/IL-3 DCs represent a promising cell population for the immunotherapy of cancer.
Keywords: cancer, cytotoxic T cells, dendritic cells, vaccines
Introduction
Tumour-specific cytotoxic T lymphocytes play a critical role in antitumour immunity and most cancer vaccine strategies are now aimed at inducing vigorous cytolytic responses against the tumour [1]. Immunization strategies based on dendritic cells (DCs) show several advantages: powerful ability of DCs to stimulate naive T cells, availability of techniques to generate large numbers of DCs, many ways of loading antigens, possibility to enhance efficacy by transfer of genes encoding cytokines into DCs. A number of DC cancer vaccine trials have shown T cell-proliferative responses but clinical efficacy has not yet been achieved [2]. To ensure that DC immunization strategies will stimulate T cell responses stronger than current vaccination strategies [3], factors will need to be optimized, including the type of DCs used and their in vitro conditioning prior to administration [4].
DCs are highly specialized antigen-presenting cells able to efficiently induce immune responses. Their unique capacity to prime naive T cells makes them an interesting target to promote antitumoral responses [5]. Large numbers of DCs can be derived in vitro from CD14+ monocytes cultured with combinations of cytokines and growth factors. The conventional protocol is a 5-day incubation in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 [6]. In vitro-generated DCs have all the features of immature DCs, able to take up and process antigen for presentation on major histocompatibility complex (MHC) molecules. When DCs maturation is induced with stimuli such as bacterial lipopolysaccharide (LPS), polyinosinic–polycytidylic acid (poly I:C) or CD40 ligation, they up-regulate presentation and co-stimulation molecules, secrete cytokines such as IL-12 and become powerful T cell stimulators.
Recently, a distinct type of DCs has been described that is generated in vitro by incubating monocytes in the presence of interferon (IFN)-β and IL-3 (IFN-β/IL-3 DCs) [7]. These cells express higher membrane levels of CD14 and lower levels of CD1a than other types of DCs and secrete lower levels of IL-12 in response to LPS. Interestingly, IFN-β/IL-3 DCs induce strong proliferative response in mixed lymphocyte reactions and are particularly efficient at eliciting IFN-γ and IL-5 production by allogeneic helper T cells. DCs with similar characteristics have been derived by incubating monocytes in the presence of IFN-α and GM-CSF, indicating that the phenotype of this type of DCs is dependent on type I interferons, IL-3 acting as a growth factor [7,8].
The aim of this study was to compare the ability of IFN-β/IL-3 DCs and GM-CSF/IL-4 DCs to generate an efficient antigen-specific immune response. For this purpose we used an in vitro cytolytic T cell priming system based on the Melan-A antigen. Melan-A is a melanocyte antigen containing an HLA-A2 restricted epitope 26–35 recognized by a high frequency of CD8+ T cells precursors that are detectable in the blood of healthy donors [9]. This large pool of antigen-specific T cells has a naive phenotype and can be primed in vitro by professional antigen-presenting cells [9]. The use of HLA-A2/Melan-A tetrameric complexes allowed us to characterize antigen-specific CD8+ T cells phenotypically and functionally. Immature DCs and DCs matured by ligation of CD40 or by poly I:C, a double-stranded RNA mimicking viral genome and acting as a ligand for Toll-like receptor (TLR)-3, were studied. Our results demonstrate definitively that IFN-β/IL-3 DCs are very efficient at presenting exogenous peptides and are able to induce activation of antigen-specific cytotoxic T lymphocytes (CTL).
Materials and methods
Cell lines and cultures
The medium used was RPMI-1640 supplemented with 2 mml-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 1% non-essential amino acids, 1% sodium pyruvate, 5 × 105m 2-mercaptoethanol (gibco, Grand Island, NY, USA) and 10% fetal calf serum (FCS) (HyClone Laboratories, Logan, UT, USA) or 5% pooled human serum AB+. JY is an HLA-A2+ lymphoblastoid cell line.
Peptides and tetramers
Melan-A26−35 peptide ELAGIGILTV, an analogue of the 26–35 epitope with an improved HLA-A2 binding affinity [10], was purchased from Sigma-Genosys (The Woodlands, TX, USA). Melan-A/HLA-A2 tetramer was synthesized as described previously [11]. In brief, purified recombinant HLA-A*0201 and β2-microglobulin were expressed in a prokaryotic expression system and allowed to refold with the Melan-A26−35 peptide by dilution. Refolded complexes were purified by FPLC, biotinylated and combined with streptavidin–phycoerythrin (PE).
Generation and stimulation of DCs
Peripheral blood mononuclear cells (PBMC) were obtained from healthy blood donors and were screened for HLA-A2 expression by fluorescence activated cell sorter (FACS) analysis. Monocytes were purified by positive selection using anti-CD14-conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), the purity was >96% CD14+ cells. DCs were generated by culturing monocytes for 5 days in RPMI-10% FCS supplemented with GM-CSF (500 U/ml) (Leucomax, Novartis Pharma, Basel, Switzerland) and IL-4 (1000 U/ml) (produced in the laboratory as a supernatant of transfected J558 cells containing 30–50·000 U/ml) or with recombinant human IL-3 (50 U/ml) (R&D Systems, Abingdon, UK) and IFN-β (100 U/ml) (Avonex, Biogen, France). DCs (5 × 105/ml) were stimulated by addition of poly I:C (20 µg/ml) (Sigma, St Louis, MO, USA) or CD40-L transfected J558 cells (at a 1 : 5 ratio) (provided by P. Lane, Birmingham, UK). The CD14-negative fraction was frozen and thawed on the day of the in vitro priming.
T cells priming
DCs were pulsed with 100 ng/ml Melan-A26−35 for 1 h in serum-free medium and washed before incubation with the autologous CD14-negative fraction at a 1 : 15 ratio in RPMI-5% human serum. Recombinant human IL-2 (R&D Systems, Minneapolis, MN, USA) was added from days 4–7 at 10 U/ml, then at 500 U/ml when cells expanded. Melan-A-specific T cells were analysed after 9–12 days of co-culture.
Flow cytometry analysis
Cells were washed in phosphate-buffered saline (PBS) supplemented with 1% FCS and stained with PE-labelled Melan-A tetramer at 37°C for 20 min, washed at room temperature and incubated on ice with the following antibodies: anti-CD8-PerCP (BD Biosciences, Mountain View, CA, USA), CD8-fluoroscein isothyocyanate (FITC), CD45RA-FITC, CD45RO-antigen-presenting cells (APC), CD28-APC and CD27-FITC (all from PharMingen, San Diego, CA, USA). The samples were analysed on a FACSCalibur (BD Biosciences). Lymphocytes were gated according to FSC/SSC (side scatter) profiles and dead cells were excluded by staining with propidium iodide (Sigma).
HLA-A2 expression was tested using mouse antihuman HLA-A2-FITC antibodies (Serotec, Oxford, UK). The phenotype of DCs was analysed by staining on ice with the following antibodies: anti-CD11c-FITC (Dako, Carpenteria, CA, USA), Class I-FITC, Class II-PE, CD14-APC, CD123-PE, CD80-PE, CD86-APC and CD83-FITC (all from PharMingen). DCs were also stained with corresponding isotype-control monoclonal antibodies.
Intracellular staining was performed on T cells labelled with Melan-A tetramer and restimulated with 20 µg/ml Melan-A26−35 peptide in RPMI-10% FCS. Control cells were either unstimulated or treated with 10−6m phorbol myristate acetate (PMA) (Sigma) and 10 µg/ml ionomycin (Sigma). After the first hour of incubation at 37°C, brefeldin A (Sigma) was added at a final concentration of 5 µg/ml. Cells were harvested after a total of 6 h, washed, fixed and permeabilized in FACS permeabilizing solution (BD Biosciences), and then stained with anti-CD8-APC and IFN-γ-FITC antibodies (PharMingen).
Cytokine measurements
DCs supernatants were collected for IL-12 p40 and p70 measurements using enzyme-linked immunosorbent assay (ELISA, sensitivity: 33 pg/ml) (PharMingen). IFN-α levels were measured using a specific commercially available ELISA (sensitivity: 10 pg/ml, Biosource International, Fleurus, Belgium).
Cytotoxic activity
A chromium-release assay was performed against JY Epstein–Barr virus (EBV) labelled with 51Cr for 60 min at 37°C. Labelled cells were washed twice and pulsed with 1 µg/ml Melan-A26−35 peptide for 1 h. Pulsed and unpulsed cells were washed and added (5000 cells/well) to a graded number of CD8+T cells purified from the in vitro priming with anti-CD8-conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Supernatants were harvested after 5 h of incubation at 37°C and 51Cr release was measured. Spontaneous and maximum releases were determined by adding to target cells, respectively, medium only or 10% sodium dodecyl sulphate (SDS). The percentage of specific lysis was calculated as follows: 100 × (experimental −spontaneous release)/(maximum − spontaneous release). Each value represents the average of triplicates.
Results
Characterization of dendritic cells derived from monocytes cultured in the presence of IFN-β and IL-3
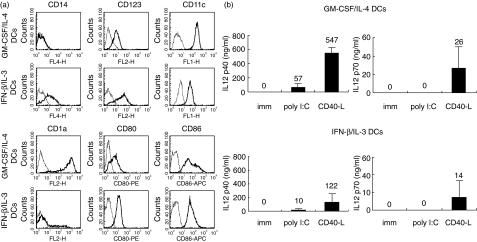
The phenotype of immature DCs generated in the presence of IFN-β/IL-3 or GM-CSF/IL-4 is presented in Fig. 1a. As reported previously, IFN-β/IL-3 DCs expressed higher levels of membrane CD14 and CD123, and lower levels of CD11c and CD1a than DCs differentiated with the conventional cytokines GM-CSF and IL-4 (GM-CSF/IL-4 DCs) [12]. The co-stimulatory molecules, CD80 and CD86, were expressed at similar levels on IFN-β/IL-3 and GM-CSF/IL-4 DCs. The production of IL-12, a cytokine promoting the development of cytolytic T cells, by immature and mature DCs is shown in Fig. 1b. Immature DCs did not secrete detectable levels of IL-12 p40 or p70. Maturation induced by CD40-L transfected cells was more efficient at inducing IL-12 p40 and p70 secretion by IFN-β/IL-3 and GM-CSF/IL-4 DCs than poly I:C. Mature IFN-β/IL-3 DCs produced lower concentrations of IL-12 p40 than GM-CSF/IL-4 DCs. Interestingly, similar concentrations of IL-12 p70 were produced by both DC types in response to CD40 ligation.
Fig. 1.
Expression of surface markers and IL-12 production by DCs derived from monocytes cultured in the presence of IFN-β and IL-3. (a) Phenotype of DCs cultured with GM-CSF and IL-4 or with IFN-β and IL-3. Thick lines show FACS profiles after staining with specific antibodies, and thin lines show FACS profile after staining with isotype control antibodies. The x axis represents fluorescence intensity and the y axis the number of events. One representative experiment of four is shown. (b) IL-12 p40 and p70 production in supernatant was measured by ELISA and results are expressed as means ± standard deviation of three independent experiments on different blood donors.
Dendritic cells derived from monocytes cultured in the presence of IFN-β and IL-3 efficiently prime antigen-specific naive CD8+ T cells
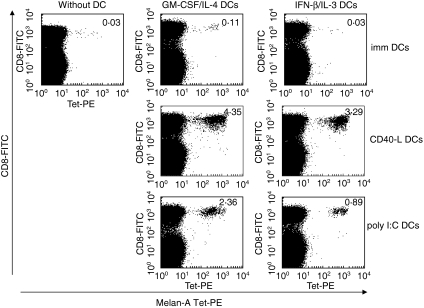
We evaluated the capacity of IFN-β/IL-3 DCs to prime antigen-specific naive CD8+ T cells, compared to GM-CSF/IL-4 DCs. DCs were generated in the presence of IFN-β/IL-3 or GM-CSF/IL-4, matured for 36 h and pulsed with the Melan-A26−35 peptide. Autologous peripheral blood lymphocytes were then co-cultured either without DC or with immature or mature DCs. When IFN-β/IL-3 DCs were matured by CD40 ligation or poly I:C, a strong proliferation of Melan-A-specific CD8+ T cells was induced, as detected by Melan-A tetramer staining (Table 1 and Fig. 2). Similar frequencies of Melan-A-specific cells were induced by mature GM-CSF/IL-4 DCs. The most potent stimulus was generally CD40 ligation but large expansions of Melan-A-specific T cells were also observed following poly I:C maturation. No significant expansions of Melan-A-specific cells were induced by immature IFN-β/IL-3 DCs. In contrast, immature GM-CSF/IL-4 DCs induced a weak expansion of antigen-specific CD8+ T cells (Table 1 and one representative experiment shown in Fig. 2).
Table 1.
Expansion of Melan-A tetramer+ cells by autologous DCs.
| GM-CSF/IL-4 DCs | IFN-β/IL-3 DCs | ||||||
|---|---|---|---|---|---|---|---|
| Without DC | imm | CD40-L | poly I:C | imm | CD40-L | poly I:C | |
| Experiment 1 | 0·07 | 0·63 | 11·17 | 9·96 | 0·13 | 5·25 | 0·42 |
| Experiment 2 | 0·07 | 0·06 | 0·73 | 0·78 | 0·06 | 0·37 | 0·10 |
| Experiment 3 | 0·06 | 0·13 | 7·02 | 13·52 | 0·09 | 11·65 | 10·78 |
| Experiment 4 | 0·03 | 0·11 | 4·35 | 2·36 | 0·03 | 3·29 | 0·89 |
DCs were generated in the presence of GM-CSF and IL-4 or IFN-β and IL-3 and were activated for 36 h with CD40-L or poly I:C. Autologous peripheral blood lymphocytes were co-cultured either without DC, or with immature or mature DCs. Values indicate the percentage of tetramer-positive cells within the CD8+ T cell population in four independent experiments.
Fig. 2.
Expansion of Melan-A tetramer+ cells by autologous DCs cultured in the presence of IFN-β and IL-3. Immature (top), CD40-L (middle) or poly I:C matured DCs (bottom) cultured in the presence of GM-CSF and IL-4 or IFN-β and IL-3 were pulsed with Melan-A peptide (100 ng/ml). Autologous peripheral blood lymphocytes were co-cultured either without DC or with immature or mature DCs at a 1 : 15 ratio and after 12 days, they were stained with Melan-A tetramer-PE and CD8-FITC. Values shown represent CD8+ tetramer+ population as a percentage of the whole CD8+ population. One representative experiment of four is shown.
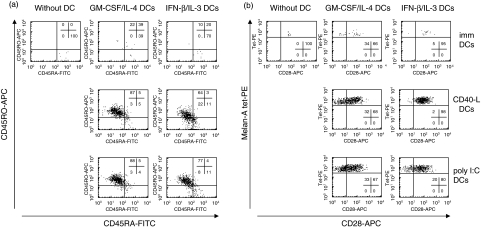
We then analysed the phenotype of Melan-A-specific T cells. One representative experiment is shown in Fig. 3. In the absence of DCs, Melan-A-specific CD8+ T cells conserved a naive phenotype, expressing CD45RA (Fig. 3a) and CD28 (Fig. 3b). Following expansion in the presence of mature DCs, Melan-A-specific T cells acquired an antigen-experienced phenotype (CD45RO+, CD45RA– and a substantial proportion of CD28– cells). This phenotype was similar when expansion of Melan-A-specific cells had been induced by IFN-β/IL-3 (ranges of CD45RO+/RA– cells in four experiments: 20–68% following CD40-L maturation and 22–77% following poly I:C maturation) or GM-CSF/IL-4 DCs (31–87% following CD40-L maturation and 58–93% following poly I:C maturation). These results demonstrate that mature IFN-β/IL-3 DCs are able to induce proliferation and differentiation of antigen-specific CD8+ T cells, in a similar way to conventional DCs.
Fig. 3.
Phenotype of Melan-A-specific CD8+ T cells primed by autologous DCs cultured in the presence of IFN-β and IL-3. Immature (top), CD40-L (middle) or poly I:C matured DCs (bottom) cultured in the presence of GM-CSF and IL-4 or IFN-β and IL-3 were pulsed with Melan-A peptide (100 ng/ml). Autologous peripheral blood lymphocytes were co-cultured either without DC or with immature or mature DCs at a 1 : 15 ratio, and after 12 days were stained with Melan-A tetramer-PE, CD8-PerCP and the following antibodies: CD45RA-FITC and CD45RO-APC (a) or CD28-FITC (b). Profiles refer to CD8+ tetramer+ cells. One representative experiment of four is shown.
Melan-A-specific CTL primed by dendritic cells cultured in IFN-β and IL-3 are functional
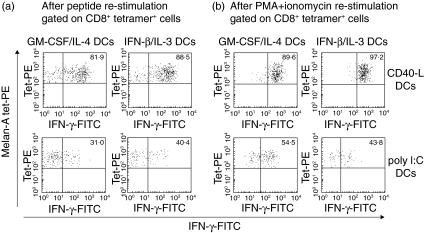
In order to assess the acquisition of effector function by expanded Melan-A-specific T cells, T cell cultures were restimulated for 6 h with Melan-A26−35 peptide before measuring the production of IFN-γ by intracellular staining (Fig. 4). We observed that a high percentage of Melan-A-specific CD8+ T cells primed with CD40-L matured IFN-β/IL-3 DCs secreted IFN-γ in a recall response to Melan-A26−35 peptide (ranges of IFN-γ-positive cells in four experiments: 33–95%) (Fig. 4a). In contrast, a minority of Melan-A-specific CD8+ T cells activated with poly I:C matured IFN-β/IL-3 DCs were able to secrete IFN-γ upon peptide restimulation (range of IFN-γ-positive cells: 7–40%). Similar proportions of IFN-γ-producing cells were detected in CD8+ T cells primed by GM-CSF/IL-4 DCs (range of IFN-γ-positive cells: 27–82% following CD40-L maturation and 14–36% following poly I:C maturation). To determine the polarization of the co-cultures, we analysed the response to PMA and ionomycin, a T cell receptor (TCR)-independent activator of T cells (Fig. 4b). Proportions of cells producing IFN-γ were similar following activation with PMA/ionomycin and with the peptide, indicating that poly I:C matured DCs induced a lower degree of functional maturation than CD40-L matured DCs.
Fig. 4.
IFN-γ production by antigen-specific CD8+ T cells primed by DCs cultured in the presence of IFN-β and IL-3. Melan-A-specific CD8+ T cells primed by autologous DCs cultured in the presence of GM-CSF and IL-4 or IFN-β and IL-3 and matured with CD40-L (top) or with poly I:C (bottom) were restimulated with 20 µg/ml of Melan-A peptide (a) or with PMA + ionomycin (b). Intracellular staining on the CD8+ tetramer+ cells is shown and the percentage of IFN-γ-producing cells is indicated. One representative experiment of four is shown.
CTL expanded by dendritic cells cultured in IFN-β and IL-3 develop cytolytic function
The cytolytic activity of Melan-A-specific CD8+ T cells expanded by CD40-L or poly I:C matured DCs was assessed in a 51Cr-release assay against an HLA-A2+ EBV-B cell line, pulsed with Melan-A26−35 peptide. As shown in Fig. 5, CD8+ T cells primed by matured IFN-β/IL-3 DCs were able to lyse peptide-pulsed target cells. At the same effector : target ratio, CTL primed by IFN-β/IL-3 or GM-CSF/IL-4 DCs showed a similar cytolytic activity (range of percentage specific lysis at the different E : T ratios observed in two experiments: 66–50% following CD40-L maturation and 81–29% following poly I:C maturation of IFN-β/IL-3 DCs; 77–48% following CD40-L maturation and 67–42% following poly I:C maturation of GM-CSF/IL-4 DCs). Moreover, despite their low capacity to produce IFN-γ, CD8+ T cells primed by poly I:C matured DCs showed a high cytolytic activity, similar to that of cells primed by CD40-L matured DCs. Thus, IFN-β/IL-3 DCs were efficient antigen-presenting cells, able to induce activation of functional antigen-specific CTL.
Fig. 5.
Cytotoxic activity of antigen-specific CTL primed by DCs cultured in the presence of IFN-β and IL-3. Melan-A-specific CTL were primed by autologous DCs cultured in the presence of GM-CSF and IL-4 or IFN-β and IL-3 and matured with poly I:C and CD40-L. CD8+ T cells were purified and tested in a 51Cr-release assay against JY, an HLA-A2+ EBV line, pulsed with 1 µg/ml Melan-A peptide (solid) or unpulsed (open). The frequencies of Melan-A tetramer+ cells after CD8+ enrichment were used to defined the effector : target ratio for each panel. One representative experiment of two is shown. Specific lysis data represent mean and standard deviation of triplicate wells.
Discussion
Immunotherapy using antigen-pulsed DCs represents a promising approach for the treatment of cancer, but the type of DCs to be used and their in vitro conditioning prior to in vivo administration remain to be optimized [3]. Large numbers of DCs can be differentiated from monocytes in vitro. Depending on the differentiation factors used, distinct populations of DCs can be generated. DCs derived from GM-CSF/IL-4 treated monocytes are efficient at priming antigen-specific CD8+ T cells in vitro[13] and are used currently in a number of clinical trials [14–16]. We have demonstrated recently that human and murine plasmacytoid DC can prime functional antigen-specific T cell responses [17,18]. Type I IFNs promote monocytes differentiation into DCs that have distinct properties but have not yet been evaluated in clinical trials [8,12]. Buelens et al. have described previously that monocytes treated with the combination of IFN-β and the survival factor IL-3 differentiate into DCs that are particularly efficient at stimulating allogeneic helper T cells [19]. We have now extended these observations by demonstrating here that IFN-β/IL-3 DCs are also potent activators of antigen-specific CD8+ T cells.
IFN-β/IL-3 DCs matured by CD40 ligation or poly I:C activated naive Melan-A-specific T cells in vitro. The magnitude of the cell expansion was similar to that induced by conventional GM-CSF/IL-4 DCs. We chose a low concentration of peptide intentionally to be able to detect potential differences in the functional capacity of the two DC populations. The antigen-experienced phenotype of antigen-specific T cells was also similar following activation with IFN-β/IL-3 or GM-CSF/IL-4 DCs. In contrast to GM-CSF/IL-4 DCs, IFN-β/IL-3 DCs did not induce detectable expansions of Melan-A-specific T cells when no DC maturation had been induced. This indicates that in vitro conditioning of IFN-β/IL-3 DCs prior in vivo administration will require a step of maturation.
Following priming by mature IFN-β/IL-3 DCs, Melan-A-specific CD8+ T cells acquired effector functions. A large proportion of Melan-A-specific T cells produced IFN-γ in response to peptide restimulation. In addition, these cells displayed potent cytolytic activity against peptide-pulsed target cells. The effector functions were similar, whether cells had been primed with IFN-β/IL-3 or GM-CSF/IL-4 DCs. Although IFN-β/IL-3 DCs were shown to stimulate allogeneic CD4+ T more efficiently than GM-CSF/IL-4 DCs, the two DC populations appear similar at activating autologous naive CD8+ T cells. Our preliminary experiments suggest that Melan-A-specific CD8+ T cells can be primed by DCs in the absence of CD4+ T cells (data not shown). An advantage of IFN-β/IL-3 DCs over GM-CSF/IL-4 DCs may therefore have been observed using a different experimental system in which the priming of antigen-specific CD8+ T cells would have been more CD4+ T cell-dependent. In addition, type I interferon could increase the capacity of DCs to cross-present antigens to CD8+ T cells [20].
Melan-A-specific CD8+ T cells displayed high and similar cytolytic activity following priming with CD40-L and poly I:C matured DCs. However, the magnitude of T cell expansion and the proportion of IFN-γ producing cells were generally higher when DCs had been matured with CD40 ligation than with TLR-3 ligation [13]. This more intense polarization correlated with a higher production of IL-12 by CD40-L matured DCs. Notably, efficient generation of tumour-antigen-specific effector T cells by CD40 ligation used as a second maturation signal was reported recently by Kalady et al. [21]. IFN-β/IL-3 DCs matured by CD40 ligation produced lower concentrations of IL-12 p40 than GM-CSF/IL-4 DCs but their production of IL-12 p70 was similar. This suggests that the p35 and p40 genes may be regulated differently in the two DC populations. Buelens et al. reported that IFN-β/IL-3 DCs produce larger concentrations of IFN-α than GM-CSF/IL-4 DCs [19]. We did not detect IFN-α in the supernatants of IFN-β/IL-3 or GM-CSF/IL-4 DCs in response to poly I:C stimulation (data not shown). This discrepancy could be related to differences in the DC preparation procedures and the presence of contaminating cells, such as natural killer (NK) cells [22]. However, we compared DCs differentiated from immunosorted CD14+ cells or from adherent cells and did not see any difference in IFN-α secretion (data not shown). Our results therefore indicate that IFN-α is not required for activation of Melan-A-specific CD8+ T cells by IFN-β/IL-3 DCs.
Potent activation of antigen-specific CD8+ T cells by DCs differentiated in the presence of type I IFNs was reported by others. Monocytes treated with a combination of IFN-α and GM-CSF were able to effectively activate virus-specific CD8+ T cells [8,23,24]. A recent report indicated that the activation of CD8+ T cells by these cells require the presence of NK cells activated by IFN-α during the process of DCs differentiation [25]. The absence of NK cells contamination in our monocytes preparation indicated that NK cell help is not required for CD8+ T cells priming by IFN-β/IL-3 DCs.
Our data demonstrate that mature DCs generated in vitro in the presence of IFN-β and IL-3 induce both expansion of antigen-specific naive T cells and acquisition of effector functions. This new subset of DCs therefore represents a promising tool for the immunotherapy of cancer and cell-based vaccination strategies.
Acknowledgments
Joelle Renneson was supported by a grant from the Fonds National de la Recherche Scientifique Télévie programme, Belgium. Naïma Mazouz was supported by Brucells S.A. and the government of the Brussels region. Arnaud Marchant is a research associate at the Fonds National de la Recherche Scientifique, Belgium. This work was funded by Cancer Research UK C399-A2291) and the Cancer Research Institute, USA.
References
- 1.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–94. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzofsky JA, Terabe M, Oh S, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–25. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5:7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- 4.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buelens C, Bartholome EJ, Amraoui Z, et al. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993–8. doi: 10.1182/blood.v99.3.993. [DOI] [PubMed] [Google Scholar]
- 8.Santini SM, Di Pucchio T, Lapenta C, Parlato S, Logozzi M, Belardelli F. A new type I IFN-mediated pathway for the rapid differentiation of monocytes into highly active dendritic cells. Stem Cells. 2003;21:357–62. doi: 10.1634/stemcells.21-3-357. [DOI] [PubMed] [Google Scholar]
- 9.Salio M, Shepherd D, Dunbar PR, et al. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J Immunol. 2001;167:1188–97. doi: 10.4049/jimmunol.167.3.1188. [DOI] [PubMed] [Google Scholar]
- 10.Romero P, Gervois N, Schneider J, et al. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201-restricted Melan-A/MART-1 antigenic peptide in melanoma. J Immunol. 1997;159:2366–74. [PubMed] [Google Scholar]
- 11.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 12.Buelens C, Bartholome EJ, Amraoui Z, et al. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993–8. doi: 10.1182/blood.v99.3.993. [DOI] [PubMed] [Google Scholar]
- 13.Salio M, Shepherd D, Dunbar PR, et al. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J Immunol. 2001;167:1188–97. doi: 10.4049/jimmunol.167.3.1188. [DOI] [PubMed] [Google Scholar]
- 14.Stift A, Sachet M, Yagubian R, et al. Dendritic cell vaccination in medullary thyroid carcinoma. Clin Cancer Res. 2004;10:2944–53. doi: 10.1158/1078-0432.ccr-03-0698. [DOI] [PubMed] [Google Scholar]
- 15.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–33. [PubMed] [Google Scholar]
- 16.Thurner B, Haendle I, Roder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–79. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salio M, Cella M, Vermi W, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–62. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 19.Buelens C, Bartholome EJ, Amraoui Z, et al. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993–8. doi: 10.1182/blood.v99.3.993. [DOI] [PubMed] [Google Scholar]
- 20.Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 21.Kalady MF, Onaitis MW, Emani S, Abdel-Wahab Z, Tyler DS, Pruitt SK. Sequential delivery of maturation stimuli increases human dendritic cell IL-12 production and enhances tumor antigen-specific immunogenicity. J Surg Res. 2004;116:24–31. doi: 10.1016/j.jss.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santodonato L, D'Agostino G, Nisini R, et al. Monocyte-derived dendritic cells generated after a short-term culture with IFN-alpha and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein–Barr virus-specific CD8+ T cell response. J Immunol. 2003;170:5195–202. doi: 10.4049/jimmunol.170.10.5195. [DOI] [PubMed] [Google Scholar]
- 24.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171:3385–93. doi: 10.4049/jimmunol.171.7.3385. [DOI] [PubMed] [Google Scholar]
- 25.Tosi D, Valenti R, Cova A, et al. Role of cross-talk between IFN-alpha-induced monocyte-derived dendritic cells and NK cells in priming CD8+ T cell responses against human tumor antigens. J Immunol. 2004;172:5363–70. doi: 10.4049/jimmunol.172.9.5363. [DOI] [PubMed] [Google Scholar]