Abstract
While most of our understanding of immune dysfunction in dialysis patients involves alterations in CD28–CD80/86 signalling, nothing is known of CD46-mediated co-stimulation of T cells in these patients. Because C3b/C4b bind to CD46 and complement activation occurs during haemodialysis (HD), we addressed whether CD46-mediated T cell activation is altered in HD (n = 9), peritoneal dialysis (PD) (n = 10) and predialysis patients (n = 8) compared to healthy controls (HC) (n = 8). T cell surface markers, T cell proliferation and interleukin (IL)-10 production were studied in CD4+T cells. In addition, CD46 splice-variants and IL-10 promoter gene polymorphisms were studied by reverse transcription (RT) or amplification refractory mutation system-polymerase chain reaction (ARMS-PCR), respectively. In all uraemic patients, irrespective of the stage of renal insufficiency or dialysis modality, a significant increase in the percentage of CD25 positivity in naive CD4+T cells was found (64% ± 21%versus 23% ± 18%, P < 0·001). Lymphocytes of HD patients proliferated in greater numbers and produced more IL-10 after co-stimulation with anti-CD46 than after co-stimulation with anti-CD28. This was also found in CD4+T cells of PD patients, albeit to a lesser extent. In contrast, with T cells of predialysis patients and of HC, co-stimulation via CD28 was more efficient. The observed alterations in T cell proliferation and IL-10 production were associated neither with CD46 splice variants nor with IL-10 promoter gene polymorphisms. Lymphocytes of HD patients show an increased response on CD46 co-stimulation. These data suggest that ongoing complement activation in HD patients may lead to alterations in acquired immunity.
Keywords: CD46, renal replacement therapy, T cell co-stimulation, uraemia
Introduction
Despite enormous improvements in the treatment of end-stage renal disease (ESRD) patients by renal replacement therapy an insidious pitfall of dialysis is still an increased risk for complications such as cardiovascular disease [1]. Uraemic toxins as well as continuous blood–membrane contact during HD sessions contribute mainly to the immune dysfunction in patients with chronic renal failure (CRF) [2]. It is generally believed that immune dysfunction in such patients is independent of the underlying disease and becomes manifest early in the course of renal insufficiency [3]. After cardiovascular disease, infections are the second most common cause of death in haemodialysis (HD) patients [4] and lead frequently to hospitalization [5]. Mortality risk due to sepsis is about 250-fold higher among HD patients than in the general population [6]. Furthermore, immune dysfunction is reflected by a poor vaccination response against T helper dependent antigens, e.g. hepatitis B or influenza, while the response against T helper independent antigens, such as pneumococci, seems to be unaffected [7–9]. Although the cause of immune dysfunction in CRF patients thus far has not completely been delineated, uraemia per se seems to play a pivotal role.
Over the past decade research has focused on the role of antigen-presenting cells (APC) that might be functionally altered in an uraemic milieu. Meuer et al. have suggested that the antigen-presenting function of monocytes is impaired in uraemic patients [10]. A few years later this was substantiated by the findings of Girndt et al., demonstrating a defective B7/CD28 pathway in haemodialysis patients [11] caused by a diminished expression of CD86 on monocytes [12].
Immune dysfunction might also occur as a consequence of uraemia-associated complement activation [13,14]. This is aggravated further by therapy modalities, especially in HD patients, due to repeated contact between mononuclear cells and the dialysis membrane. This might result in the activation of mononuclear cells and release of large quantities of cytokines [15,16]. The transmembrane protein CD46 is a ubiquitously expressed complement regulatory protein that acts as a co-factor for the cleavage of C3b and C4b complement products by factor I [17]. Recently, it has emerged that besides its binding function, CD46 also acts as alternative co-stimulatory molecule for T cells inducing interleukin (IL)-10 secreting regulatory T cells [18–20].
Among the cytokines, IL-10 has a special position by virtue of its propensity to control chronic inflammation [21,22]. High and low IL-10 producers can be distinguished genetically by single nucleotide polymorphisms (SNPs) in the promoter region of the IL-10 gene [23]. In dialysis patients low IL-10 production is associated with increased levels of C-reactive protein [24]. Moreover, a diminished vaccination response against hepatitis B is generally found in low IL-10 producers, and cardiovascular complications occurs significantly more often in this group of dialysis patients.
Paradoxically, although the role of activated complement components as endogenous adjuvants in vaccination responses has been recognized widely [25], in HD patients impairment of these responses occurs despite complement activation during dialysis sessions [8]. Therefore involvement of complement activation in immune dysfunction needs to be elucidated in more detail.
We reasoned that an increased CD46-mediated T cell activation might explain the immune dysfunction in HD patients. We therefore investigated phenotypically and functionally CD4+ T cells in HD, PD and predialysis patients and healthy controls.
Materials and methods
Patients
Nineteen chronic dialysis patients (nine HD, 10 PD patients) from our out-patient dialysis clinic and eight patients with CRF (CrCl < 20 ml/min) not yet undergoing renal replacement therapy were included in this study. The study was approved by the local ethic committee and all patients gave informed consent. Exclusion criteria were clinical signs of the presence of an acute inflammatory process, the use of immunosuppressive drugs, evidence of active malignancy, dialysis duration less than 12 months and spKt/V < 1·19 (urea) in patients undergoing chronic intermittent haemodialysis and weekly Kt/V < 2·1 (urea) in patients on PD. Patients were also excluded if leucocytes exceeded 11 000/µl or if CRP values were higher than 15 mg/l. HD patients were treated with polysulphone membranes (one of nine F60 S, one of nine F8 HPS, six of nine F7 HPS all from Fresenius, Bad Homburg, Germany, one of nine Arylane, Hospal, München, Germany), three of 10 PD patients with ‘Fresenius Stay Safe’ (Fresenius, Bad Homburg, Germany), four of 10 with ‘Baxter IDS’ and five of 10 with ‘Baxter Home Choice Pro’ (Baxter, Unterschleißheim, Germany). Pre-dialysis patients included in this study had a creatinine-clearance < 20 ml/min calculated with the Cockrofft formula and showed no clinical signs of uraemia. The underlying diseases and further clinical and laboratory characteristics are shown in Table 1. Healthy control individuals were recruited from our clinic and laboratory personnel (four female, four male) and had a mean age of 42·8 ± 15·4 years. As the age distribution was different in healthy controls and uraemic patients and to exclude an age-related bias in our analysis, we compared a group of healthy people aged 58 ± 3 years with a group aged 28 ± 3 years (n = 4 each). No age-related differences were observed.
Table 1.
Patient characteristics.
| HD patients | PD patients | Predialysis patients | |
|---|---|---|---|
| n | 9 | 10 | 8 |
| Age | 68·2 ± 13·4 | 61·6 ± 15·8 | 66·3 ± 14·1 |
| Gender (m/f) | 7/2 | 6/4 | 6/2 |
| RRT (years) | 2·4 ± 1·6 | 2·2 ± 1·6 | – |
| Diagnosis | |||
| DN | 1 | 2 | 4 |
| SLE | 1 | 0 | 0 |
| Hypernephroma | 1 | 0 | 0 |
| IgA nephropathy | 1 | 1 | 0 |
| Nephrolithiasis | 1 | 0 | 1 |
| Hypertension | 2 | 1 | 1 |
| PKD | 0 | 1 | 0 |
| GN | 0 | 2 | 0 |
| Unknown | 2 | 3 | 2 |
| Hb (g/dl) | 11·2 ± 0·9 | 12·4 ± 1·8 | 10·5 ± 1·4 |
| WBC (109 cells/l) | 7·2 ± 1·5 | 7·2 ± 1·8 | 6·5 ± 1·5 |
| CRP (mg/l) | 5·2 ± 3·9 | 3·8 ± 3·3 | 8·6 ± 4·3 |
| Albumin (g/l) | 33·2 ± 3·8 | 30·7 ± 2·3 | 30·4 ± 4·8 |
| Ca2+ (mmol/l) | 2·3 ± 0·3 | 2·3 ± 0·3 | 2·1 ± 0·2 |
 (mmol/l) (mmol/l) |
1·4 ± 0·3 | 1·4 ± 0·4 | 1·7 ± 0·2 |
| PTH (ng/l) | 96 ± 32 | 168 ± 126 | 280 ± 159 |
| Urea | |||
| (mg/dl) | 107·5 ± 39·3 | 84·9 ± 35·6 | 155·0 ± 31·7 |
| (mmol/l) | 17·9 ± 6·5 | 14·1 ± 5·9 | 25·8 ± 5·3 |
| CrCl (ml/min) | – | 105·0 ± 42·6a | 12·2 ± 5·2 |
| Creatinine | |||
| (mg/dl) | 7·1 ± 2·5 | 7·7 ± 2·9 | 5·6 ± 1·7 |
| (µmol/l) | 627·8 ± 221·0 | 687·8 ± 256·4 | 495·1 ± 150·3 |
| Kt/V | 1·6 ± 0·2 b | 2·9 ± 0·7c | – |
In PD patients combined renal and peritoneal CrCl was calculated on a weekly basis (litre*1·73 m2/week).
spKt/V (urea) was calculated according to Daugirdas and Ing.
Weekly, combined renal and peritoneal Kt/V (urea). RRT: renal replacement therapy, DN: diabetic nephropathy, SLE: systemic lupus erythematodes, PKD: polycystic kidney disease, GN: glomerulonephritis. There were no statistically significant differences between the three patient groups. Statistical analysis was performed by anova with Tukey–Kramer adjustment for multiple comparisons.
Isolation of peripheral blood mononuclear cells (PBMC) and CD4+ T cells
Peripheral blood was obtained from HD patients through the arterial access prior to dialysis session or by venipuncture in PD and predialysis patients and healthy controls. PBMCs were prepared by gradient centrifugation using Ficoll-Hypaque (Amersham Biosciences, Freiburg, Germany). For functional assays CD4+ T cells were isolated from PBMC by negative selection (Miltenyi Biotec, Bergisch-Gladbach, Germany). Overall purity of the isolated CD4+ T cells was above 95%.
Flow cytometry
Antigen expression on T lymphocyte subsets was determined by quadruple immunofluorescence staining using directly conjugated antibodies. To this end, PBMC were incubated for 30 min with specific monoclonal antibodies directed against CD4, CD45RA, CD45RO, CD25, CD28, CD46, CD69, HLA-DR and CCR7 (all from BD Biosciences, Heidelberg, Germany). The antibodies were conjugated either to flouroisothyocyanate (FITC), R-phycoerythrin (RPE), peridinin chlorophyll (PerCP) or allophycocyanin, depending on the combination of specific antibodies used. The cells were washed three times to remove unbound antibodies and finally resuspended in 400 µl of FACS solution (BD Biosciences, Heidelberg, Germany). Four-colour analysis was performed on a FACSCalibur flowcytometer (BD Biosciences, Heidelberg, Germany) and the data were analysed using winmdi 2·8 software.
T cell stimulation assays
Purified CD4+ T cells were seeded in high-binding 96-well flat-bottomed plates (Greiner Bio-One, Frickenhausen, Germany) coated with various concentrations of anti-CD3 (clone UCHT 1, R&D Systems, Wiesbaden, Germany) alone or coated with anti-CD3 in combination with either 1 µg/ml anti-CD28 (clone 37407·11, R&D Systems) or 5 µg/ml anti-CD46 (clone E4·3, BD Biosciences). The cells were cultured for 5 days in Iscove's modified Dulbecco's medium containing 10% fetal calf serum (FCS) (both from PAN Biotech, Aidenbach, Germany) and 1% penicillin/streptomycin (Sigma, St Louis, MO, USA). After 4 days 50 µl of supernatant were collected to assess IL-10 production. Subsequently 50 µl of [3H]-thymidine (1 µCi) (Amersham, Freiburg, Germany) containing culture medium was added during the final 16 h of the culturing period. The cells were harvested on special glass fibre filters (Wallac Oy, Turku, Finland) by an automatic cell harvesting system (Inotech, Dottikon, Switzerland). [3H]-thymidine incorporation was assessed by scintillation counting in a liquid scintillation counter (LS 6500, Beckman Coulter, Krefeld, Germany). IL-10 production in the supernatants was assessed by ELISA (BD Biosciences) according to the manufacturer's instructions.
DNA isolation and genotyping of the IL-10 promoter gene polymorphisms
DNA was extracted from 5 ml venous blood anticoagulated with EDTA using the Wizard® Genomic DNA Purification Kit (Promega, Mannheim, Germany) according to the manufacturer's instructions.
Genotype analysis was performed by a highly specific amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) as described previously by Girndt et al. [16]. PCR products were separated on 1·5% agarose gel.
Reverse transcription (RT)-PCR
Total RNA was extracted from PBMC and CD4+T cells using RNA-Trizol (Gibco BRL, Eggenstein, Germany) and finally dissolved in diethyl-pyrocarbonate (DEPC)-treated water. To exclude amplification of contaminating genomic DNA, DNAse I (Roche Diagnostics, Mannheim, Germany) treatment was performed on all samples prior to cDNA synthesis. Total RNA (0·5 µg) was reverse transcribed in cDNA following the instructions of SuperScript TM II Preamplification System (Life Technologies, Karlsruhe, Germany).
PCR reactions were performed as described previously [26]. Amplification was performed in a total volume of 25 µl containing 0·5 µl of cDNA, 2·5 m m of each dNTP, 2·5 U Taq DNA polymerase, 20 pmol of each primer, 1·0 m m Tris-HCl (pH 8·8), 0·15 m m MgCl2, 7·5 m m KCl. After 1·5 min denaturation at 94°C, amplification was initiated using 35 cycles, each consisting of denaturation (94°C, 0·5 min), primer annealing (65°C, 0·5 min) and primer extension (72°C, 1·5 min). PCR products were separated on precast polyacrylamide gels (Amersham, Freiburg, Germany) and stained using a sensitive silver-staining technique according to the manufacturer's manual (Amersham).
Statistical analysis
For statistical analysis Fisher's exact test, unpaired Student's t-test, Mann–Whitney U-test and anova with Tukey–Kramer adjustment for multiple comparisons (stats direct 2·2.2) were applied, when appropriate. A P-value of P < 0·05 was considered to be significant.
Results
Phenotypic changes in T cell subsets of uraemic patients
The percentage of CD4+ T cells did not differ between uraemic patients and healthy controls. Although the overall percentage of memory CD4+CD45RO+ T cells was slightly increased in uraemic patients, this did not reach statistical significance. Similarly, no differences were found between the groups in the percentage of central memory CD4+ T cells expressing the CCR7 receptor (Table 2). In contrast, the percentage of CD25 expressing cells in the naive CD4+CD45RA+ population was increased significantly in all groups of uraemic patients (P < 0·001). There was no statistically significant difference in the percentage of CD25 between the groups in the memory population.
Table 2.
FACS analysis: influence of uraemia on CD4+ T lymphocytes.
| HD patients | PD patients | Predialysis patients | Healthy controls | P-value | |
|---|---|---|---|---|---|
| CD4+ | 37 ± 10 | 50 ± 14 | 58 ± 16 | 42 ± 18 | |
| CD45RA+ | 27 ± 17 | 25 ± 11 | 30 ± 24 | 41 ± 15 | |
| CD45RA+CD25+ | 67 ± 23 | 60 ± 22 | 64 ± 16 | 23 ± 18 | P < 0·001a |
| CD45RA+CD28+ | 92 ± 10 | 96 ± 8 | 94 ± 5 | 97 ± 3 | |
| CD45RA+CCR7+ | 93 ± 9 | 94 ± 5 | 92 ± 6 | 94 ± 5 | |
| CD45RA+CD46+ | 100 ± 1 | 99 ± 2 | 99 ± 1 | 99 ± 1 | |
| CD45RO+ | 73 ± 17 | 75 ± 11 | 71 ± 24 | 59 ± 15 | |
| CD45RO+CD25+ | 74 ± 16 | 76 ± 13 | 76 ± 14 | 54 ± 28 | |
| CD45RO+CD28+ | 91 ± 9 | 94 ± 7 | 93 ± 6 | 95 ± 9 | |
| CD45RO+CCR7+ | 57 ± 17 | 65 ± 13 | 66 ± 18 | 60 ± 13 | |
| CD45RO+CD46+ | 100 ± 1 | 99 ± 1 | 99 ± 1 | 100 ± 1 |
Results are expressed as percentage of positive cells ± s.d. To analyse the expression of different surface markers four-colour FACS stainings were performed. The percentages were calculated from the total amount of CD4+ T cells positive for the specific antibodies as analysed by winmdi 2·8 software.
Statistical analysis was performed by anova with Tukey–Kramer adjustment for multiple comparisons.
The surface expressions of CD28 and CD46 were analysed to assess a potential influence on CD28- and CD46-mediated co-stimulation. Both CD28 and CD46 were expressed uniformly on CD4+T cells from both uraemic patients and healthy controls.
CD46-mediated T cell proliferation
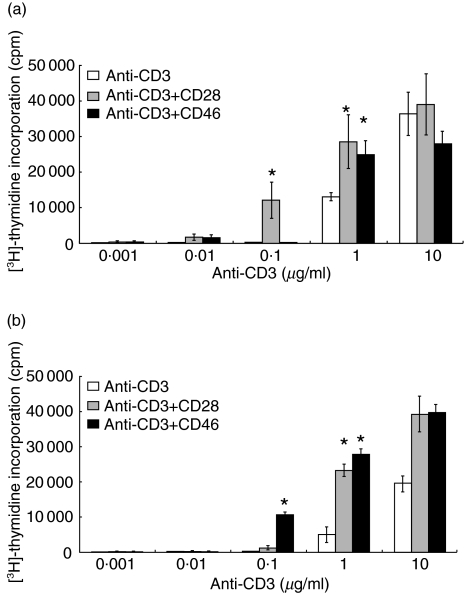
Because complement activation occurs during dialysis sessions in HD patients, we next investigated whether CD46-mediated T cell co-stimulation was different in uraemic patients and whether this was associated with dialysis modality. To establish co-stimulatory conditions, we first used different concentrations of plate-bound anti-CD3 either alone or in conjunction with a fixed amount of plate-bound anti-CD46 (5 µg/ml) or anti-CD28 (1 µg/ml). We used fixed amounts of antibodies for CD28 (1 µg/ml) and CD46 (5 µg/ml). In our system these concentrations appeared to be optimal, i.e. higher concentrations of either CD28 or CD46 did not result in a stronger co-stimulatory acitivity. In contrast, decreasing the concentrations of the antibodies directly affected the co-stimulatory capacities (data not shown). Although complement activation is occurring in all uraemic patients, HD patients are most affected. Therefore CD4+ T cells obtained from three HD patients and healthy controls, both selected randomly, were used to set up the experimental conditions (Fig. 1). While, at a concentration of 0·1 µg/ml of anti-CD3, the addition of anti-CD28 increased T cell proliferation strongly in healthy controls (Fig. 1a), this was not observed in HD patients (Fig. 1b). However, using this concentration of anti-CD3, the addition of anti-CD46 resulted in a significant increase in T cell proliferation in HD patients (Fig. 1). This was not observed in healthy controls. At high concentrations of anti-CD3 there were no differences in T cell-mediated co-stimulation, either for CD28 or for CD46 between uraemic patients and healthy controls. The co-stimulatory capacity of anti-CD28 or anti-CD46 was most efficient under conditions of 0·1 µg/ml of anti-CD3. High concentrations of anti-CD3 (1·0 µg/ml or above) resulted in T cell activation that was partially independent of co-stimulation. Therefore, all further experiments were performed using 0·1 µg/ml anti-CD3.
Fig. 1.
Effects of co-stimulatory molecules on CD4+ T cell proliferation. To determine an anti-CD3 concentration requiring co-stimulatory molecules implicitly, various concentrations with or without antibodies for the examined co-stimulatory molecules were assessed in healthy controls (a) and HD patients (b). Results are depicted as mean value ± s.d. *P < 0·001 compared to stimulation with anti-CD3 alone. Data are shown from single representative experiments that have been repeated in three subjects. Statistical analysis was performed by unpaired t-test.
In HD patients CD46-mediated T cell proliferation was significantly stronger compared to CD28-mediated T cell proliferation (P < 0·0001, Fig. 2). T cells from PD patients also showed a tendency towards an increased stimulation through CD46. This was found neither in predialysis patients nor in healthy controls. In healthy controls CD28-mediated T cell proliferation was significantly stronger than in HD patients (P = 0·035), while there was a tendency towards a more efficient T cell activation through CD46 in HD patients than in healthy controls. Statistically significant differences were demonstrated in CD46-mediated T cell proliferation between HD and predialysis patients (P = 0·027, Fig. 2). To extend these findings the co-stimulatory index, defined by the quotient of T cell proliferation under conditions of anti-CD3 and anti-CD46 or anti-CD28 stimulation divided by T cell proliferation using anti-CD3 alone, was determined in HD, PD and predialysis patients and in healthy controls. In HD patients, CD46-mediated co-stimulation was more pronounced compared to co-stimulation through CD28 (P < 0·001, data not shown). This was also found to a lesser extent in PD patients. In contrast, both in predialysis patients and healthy controls, CD28-mediated co-stimulation was significantly stronger when compared to co-stimulation through CD46. Moreover, CD46-mediated co-stimulation was significantly stronger in HD patients compared to predialysis patients or healthy controls, while CD28-mediated co-stimulation was significantly higher in healthy controls compared to HD patients (data not shown).
Fig. 2.
CD4+ T cell proliferation: influence of CD28- and CD46-mediated co-stimulation. CD4+T cell proliferation was determined by [3H]-thymidine incorporation after 5 days. CD4+ T cells were stimulated with anti-CD3, anti-CD3 and anti-CD28 or anti-CD3 and anti-CD46, respectively. Mean values are marked as black line (−). Statistical analysis was performed by unpaired t-testa and anova with Tukey–Kramer adjustment for multiple comparisonsb.
CD46-mediated IL-10 production
Similarly to CD46-mediated T cell proliferation, we tested if CD46-mediated IL-10 production was different in uraemic patients compared to healthy controls. IL-10 production using either anti-CD3 alone or in combination with CD28 or anti-CD46 was heterogeneous in the studied groups and ranged from 161 to 1750 pg/ml for anti-CD3 alone, from 288 to 9754 pg/ml for anti-CD3 and anti-CD28 and from 101 to 8403 pg/ml for anti-CD3 and anti-CD46. No statistical differences in the absolute amount of IL-10 were found between the groups (data not shown). However, in lymphocytes of HD patients significantly more IL-10 was produced after CD46 than after CD28 co-stimulation (P = 0·007, Table 3). No association between IL-10 production and SNP genotypes was found. The analysed SNP genotypes were distributed equally between uraemic patients and healthy controls (data not shown). When the co-stimulatory effect on IL-10 production was assessed by calculating the co-stimulatory index, again in HD patients co-stimulation via CD46 was significantly stronger compared to co-stimulation through CD28 (data not shown).
Table 3.
Effects of co-stimulatory molecules on IL-10 production of CD4+T cells.
| Anti-CD3 | Anti-CD3 + anti-CD46 | Anti-CD3 + anti-CD28 | P-value | |
|---|---|---|---|---|
| HD patients | 443 ± 209 | 2130 ± 1306 | 480 ± 114 | P = 0·007a |
| PD patients | 634 ± 419 | 2870 ± 2495 | 2195 ± 2352 | |
| Predialysis patients | 670 ± 313 | 1415 ± 1517 | 1809 ± 1828 | |
| Healthy controls | 541 ± 352 | 1935 ± 2641 | 2629 ± 1935 |
CD4+ T cells from HD patients (n = 9), PD patients (n = 10), predialysis patients (n = 8) and healthy controls (n = 8) were stimulated with anti-CD3, anti-CD3 and anti-CD28 or anti-CD3 and anti-CD46. The effects of co-stimulatory molecules on IL-10 production of CD4+ T cells were assessd by the ELISA technique. Results are expressed as mean value ± s.d. (pg/ml).
Two-sided Student's t test was used for statistical analysis.
CD46 splice variants
Inasmuch as we could demonstrate that co-stimulation through CD46 was significantly stronger in HD patients compared to healthy controls, we next addressed the question, if this was associated with different CD46 isoforms generated by alternative splicing. Four major splice variants can be detected by RT-PCR, i.e. the extracellular B domain is either or not present resulting in BC or C variants that can couple to one of the cytoplasmic tails Cyt1 or Cyt2. Based on the distribution of these splice variants individuals can be divided into three phenotypes [27]:
In type L the C containing splice variants, coupled either to Cyt1 or Cyt2, are expressed predominantly.
In type E the BC or C containing splice variants, coupled predominantly to Cyt2, are expressed equally.
In contrast, the BC containing splice variants, either coupled to Cyt1 or Cyt2, are represented more in type U.
T cell stimulation itself, irrespective of the antibodies used, did not influence CD46 splicing (data not shown). As isoform specific tyrosine phosphorylation has been described for Cyt2 only, this could account theoretically for differences in co-stimulatory capacity. Therefore all tested individuals were grouped according to their distribution of CD46 splice variants and the co-stimulatory index calculated for T cell proliferation and IL-10 production using the combination of anti-CD3 and anti-CD46. No differences in CD46-mediated co-stimulation were found between CD46 phenotypes (data not shown).
Discussion
The main findings of this study are: first, T cell activation occurs in uraemic patients independent of dialysis modality, as demonstrated by an increased CD25 expression in the naive population of CD4+ T cells only. Secondly, in HD patients CD46-mediated T cell co-stimulation resulted in a significantly increased T cell proliferation compared to CD28-mediated co-stimulation, while the reverse was true for predialysis patients and healthy controls. Thirdly, lymphocytes of HD patients produced significantly more IL10 after co-stimulation with anti-CD46 than after co-stimulation with anti-CD28.
While an increased CD25 expression in T cells of HD patients has been observed previously [28–33], the role of uraemia versus dialysis in this abnormality remain to be elucidated in more detail. In our study, the increased CD25 expression in CD4+ T cells was found in all uraemic patients, irrespective of dialysis modality, suggesting an influence of uraemia per se. CD25 expression in the naive CD45RA population is believed to be an early sign of T cell activation, which normally may progress to the generation of post-activated memory CD45RO+ T cells. No differences in the percentage of memory CD45RO+ T cells, however, were found between uraemic patients and healthy controls. This might indicate that the type of activation observed in uraemic patients was somewhat aborted or insufficient and is most probably not indicating activated T cells directed against certain specific antigens. To provide further evidence for the existence of an insufficient T cell activation in uraemic patients, we also investigated the expression of CD69, another early marker of T cell activation, and HLA-DR, thought to be expressed late in T cell activation. Although CD4+ T cells from uraemic patients revealed increased levels of CD25 in the naive CD45RA population, we did not observe an increased expression either of CD69 or of HLA-DR (data not shown).
The expression of the co-stimulatory molecules CD28 and CD46 was not altered among the individuals tested. However, lymphocytes of HD patients proliferated more strongly and produced more IL-10 than lymphocytes from healthy controls after stimulation with 0·1 µg/ml of anti-CD3 and anti-CD46 (5 µg/ml). It thus seems that HD patients and healthy controls are the outer edges of a continuum whose centre is formed by PD and predialysis patients. The alterations in PD patients tend towards those seen in HD patients, whereas predialysis patients showed a co-stimulation pattern more similar to healthy controls. These data do not imply that CD46 does not co-stimulate T cells in healthy controls, as in the presence of higher concentrations of anti-CD3 monoclonal antibodies CD46-mediated co-stimulation was also observed in healthy controls. Similarly, CD28-mediated co-stimulation in HD patients was also found using 1 µg/ml of anti-CD3.
The increased IL-10 production could not be explained by differences in IL-10 promoter gene polymorphisms, as the genotype distribution of analysed SNPs was equal in HD patients and healthy controls. In addition, it must be stressed that the findings of different types of IL-10 producers has been demonstrated only for monocytes, while similar data for T cells are lacking thus far. In fact, regulation of IL-10 production in T cells and monocytes seems to differ, as only in monocytes but not in T cells enhancement in IL-10 production is accomplished through binding of transcription factors to cAMP-responsive elements in the IL-10 promoter [34].
Our data are somewhat in conflict with previously published data of Kemper et al. [20], who suggested that IL-10 production by T cells is mediated predominantly by CD46 engagement. In contrast, our data indicate clearly that CD4+ T cells also produce IL-10 when stimulated with anti-CD3 and anti-CD28. The amount of anti-CD3 used to stimulate T cells in the study of Kemper et al. was 100 times higher than in our study, which makes a direct comparison difficult. Moreover, CD46-mediated IL-10 production was accomplished either by adding exogenous IL-2 or by including anti-CD28 as additional T cell stimulatory factor [20]. Because the aim of our study was primarily to analyse the co-stimulatory capacity of CD46 and CD28, we have chosen to use low concentrations of anti-CD3 (0·1 µg/ml) without addition of exogenous IL-2, to ascertain that the measured stimulation was maximally co-stimulation-dependent.
Several CD46 isoforms can be expressed on T cells as a result of alternative splicing [26,27]. These isoforms contain one of two cytoplasmic domains termed CyT 1 and CyT 2. The ability of these cytoplasmic sequences to couple with intracellular signalling pathways is likely to determine the nature of the cellular response upon ligand binding. Based on the relative distribution of the isoforms, three phenotypes can be distinguished. Uraemic patients express all known CD46 splice variants. As tyrosine phosphorylation by scr kinases occurs only for CyT 2 [26], and CyT 2 is represented more strongly in phenotype E compared to phenotype U, this could account theoretically for the differences in CD46-mediated co-stimulation. Indeed, Marie et al. showed in a transgenic mice model that contact hypersensitivity reaction was decreased upon co-stimulation through CD46 CyT 1. In contrast, CD46 CyT 2 co-stimulation resulted in an increased reaction [35]. However, no differences in co-stimulatory capacity of CD46 were found between individuals with phenotype E or phenotype U, sugggesting that quantitative differences in CyT 2 containing isoforms were not likely to explain our results.
Thus renal insufficiency per se seems to have a major impact on T cell co-stimulation, which is influenced strongly by therapy modalities. Given the co-stimulatory characteristics of CD46 and the presence of chronic complement activation, T cells in uraemic patients may have a lower activation threshold. It remains to be elucidated if the altered CD46-mediated co-stimulatory pathway is misguiding and consequently affecting the cellular immune response in uraemic patients.
The inclusion of patients with immunologically mediated primary renal disease may be a confounding factor in our study. However, the T cell response from these patients did not differ from those with non-immunologically mediated renal diseases. Furthermore, these patients did not show any signs of active immunological disease at the time of this study.
We conclude that lymphocytes of HD patients proliferate more strongly and produce more IL-10 after co-stimulation with CD46 than after co-stimulation with CD28. In healthy controls and predialysis patients the reverse phenomenon was observed. Our findings suggest that ongoing complement activation in uraemic patients, particularly in HD patients, may contribute substantially to a dysregulation of acquired immunity. The presence of insufficient activated naive T cells and poor vaccination response in HD patients could, at least partially, be explained by this phenomenon.
Acknowledgments
The authors would like to thank M. Girndt and C. Ulrich (Medical Department IV, University Homburg/Saar, Germany) for their useful information and assistance on IL-10 genotyping.
References
- 1.Tsakiris D, Jones EHP, Briggs JD, et al. Deaths within 90 days from starting renal replacement therapy in the ERA–EDTA Registry between 1990 and 1992. Nephrol Dial Transplant. 1999;14:2343–50. doi: 10.1093/ndt/14.10.2343. [DOI] [PubMed] [Google Scholar]
- 2.Opatrny K., Jr Clinical importance of biocompatibility and its effect on haemodialysis treatment. Nephrol Dial Transplant. 2003;18:41v–44. doi: 10.1093/ndt/gfg1044. [DOI] [PubMed] [Google Scholar]
- 3.Goldblum SE, Reed WP. Host defences and immunologic alterations associated with chronic hemodialysis. Ann Intern Med. 1980;93:597–613. doi: 10.7326/0003-4819-93-4-597. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System (USRDS) US Renal Data System 2003 annual report. Available at: http://www.usrds.org.
- 5.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients. Incidence, risk factors, and prognosis. Kidney Int. 1999;55:1081. doi: 10.1046/j.1523-1755.1999.0550031081.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–64. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Cavdar C, Sayan M, Sifil A, et al. The comparison of antibody response to influenza vaccination in continuous ambulatory peritoneal dialysis, hemodialysis and renal transplantation patients. Scand J Urol Nephrol. 2003;37:71–6. doi: 10.1080/00365590310008749. [DOI] [PubMed] [Google Scholar]
- 8.Koehler H, Arnold W, Renschin G, Dormeyer HH, Meyer zum Büschenfelde KH. Active hepatitis B vaccination of dialysis patients and medical staff. Kidney Int. 1984;25:124–8. doi: 10.1038/ki.1984.18. [DOI] [PubMed] [Google Scholar]
- 9.Friedman EA, Beyer MM, Hirsch SR, Schiffman G. Intact antibody response to pneumo-coccal capsular polysaccharides in uraemia and diabetes. JAMA. 1980;244:2310–11. [PubMed] [Google Scholar]
- 10.Meuer SC, Hauer M, Kurz P, Meyer zum Buschenfelde KH, Kohler H. Selective blockade of the antigen-receptor-mediated pathway of T cell activation in patients with impaired primary immune responses. J Clin Invest. 1987;80:743–9. doi: 10.1172/JCI113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girndt M, Kohler H, Schiedhelm-Weick E, Meyer zum Buschenfelde KH, Fleischer B. T cell activation defect in hemodialysis patients: evidence for a role of the B7/CD28 pathway. Kidney Int. 1993;44:359–65. doi: 10.1038/ki.1993.252. [DOI] [PubMed] [Google Scholar]
- 12.Girndt M, Sester M, Sester U, Kaul H, Kohler H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uraemia-associated immune defect. Kidney Int. 2001;59:1382–9. doi: 10.1046/j.1523-1755.2001.0590041382.x. [DOI] [PubMed] [Google Scholar]
- 13.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int. 2001;59:407–14. doi: 10.1046/j.1523-1755.2001.059002407.x. [DOI] [PubMed] [Google Scholar]
- 14.Krediet RT, Asghar SS, Koomen GC, Venneker GT, Struijk DG, Arisz L. Effects of renal failure on complement C3d levels. Nephron. 1991;59:41–5. doi: 10.1159/000186515. [DOI] [PubMed] [Google Scholar]
- 15.Girndt M, Sester U, Kaul H, Kohler H. Production of proinflammatory and regulatory mo-nokines in hemodialysis patients shown at a single-cell level. J Am Soc Nephrol. 1998;9:1689–96. doi: 10.1681/ASN.V991689. [DOI] [PubMed] [Google Scholar]
- 16.Koehler H, Girndt M, Dumann H, Klingel R. Immundefekt bei Niereninsuffizienz, Teil II. Mechanismen des ‘urämischen’ Immundefektes. Dtsch Med Wochenschr. 1993;118:790–5. doi: 10.1055/s-2007-1024153. [DOI] [PubMed] [Google Scholar]
- 17.Kojima A, Iwata K, Seya T, et al. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol. 1993;151:1519–27. [PubMed] [Google Scholar]
- 18.Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge. CD46, a new co-stimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–5. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- 19.Zaffran Y, Destaing O, Roux A, et al. CD46/CD3 co-stimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–5. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- 20.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 21.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy − review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 22.Girndt M, Kohler H. Interleukin-10 (IL-10): an update on its relevance for cardiovascular risk. Nephrol Dial Transplant. 2003;18:1976–9. doi: 10.1093/ndt/gfg311. [DOI] [PubMed] [Google Scholar]
- 23.Girndt M, Sester U, Sester M, et al. The interleukin-10 promoter genotype determines clinical immune function in hemodialysis patients. Kidney Int. 2001;60:2385–91. doi: 10.1046/j.1523-1755.2001.00062.x. [DOI] [PubMed] [Google Scholar]
- 24.Girndt M, Kaul H, Sester U, et al. Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int. 2002;62:949–55. doi: 10.1046/j.1523-1755.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Stager S, Alexander J, Kirby AC, et al. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T cell responses. Nat Med. 2003;9:1287–92. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Liszewski MK, Chan AC, Atkinson JP. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J Immunol. 2000;64:1839–46. doi: 10.4049/jimmunol.164.4.1839. [DOI] [PubMed] [Google Scholar]
- 27.Ballard L, Seya T, Teckman J, Lublin DM, Atkinson JP. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45–70) J Immunol. 1987;138:3850–5. [PubMed] [Google Scholar]
- 28.Beaurain G, Naret C, Marcon L, et al. In vivo T cell preactivation in chronic uraemic hemodialyzed and non-hemodialyzed patients. Kidney Int. 1989;36:636–44. doi: 10.1038/ki.1989.240. [DOI] [PubMed] [Google Scholar]
- 29.Dumann H, Meuer S, Meyer zum Buschenfelde KH, Kohler H. Hepatitis B vaccination and interleukin 2 receptor expression in chronic renal failure. Kidney Int. 1990;38:1164–8. doi: 10.1038/ki.1990.328. [DOI] [PubMed] [Google Scholar]
- 30.Donati D, Degiannis D, Homer L, Gastaldi L, Raskova J, Raska K., Jr Immune deficiency in uraemia: interleukin-2 production and responsiveness and interleukin-2 receptor expression and release. Nephron. 1991;58:268–75. doi: 10.1159/000186435. [DOI] [PubMed] [Google Scholar]
- 31.Libetta C, Rampino T, Dal Canton A. Polarization of T helper lymphocytes toward the Th2 phenotype in uraemic patients. Am J Kidney Dis. 2001;38:286–95. doi: 10.1053/ajkd.2001.26092. [DOI] [PubMed] [Google Scholar]
- 32.van Riemsdijk IC, Baan CC, Loonen EHM, et al. T cells activate the tumor necrosis factor system during hemodialysis, resulting in tachyphylaxis. Kidney Int. 2001;59:883–92. doi: 10.1046/j.1523-1755.2001.059003883.x. [DOI] [PubMed] [Google Scholar]
- 33.Kelly CJ. T cell function in chronic renal failure and dialysis. Blood Purif. 1994;12:36–41. doi: 10.1159/000170143. [DOI] [PubMed] [Google Scholar]
- 34.Riese U, Brenner S, Docke WD, et al. Catecholamines induce IL-10 release in patients suffering from acute myocardial infarction by transactivating its promoter in monocytic but not in T cells. Mol Cell Biochem. 2000;212:45–50. [PubMed] [Google Scholar]
- 35.Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–66. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]