Abstract
The fibrotic and antiapoptotic effects of insulin-like growth factors (IGF) are mediated by type I IGF receptor (IGF-1R). IGFs could play a role in intestinal stricturing and in the maintenance of inflammation in Crohn's disease (CD). We aimed to describe IGF-1R expression in CD intestinal lesions, to compare it to other intestinal inflammatory diseases and to correlate it with fibrosis and apoptosis. IGF-1R expression and apoptosis (active caspase-3) were studied by immunohistochemistry. Surgical intestinal specimens [17 CD, nine controls, six diverticulitis and four ulcerative colitis (UC)] were used. IGF-1R was expressed transmurally mainly by inflammatory cells (IC) and smooth muscle cells, both in diseased intestine and controls. IGF-1R positive IC were increased in the mucosa and the submucosa of CD (P < 0·007), and in involved areas compared to uninvolved areas (P = 0·03). In UC, the number of IGF-1R positive IC was only increased in the mucosa, and was not different from controls in the submucosa. In diverticulitis, the number of IGF-1R positive IC did not differ from controls. In CD submucosa, IGF-1R expression in IC was inversely correlated with apoptosis in uninvolved areas (P = 0·01). Expression of IGF-1R in submucosal fibroblast-like cells, subserosal adipocytes and hypertrophic nervous plexi was specific for CD. We have shown a transmural altered expression of IGF-1R in CD. This may suggest a role for IGF-1R in the maintenance of chronic inflammation and stricture formation in CD.
Keywords: apoptosis, Crohn's disease, inflammation, insulin-like growth factor receptor
Introduction
Crohn's disease (CD) is a chronic inflammatory disorder of the digestive tract. Intestinal lesions of CD are characterized by a transmural inflammatory infiltrate, mucosal ulcerations and non-caseating granulomas. The precise mechanisms underlying this chronic inflammation are unknown, but the relative resistance of inflammatory cells (IC) to apoptosis may play a critical role [1,2]. Chronic inflammation of the bowel wall in CD may lead to stricture formation. These strictures are characterized by thickening of the intestinal wall, diffuse fibrosis, increased collagen deposition and smooth muscle cells hyperplasia [3]. This complication is the main reason for intestinal surgical resection in CD.
Insulin-like growth factors 1 (IGF-1) and 2 (IGF-2) are potentially relevant mediators in the chronic inflammation characterizing CD because of their profibrogenic actions [4] and their antiapoptotic effect [5]. IGF-1 and -2 mediate the majority of their biological actions through type I insulin-like growth factor receptor (IGF-1R) [6]. IGF-1 increases collagen synthesis in rat intestinal smooth muscle cells [7]. Insulin at concentrations that activate IGF-1R is also able to stimulate collagen synthesis by fibroblasts isolated from the intestinal wall in CD [8]. IGFs stimulate proliferation of fibroblasts [9], myofibroblasts [10] and smooth muscle cells [11]in vitro. IGFs, through activation of IGF-1R, can protect several types of cells from apoptosis, including fibroblasts [12], muscle cells [13], lymphocytes [14] and colon carcinoma cells [15]. In contrast, decreased expression of IGF-1 and IGF-1R in advanced atherosclerotic intima may contribute to apoptosis of smooth muscle cells leading to plaque weakening and rupture [16].
IGF-1 was detected by enzyme-linked immunosorbent assay (ELISA) in whole gut fluid lavage in 78·6% of patients with intestinal strictures associated with CD and other diseases versus 11·6% of controls [17]. Moreover, IGF-1 and IGF-2 mRNA were shown to be overexpressed in diseased intestine of CD compared to uninvolved areas and, for IGF-1 mRNA, mainly in strictured compared to ulcerated segments [18]. IGF-1 has been detected by immunohistochemistry in normal intestine in lamina propria mononuclear cells and in CD throughout the bowel wall, following the intensity of the inflammatory infiltrate [19]. Another study localized IGF-1 mRNA in CD to immune cells of the lamina propria, fibroblast-like cells of the submucosa and muscularis propria of both normal-appearing and fibroinflammatory CD, whereas positive smooth muscle cells were seen only in fibroinflammatory regions [20].
IGF-1R is expressed in human intestinal smooth muscle cells [21] and in human colon adenocarcinoma cell line Caco2. IGF-1R is also present on T cells and its expression is increased in activated T cells [14]. In normal rat intestine, IGF-1R expression was localized mainly to the muscularis propria and the mucosa [22]. This expression of IGF-1R in the intestinal wall could be relevant to Crohn's disease through IGF's antiapoptotic and profibrotic actions.
Our aims were to describe intestinal expression of IGF-1R in CD, to compare it with other inflammatory states [ulcerative colitis (UC) and diverticulitis] and to correlate it with fibrosis and apoptosis.
Materials and methods
We used paraffin-embedded surgical tissue fragments from patients undergoing intestinal resection for a complicated CD or UC, for diverticulitis and for colonic adenocarcinoma. From our hospital archives of pathology, we selected 17 cases of colonic CD, six diverticulitis, four UC and nine colon carcinoma patients. The slides used for diagnosis of CD were reviewed in order to identify histologically involved (n = 17) or uninvolved areas (n = 15). For 15 cases of CD a tissue sample was selected in both involved and uninvolved areas. The corresponding paraffin-embedded bloc was used. Normal tissue from colonic resections for adenocarcinoma was used as controls. Inflamed tissue from UC and diverticulitis was used as inflammatory controls. Serial sections were carried out for each paraffin bloc.
Immunohistochemistry for IGF-1R and active caspase-3
Tissue slides were deparaffinized, rehydrated, preincubated in goat serum for 30 min and then incubated with primary antibody at room temperature for 2 h at a 1 : 75 dilution for anti-IGF-1R (IGF-1Rα, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and for 30 min at a 1 : 700 dilution for anticaspase3 (rabbit antihuman/mouse caspase-3 active, R&D Systems, Oxon, UK). Sections were then incubated for 30 min with the secondary biotinylated goat antirabbit antibody (Dako, Heverlee, Belgium). Binding of the primary antibody was detected with the streptavidine–HRP complex for 30 min. Each washing step was performed using phosphate buffered saline/Tween (3 × 5 min). Revelation occurred after an 8-min exposure with DAB (Dako) for IGF-1R and 30 min with AEC (Dako) for caspase. Slides were counterstained with haematoxylin for 10 s.
Negative controls for IGF-1R consisted in neutralizing the primary antibody by preabsorption (for 2 h at room temperature) with an excess (fivefold by weight) of blocking peptide (Santa Cruz Biotechnology). For caspase-3 we omitted the primary antibody as a negative control. These negative controls were uniformly and strictly negative.
Immunohistochemistry evaluation
IGF-1R
IGF-1R immunostaining was analysed semiquantitatively by counting positive IC with the help of a computerized image-analysis system at a magnification of ×400. Because of a clustered distribution of IGF-1R-positive IC, a mean count of three highly stained areas (‘hot spots’) was performed as described previously [23]. For each slide, three fields were captured in both the lamina propria and the submucosa and the IC were counted. A mean value was calculated for total and positive cells by field. Results were expressed as absolute and relative values (% of positive IC by field). Quantitative evaluation could not be performed on fibroblasts expressing IGF-1R because these cells were absent from normal colon and scattered in CD.
Active caspase-3
Detection of apoptotic cells by immunohistochemistry with anti-active caspase-3 has been shown to be a validated method to study apoptosis [24]. Because active caspase-3 positive cells were distributed in a more diffuse way, the number of positive cells was evaluated for each layer (mucosa, submucosa) after viewing the whole slide (at magnification × 200). The slides used for this evaluation were immediately adjacent to the ones used for IGF-1R. The mean number of apoptotic IC cells by field was established. Relative values were calculated by dividing these numbers with the total number of IC by field.
Fibrosis evaluation (Table 1)
Sections immediately adjacent to those used for immunohistochemistry were stained with Masson's trichrome and counterstained with haematoxylin to analyse collagen deposition. Fibrosis was evaluated using a 1–3 scale (1, negative or discrete staining; 2, moderate staining; 3, strong staining).
Table 1.
Fibrosis status in involved (n = 17) and uninvolved (n = 15) areas of CD cases. Slides were stained with Masson's trichrome; fibrosis was evaluated using a scale from 1 to 3 (1: negative or discrete staining; 2: moderate staining; 3: strong staining)
| 1 | 2 | 3 | |
|---|---|---|---|
| Uninvolved CD | 15 | 0 | 0 |
| Involved CD | 5 | 4 | 8 |
CD: Crohn's disease.
Statistics
Absolute and relative numbers of IGF-1R positive cells were compared for significant difference using the Mann–Whitney U-test or Student's unpaired t-test with Welch correction (when the values passed normality, the Kolmogorov–Smirnov test), between CD, controls, UC and diverticulitis, and between involved and uninvolved CD. Paired comparison between involved and uninvolved areas of CD on the same patient was performed using Wilcoxon's matched-pairs signed-rank test. A correlation was searched between numbers (absolute and relative) of IGF-1R positive cells and caspase-3 positive cells, on one hand, or fibrosis score on the other hand, using Spearman's rank test. P < 0·05 was considered statistically significant.
Results
Various types of cells express IGF-1R
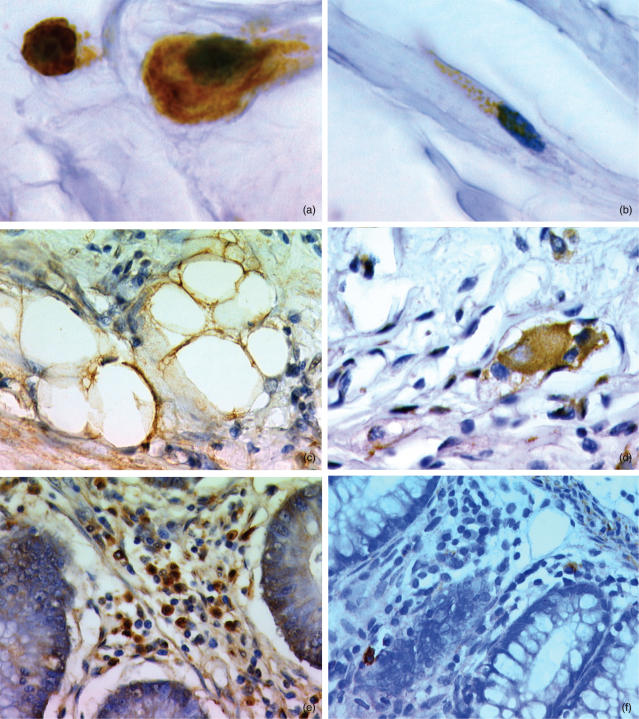
Expression of IGF-1R was found in all the layers of the intestinal wall (mucosa, submucosa and muscularis propria) in both the normal and diseased intestine. The glandular epithelium, however, was negative in all the specimens analysed. Positivity was mainly cytoplasmic, but membranes were also stained. The majority of IGF-1R positive cells had a morphology of inflammatory mononuclear cells (Fig. 1a). Inflammatory IGF-1R positive cells were observed within each layer of the intestinal wall. They were distributed in clusters following inflammatory infiltrate. Intraepithelial lymphocytes were sporadically positive. In the submucosa and subserosa, positive IC were frequently gathered around vessels and some were intravascular. Lymphoid follicles very rarely contained positive cells. In fibroinflammatory regions of CD, disorganized muscularis propria contained many positive IC within the septas and at the margins of the longitudinal and circular muscular layer. Both in diseased intestine and controls, smooth muscle cells also expressed IGF-1R. Positive smooth muscle cells were observed within vessel walls, muscularis mucosa and muscularis propria. IGF-1R positive fibroblast-like cells were recognized in the submucosa and subserosa in CD (Fig. 1b), but not in controls. These cells were encountered more often in fibrosed areas of CD. Finally, IGF-1R was also found in adipocytes within fibrolipomatous regions of CD (Fig. 1c) and, sporadically, in the hypertrophic nervous plexus of CD intestinal wall (Fig. 1d).
Figure 1.
IGF-1R positive cells in Crohn's disease: (a) inflammatory cells, (b) fibroblast-like cell, (c) adipocytes and (d) hypertrophied nerve plexus. IGF-1R positive cells in the lamina propria of a specimen with Crohn's disease in an uninvolved area (e) and in a diseased area (f).
Semi-quantitative analysis of inflammatory cells expressing IGF-1R and correlation with apoptosis and fibrosis
The absolute number of inflammatory IGF-1R positive cells was increased in CD lamina propria (P = 0·007) and submucosa (P = 0·003) compared to controls (Table 2, Fig. 2a). In relative values, the difference between CD and controls was still significant in the lamina propria (P = 0·028) and borderline for significance in the submucosa (P = 0·066). In CD samples, the absolute number of IGF-1R positive IC was significantly higher in diseased areas compared to uninvolved areas of the same patients in the lamina propria (P = 0·03) (Fig. 1e,f) and in the submucosa (P = 0·03) (Table 2). In relative values, however, there was no significant difference.
Table 2.
Mean number of inflammatory cells by field, mean absolute number and mean relative number of IGF-1R positive inflammatory cells by field (± s.d.)
| Inflammatory cells | ||||||
|---|---|---|---|---|---|---|
| Lamina propria | Submucosa | |||||
| IGF-1R+ | IGF-1R+ | |||||
| Total | Absolute | Relative | Total | Absolute | Relative | |
| Controls | 103·6 ± 19·3 | 9·9 ± 6·5 | 6·56 ± 6·2 | 13·7 ± 7·9 | 1 ± 0·9 | 9·1 ± 9·4 |
| Uninvolved CD | 93·2 ± 45·3 | 15·5 ± 16·3 | 16·6 ± 17·5 | 18·2 ± 19·4 | 3·8 ± 6·6 | 17·7 ± 14·8 |
| Involved CD | 131·1 ± 46·4 | 20·2 ± 11·4a | 16·6 ± 8·9b | 95·7 ± 75·7 | 12·7 ± 11·6c | 15·6 ± 8 |
| UC | 156·7 ± 54·3 | 19 ± 14·6 | 11·3 ± 4·9 | 63·9 ± 53·2 | 3·6 ± 2·5 | 9·1 ± 9·4 |
| Diverticulitis | 104·1 ± 16·7 | 4·3 ± 3·6 | 4·1 ± 3·2 | 27·1 ± 17·5 | 2·5 ± 2·3 | 9·4 ± 5·3 |
Compared to controls (P = 0·007); compared to uninvolved CD using paired test (P = 0·03), compared to diverticulitis (P = 0·003).
Compared to controls (P = 0·028), compared to diverticulitis (P = 0·0004).
Compared to controls (P = 0·03); compared to uninvolved CD using paired test (P = 0·03); compared to UC (P = 0·03); compared to diverticulitis (P = 0·003). CD: Crohn's disease; UC: ulcerative colitis.
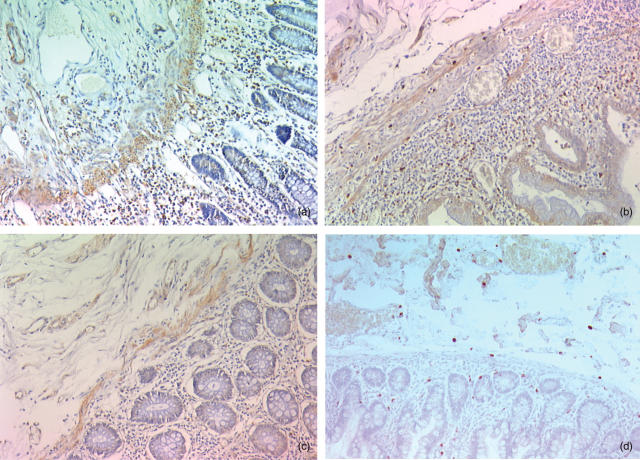
Figure 2.
Immunohistochemistry with an anti-IGF-1R antibody: (a) increased number of IGF-1R positive inflammatory cells (in brown) in the mucosa and the submucosa in an involved area of Crohn's disease. (b) Increased number of IGF-1R positive inflammatory cells limited to the mucosa in an involved area of ulcerative colitis. (c) No overexpression of IGF-1R in diverticulitis. (d) Immunohistochemistry with an antiactive caspase 3 antibody: positive inflammatory cells (in red) undergoing apoptosis in an uninvolved area of Crohn's disease.
The absolute number of IGF-1R positive IC in UC did not differ significantly from CD in the lamina propria, but was significantly lower than in involved CD in the submucosa (P = 0·03) (Fig. 2b). This was no longer significant in relative values. The absolute number of IGF-1R positive cells was significantly lower in diverticulitis than in CD in the lamina propria (P = 0·003) and in the submucosa (P < 0·03) (Fig. 2c). In relative values, the number of IGF-1R positive cells in diverticulitis was still lower than in CD in the lamina propria (P = 0·0004)
Most of the cells expressing active caspase-3 had a morphology of IC (Fig. 2d). Fibroblast-like cells positive for active caspase-3 were not identified. Table 3 shows the relative numbers of caspase-3 positive IC in the lamina propria and the submucosa, in the various conditions studied. There was no significant difference between controls, involved or uninvolved CD. The number of apoptotic cells (positive for active caspase-3) was correlated inversely to the number of IGF-1R-positive IC in uninvolved submucosa of CD (P = 0·01). In involved submucosa, there was only a trend towards this correlation (P = 0·09). No significant correlation was found in the lamina propria.
Table 3.
Mean relative number (%) of apoptotic (active caspase-3 positive) inflammatory cells by field (± s.d.)
| Apoptotic inflammatory cells | ||
|---|---|---|
| Lamina propria | Submucosa | |
| Controls | 1·13 ± 1·62 | 20·67 ± 25·12 |
| Uninvolved CD | 2·83 ± 2·92 | 19·77 ± 30·44 |
| Involved CD | 2·43 ± 2·97 | 15·61 ± 18·46 |
CD: Crohn's disease.
Furthermore, in the submucosa of involved CD there was also a weak trend towards a correlation between the number of IGF-1R positive IC and the severity of fibrosis (P = 0·091). No correlation was found in the lamina propria.
Discussion
Our study describes, for the first time, the expression of IGF-1R in the bowel wall of CD patients. Previously, expression of IGF-1 mRNA and pro-IGF-1 has been shown to be increased at sites of inflammation and fibrosis in active CD [25], suggesting a role for IGF-1 in this process. However, biological activity of IGF is dependent on the expression of IGF-1R. We show that various cells express IGF-1R protein throughout the bowel wall in controls and CD. Smooth muscle cells and immune cells are the most numerous. However, other cell types were found to express it in CD: fibroblast-like cells in the fibrosed submucosa, adipocytes in fibrolipomatous tissue wrapping the intestine, also in the fibrosed area, and nerve cells in hypertrophic ganglia.
The increase in absolute number of IGF-1R positive IC in lamina propria and submucosa of CD was parallel to the increase of the immunoinflammatory infiltrate. However, there was also an increase in the proportion (relative numbers) of IC positive for IGF-1R in CD. This may suggest a true overexpression of IGF-1R in the lamina propria and, to a lesser extent, in the submucosa of CD. One of the mechanisms by which IGF-1R expression may contribute to the increase of the immunoinflammatory infiltrate is by inducing a resistance to apoptosis. It has been reported that IGF-1-mediated activation of PI3-kinase/Akt contributes to normal T cell survival [26] and that IGF-1R expression is increased in activated T cells, protecting them from Fas-induced apoptosis [14]. We found an inverse correlation between IGF-1R expression and apoptosis detected by caspase-3 positivity in only CD submucosa, mainly in uninvolved areas. This may suggest that in the mucosa or when active inflammation is present the role of IGF-1R expression in protection against apoptosis is less prominent. The mechanisms of reduced apoptosis of IC in CD are still unclear, although some data show that a decrease in Fas signalling could be implicated [1]. However, functional studies would be necessary to demonstrate a specific role for IGF-1R in IC protection from apoptosis in CD. As well as an antiapoptotic effect, IGF-1 and -2 may also have a direct effect on lymphocytes proliferation, as has been shown with human activated peripheral blood T cells in vitro, in which this proliferative effect is correlated with the appearance of IGF-1R [27]. Along with this, in CD, double staining with CD3 and Ki67 identified an increased number of dividing T cells in submucosa and muscularis propria [28].
IGF-1R may also have a trophic effect in CD, potentially favouring fibrosis, particularly through its expression and activation on mesenchymal cells. The implication of the IGF system in fibrosis is also described in other diseases such as pulmonary fibrosis [29] and hepatic fibrosis [30]. We found a significant IGF-1R expression on fibroblast-like cells in submucosa and subserosa exclusively in CD samples. These CD fibroblasts may be stimulated by local IGF-1 to produce collagens. In inflammatory bowel disease, fibroblasts isolated from affected intestinal mucosa display faster doubling time and greater proliferation in response to mediators (including IGF-1) and secrete higher levels of collagen compared to control fibroblasts [31]. As well as this increased expression of IGF-1R in fibroblast-like cells in CD, our data also showed that numerous IGF-1R positive IC are found at margins of fibrosed muscularis propria and within septas. The means by which activation of IGF-1R on immunoinflammatory submucosal cells may favour fibrosis remains to be elucidated. The absence of significant correlation between the degree of fibrosis and the number of IGF-1R positive IC suggests that these cells do not play an exclusive or determinant role in this phenomenon. Overexpression of IGF-1 and IGF-1R in IC, associated with fibrosis, has also been described in other pathologies such as fibroproliferative acute respiratory distress syndrome [32], interstitial lung disease [33] and fibrotic lesions of asbestosis [34].
Through its ability to promote cell proliferation, the IGF system may have a role in smooth muscle cell hyperplasia of the muscularis propria, which is a prominent feature of CD intestinal strictures. Mice homozygous for IGF-1R null mutant gene have generalized organ hypoplasia including muscles [35], whereas SMP8-IGF-1 transgenic mice, which specifically overexpress IGF-1 in alpha-smooth muscle actin positive cells [36,37], exhibit a thickened intestinal muscularis propria [37]. In CD, IGF-1 local intestinal overexpression could act in an autocrine way on IGF-1R positive smooth muscle cells to stimulate their proliferation or in a paracrine way when it is produced by fibroblasts [20] and IC [19]. In our study, we observed an important expression of IGF-1R in smooth muscle cells from muscularis mucosae and muscularis propria, without a dramatic difference between normal and CD intestine, even in strictured areas. However, this constitutively high degree of IGF-1R expression in the muscularis propria together with previously demonstrated increased expression of IGF-1 may suggest a role for IGF-1R hyperactivation in muscularis propria hyperplasia observed in strictures of CD.
Overexpression of IGF-1R was also found in the lamina propria in UC, another chronic inflammatory bowel disease (Fig. 2b). In this disease, however, the overexpression of IGF-1R was limited to this layer as no overexpression was found in the submucosa or muscularis propria. This is in keeping with the pure mucosal, and not transmural, involvement characterizing UC. In contrast, in diverticulitis, an acute inflammatory disorder of the colon, no overexpression of IGF-1R was found in any of the layers of the bowel wall (Fig. 2c).
Finally, another specific overexpression of IGF-1R was found in two pathological features often associated with CD: adipous cells in peri-intestinal fibrolipomatous areas and hypertrophic intestinal nerve plexi. Interestingly, adipocytes and myocytes derive from a common precursor and IGF-1 promotes the differentiation of both cell types [38]. Moreover, it has been shown that macroscopic intestinal fat-wrapping in CD is correlated with transmural inflammation, fibrosis and muscle thickening [39]. This suggests that IGF may have a role in the genesis of fibrolipomatous tissue in CD. However, the precise mechanism of this fibrolipomatosis in CD is largely unknown [40], although mesenteric PPARγ overexpression has been described [41]. Along the same lines, IGF-1 hyperactivation may be implicated in nerve hypertrophy in CD as it has been reported that transgenic mice overexpressing IGF-1 exhibit brain overgrowth [42].
In conclusion, we have shown an altered expression of IGF-1R transmurally in the bowel wall in CD, while it was present only in the mucosa in UC and absent in acute diverticulitis. This overexpression in the lamina propria of chronic inflammatory bowel diseases may participate in the maintenance of inflammation. In CD alone, altered expression in the other layers of the bowel wall, particularly increased numbers of IGF-1R positive IC and fibroblast-like cells in the submucosa and the muscularis propria, may also play a role in the bowel wall fibrosis characterizing this disease. New therapeutic strategies targeting IGF-1R activation may be a worthwhile study, aiming at controlling either chronic inflammation or fibrosis in CD.
Acknowledgments
Fabienne El Yafi is Research Fellow and Edouard Louis Research Associate at the FNRS Belgium.
References
- 1.Boirivant M, Marini M, Di Felice G, et al. Lamina propria T cells in Crohn's disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology. 1999;116:557–65. doi: 10.1016/s0016-5085(99)70177-0. [DOI] [PubMed] [Google Scholar]
- 2.Brannigan AE, O'Connell PR, Hurley H, et al. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock. 2000;13:361–6. doi: 10.1097/00024382-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Graham MF, Diegelmann RF, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–65. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- 4.Lund PK, Zimmermann EM. Insulin-like growth factors and inflammatory bowel disease. Baillière's Clin Gastroenterol. 1996;10:83–96. doi: 10.1016/s0950-3528(96)90041-x. [DOI] [PubMed] [Google Scholar]
- 5.Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12:193–7. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 6.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann EM, Li L, Hou YT, Cannon M, Christman GM, Bitar KN. IGF-1 induces collagen and IGFBP-5 mRNA in rat intestinal smooth muscle. Am J Physiol. 1997;273:G875–82. doi: 10.1152/ajpgi.1997.273.4.G875. [DOI] [PubMed] [Google Scholar]
- 8.Stallmach A, Schuppan D, Riese HH, Matthes H, Riecken EO. Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn's disease. Gastroenterology. 1992;102:1920–9. doi: 10.1016/0016-5085(92)90314-o. [DOI] [PubMed] [Google Scholar]
- 9.Simmons JG, Pucilowska JB, Lund PK. Autocrine and paracrine actions of intestinal fibroblast-derived insulin-like growth factors. Am J Physiol. 1999;276:G817–27. doi: 10.1152/ajpgi.1999.276.4.G817. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Smartt H, Holgate ST, Roche WR. Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: an in vitro co-culture model of airway remodeling in asthma. Lab Invest. 1999;79:395–405. [PubMed] [Google Scholar]
- 11.Kuemmerle JF. Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology. 1997;113:817–24. doi: 10.1016/s0016-5085(97)70176-8. [DOI] [PubMed] [Google Scholar]
- 12.Valentinis B, Morrione A, Peruzzi F, Prisco M, Reiss K, Baserga R. Anti-apoptotic signaling of the IGF-I receptor in fibroblasts following loss of matrix adhesion. Oncogene. 1999;18:1827–36. doi: 10.1038/sj.onc.1202471. [DOI] [PubMed] [Google Scholar]
- 13.Stewart CE, Rotwein P. Insulin-like growth factor-II is an autocrine survival factor for differentiating myoblasts. J Biol Chem. 1996;271:11330–8. doi: 10.1074/jbc.271.19.11330. [DOI] [PubMed] [Google Scholar]
- 14.Walsh PT, O'Connor R. The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol. 2000;30:1010–18. doi: 10.1002/(SICI)1521-4141(200004)30:4<1010::AID-IMMU1010>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Remacle-Bonnet MM, Garrouste FL, Heller S, Andre F, Marvaldi JL, Pommier GJ. Insulin-like growth factor I protects colon cancer cells from death factor-induced apoptosis by potentiating tumor necrosis factor alpha-induced mitogen-activated protein kinase and nuclear factor kappaB signaling pathways. Cancer Res. 2000;60:2007–17. [PubMed] [Google Scholar]
- 16.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol. 2001;33:1777–89. doi: 10.1006/jmcc.2001.1441. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Humphreys K, Papachrysostomou M, Ferguson A. Detection of insulin-like growth factor-I and transforming growth factor-beta in whole gut lavage fluid: a novel method of studying intestinal fibrosis. Eur J Gastroenterol Hepatol. 1997;9:505–8. doi: 10.1097/00042737-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JA, Zimmermann EM, Sartor RB, Lund PK. IGF-1 and IGF-2 are overexpressed in inflamed and strictured intestine in CD. Gastroenterology. 1993;104:A683. [Abstract] [Google Scholar]
- 19.Lawrance IC, Maxwell L, Doe W. Inflammation location,but not type, determines the increase in TGF-β1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16–26. doi: 10.1097/00054725-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1022–9. doi: 10.1152/ajpgi.2001.280.5.G1022. [DOI] [PubMed] [Google Scholar]
- 21.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle. Gbetagamma-dependent mitogen activated protein kinase activation and growth. J Biol Chem. 2001;276:7187–94. doi: 10.1074/jbc.M011145200. [DOI] [PubMed] [Google Scholar]
- 22.Heinz-Erian P, Kessler U, Funk B, Gais P, Kiess W. Identification and in situ localization of the insulin-like growth factor-II/mannose-6-phosphate (IGF-II/M6P) receptor in the rat gastrointestinal tract: comparison with the IGF-1 receptor. Endocrinology. 1991;129:1769–78. doi: 10.1210/endo-129-4-1769. [DOI] [PubMed] [Google Scholar]
- 23.Fridman V, Humblet C, Bonjean K, Boniver J. Assessment of tumor angiogenesis in invasive breast carcinomas: absence of correlation with prognosis and pathological factors. Virchows Arch. 2000;437:611–17. doi: 10.1007/s004280000292. [DOI] [PubMed] [Google Scholar]
- 24.Groos S, Reale E, Luciano L. General suitability of techniques for in situ detection of apoptosis in small intestinal epithelium. Anat Rec. 2003;272A:503–13. doi: 10.1002/ar.a.10063. [DOI] [PubMed] [Google Scholar]
- 25.Pucilowska JB, McNaughton KK, Mohapatra NK, et al. IGF-1 and procollagen alpha 1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1307–22. doi: 10.1152/ajpgi.2000.279.6.G1307. [DOI] [PubMed] [Google Scholar]
- 26.Walsh PT, Smith LM, O'Connor R. Insulin-like growth factor-1 activates Akt and Jun N-terminal kinases (JNKs) in promoting the survival of T lymphocytes. Immunology. 2002;107:461–71. doi: 10.1046/j.1365-2567.2002.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson EW, Jones LA, Kozak RW. Expression and function of insulin-like growth factor receptors on anti-CD3-activated human T lymphocytes. J Immunol. 1992;148:63–71. [PubMed] [Google Scholar]
- 28.Fell JM, Walker-Smith JA, Spencer J, MacDonald TT. The distribution of dividing T cells throughout the intestinal wall in inflammatory bowel disease (IBD) Clin Exp Immunol. 1996;104:280–5. doi: 10.1046/j.1365-2249.1996.999701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pala L, Giannini S, Rosi E, et al. Direct measurement of IGF-1 and IGFBP-3 in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis. J Endocrinol Invest. 2001;24:856–64. doi: 10.1007/BF03343942. [DOI] [PubMed] [Google Scholar]
- 30.Wang XZ, Chen ZX, Zhang LJ, et al. Expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor and it's intervention by interleukin-10 in experimental hepatic fibrosis. World J Gastroenterol. 2003;9:1287–91. doi: 10.3748/wjg.v9.i6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:226–36. doi: 10.1097/00054725-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Krein PM, Sabatini PJ, Tinmouth W, Green FH, Winston BW. Localization of insulin-like growth factor-1 in lung tissues of patients with fibroproliferative acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167:83–90. doi: 10.1164/rccm.2201012. [DOI] [PubMed] [Google Scholar]
- 33.Rom WN, Paakko P. Activated alveolar macrophages express the insulin-like growth factor-I receptor. Am J Respir Cell Mol Biol. 1991;4:432–9. doi: 10.1165/ajrcmb/4.5.432. [DOI] [PubMed] [Google Scholar]
- 34.Lee TC, Gold LI, Reibman J, et al. Immunohistochemical localization of transforming growth factor-beta and insulin-like growth factor-I in asbestosis in the sheep model. Int Arch Occup Environ Health. 1997;69:157–64. doi: 10.1007/s004200050132. [DOI] [PubMed] [Google Scholar]
- 35.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF-1) and type 1 IGF receptor. Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 36.Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-1 overexpression: paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol. 2002;283:G875–85. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Niu W, Nikiforov Y, et al. Targeted overexpression if IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest. 1997;100:1425–39. doi: 10.1172/JCI119663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signalling regulates a switch between adipogenesis and myogenesis. Cell. 2003;18:147–58. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–8. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 40.Colombel JF, Tampoli CP, Dubuquoy L. New hypothesis on fat tissue and Crohn's disease: is it time to rectify an oversight? Inflamm Bowel Dis Monitor. 2003;4:78–81. [Google Scholar]
- 41.Desreumaux P, Ernst O, Geboes K, et al. Inflammation alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 42.D’Ercole AJ, Ye P, O'Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides. 2002;36:209–20. doi: 10.1054/npep.2002.0893. [DOI] [PubMed] [Google Scholar]