Abstract
Although enhanced lymphocyte trafficking is associated with colitis formation, little information about its regulation is available. The aim of this study was to examine how the murine liver and activation-regulated chemokine (mLARC/CCL20) contributes to lymphocyte recruitment in concert with vascular adhesion molecules in murine chronic experimental colitis. T and B lymphocytes isolated from the spleen were fluorescence-labelled and administered to recipient mice. Lymphocyte adhesion to microvessels of the colonic mucosa and submucosa was observed with an intravital microscope. To induce colitis, the mice received two cycles of treatment with 2% dextran sodium sulphate (DSS). In some of the experiments antibodies against the adhesion molecules or anti-mLARC/CCL20 were administered, or CC chemokine receptor 6 (CCR6) of the lymphocytes was desensitized with excess amounts of mLARC/CCL20. Significant increases in T and B cell adhesion to the microvessels of the DSS-treated mucosa and submucosa were observed. In chronic colitis, the accumulation of lymphocytes was significantly inhibited by anti-mucosal addressin cell adhesion molecule (MAdCAM)-1 mAb, but not by anti-vascular cell adhesion molecule-1. In DSS-treated colonic tissue, the expression of mLARC/CCL20 was significantly increased, the blocking of mLARC/CCL20 by monoclonal antibody or the desensitization of CCR6 with mLARC/CCL20 significantly attenuated the DSS-induced T and B cell accumulation. However, the combination of blocking CCR6 with MAdCAM-1 did not further inhibit these accumulations. These results suggest that in chronic DSS-induced colitis, both MAdCAM-1 and mLARC/CCL20 may play important roles in T and B lymphocyte adhesion in the inflamed colon under flow conditions.
Keywords: inflammatory bowel disease, dextran sodium sulphate, lymphocyte migration, MAdCAM-1, VCAM-1, murine liver and activation-regulated chemokine (mLARC/CCL20), CC chemokine receptor 6 (CCR6)
Introduction
There is now accumulating evidence that inflammation of the intestine is associated with altered lymphocyte trafficking in the intestinal mucosa. It is also becoming apparent that colonic inflammation is associated with enhanced expression of adhesion molecules. Mucosal addressin cell adhesion molecule-1 (MAdCAM-1) has been implicated in the selective recruitment of lymphocytes to the gut [1,2]. In human ulcerative colitis and Crohn's disease, the expression of MAdCAM-1 is up-regulated in the inflamed colonic mucosa [1,3]. We have also demonstrated the functional importance of elevated MAdCAM-1 levels in different animal models of colitis after immunoneutralization of MAdCAM-1 [4,5]. Although other adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) are up-regulated in the intestinal mucosa during inflammatory bowel diseases [3,6,7], the possible involvement of VCAM-1 in lymphocyte recruitment to the inflamed gut is still controversial [4,8]. The relative functional importance of MAdCAM-1 and VCAM-1 in altered lymphocyte trafficking should thus be elucidated.
Receptors for chemokines are fundamentally important in determining the trafficking properties of specific lymphocyte subsets. In humans, macrophage inflammatory protein-3 alpha (MIP-3α/CCL20) is expressed in the epithelial crypts of inflamed tonsils and appendices [9], while in mice it is expressed in the intestinal epithelium [10]. Expression of human CC chemokine receptor 6 (CCR6, a ligand for MIP-3α) was originally demonstrated in dendritic cells, peripheral blood leucocytes, and the spleen, thymus, and small intestine, and it was later shown to be expressed in B cells and memory T cells, including α4β7-expressing cells [11]. The expression patterns suggest that these molecules might mediate the migration of lymphocytes during mucosal immune responses [12]. Recently MIP-3α/CCL20 was found to trigger the arrest of a subset of peripheral lymphocytes rolling on a surface coated with MAdCAM-1 plus ICAM-1 [13], suggesting that MIP-3α/CCL20 can induce rapid integrin-dependent arrest of certain subsets of lymphocytes in intestinal microvessels especially under inflammation. There is a lack of information however, on how the murine liver and activation-regulated chemokine (mLARC), the orthologue of human MIP-3α/CCL20 in mice [10], actually contribute to lymphocyte recruitment in the colonic mucosa during experimental colitis.
We have employed the technique of intravital videoendoscopy to quantify the homing of T-lymphocytes in venules of lymphoid and nonlymphoid regions of the small intestine and in colonic mucosa [2,14,15]. In using this technique, the main objectives of this study were
to examine the spatial distribution of lymphocyte trafficking in DSS colitis, focusing especially on differences between the mucosal and submucosal region, and between the T and B lymphocyte subsets;
to define the relative importance of MAdCAM-1 and VCAM-1 in the lymphocyte-endothelial cell adhesion induced by dextran sodium sulphate (DSS);
to determine the possible contribution of mLARC/CCL20-CCR6 system in lymphocyte arrest on endothelial cells of colonic microvessels during chronic colitis.
Materials and methods
Administration of DSS and assessment of colonic inflammation
Male C57Bl6 mice at 8 weeks received two cycles of DSS treatment. Each cycle consisted of 2% DSS (molecular weight 40,000, ICN Biochemicals, Cleveland, OH, USA) in drinking water for 7 days, followed by a 7-day interval with normal drinking water. The care and use of the laboratory animals were in accordance with the guidelines of Keio University's Animal Research Committee. Under pentobarbital anaesthesia, the colon was removed, and fixed in 10% buffered formalin for H&E staining. Another part was fixed in periodate-lysine-paraformaldehyde, and immunohistochemistry was performed on cryostat sections by the labelled streptavidin biotin technique. Primary monoclonal antibodies that react to MAdCAM-1 (MECA367), β7-integrin (M293), CD4 (RM4-5), CD8 (53–6·7), and CD45R/B220 (RA3–6B2) were obtained from PharMingen, San Diego, CA, USA. They were visualized by streptavidin-FITC and observed with a fluorescent microscope (Olympus, Tokyo).
Intravital observation of lymphocyte migration to colonic microvessels
Lymphocytes were isolated from the spleen after red blood cells were lysed in ammonium phosphate/chloride lysis buffer. T- and B-cell-rich fractions of lymphocytes were obtained by using a separation column (Mouse T Cell Enrichment Columns, R & D Systems Inc. Minneapolis, MN, USA; Mouse B Cell Recovery Column Kit, Cedaelane, Ontario, Canada). The cell suspensions were washed and stored in RPMI 1640 medium with 5% fetal calf serum on ice until used. The lymphocytes were labelled with CFSE (carboxyfluorescein diacetate succinimidyl ester) as previously reported [14]. The cells were resuspended in 0·2 ml of the medium and used within 30 min
The microcirculation in the colonic mucosa was observed from the mucosal surface with an inverted-type fluorescence microscope (Diaphot TMD-2S, Nikon, Tokyo) equipped with a silicon intensifier target (SIT) image tube camera (C-2400–08, Hamamatsu Photonics Co., Shizuoka, Japan) as previously reported [15]. Lymphocytes (3 × 107 dissolved in 0·3 ml) were injected into tail veins of the recipient mice for 5 min. The interaction of the infused lymphocytes with the colonic microvascular beds was monitored and continuously recorded on S-VHS videotapes for 60 min. Lymphocytes that adhered to the wall at the same position in the microvessels without moving for more than 30 s were defined as sticking lymphocytes. By changing the focal plane one can mainly observe at the depth of the mucosal microvessels. In another set of experiments, the colonic wall was observed from the serosal surface. With this setting we could observe at the depth of the entire submucosal venules.
Administration of antibodies and desensitization of CCR6 on lymphocytes with mLARC
Anti-MAdCAM-1 mAb (MECA-367, PharMingen, 2 mg/kg) or anti-vascular cell adhesion molecule (VCAM)-1 (429, PharMingen, 2 mg/kg) dissolved in 0·2 ml of saline was infused from tail veins at 30 min before injecting T and B lymphocytes. For the experiment of in vivo inhibition of mLARC/CCL20, mice received anti-mLARC/CCL20 mAb (MAB7601, R & D systems Inc., 2 mg/kg) 30 min before the cell infusion from tail veins. As control rat IgG1 or IgG2a was infused. For desensitization after labelling with CFSE, splenic T and B lymphocytes (3·0 × 107 cells/ml) in medium were incubated with 1-µM mLARC/CCL20 (760-M3-025, R & D systems Inc.) for 45 min before infusion. Meanwhile, control cells were incubated with saline for 45 min. After washing through serum, the lymphocytes were re-suspended at 0·3 ml in RPMI, and used immediately. In our preliminary study the effect of CCR6 blocking with high concentration (1 µm) of mLARC/CCL20 was confirmed by in vitro using lymphocyte chemotaxis assay [16]. Desensitization of lymphocytes showed a drastic (about 76%) inhibition of chemotaxis ability toward mLARC/CCL20 at least for 60 min (data not shown).
Determination of mLARC expression in colon by RT-PCR
Total RNA was isolated from whole colon samples by using RNA-zol B (Tel.test Inc., Friendwood, TX). For RT-PCR, 1 µg of RNA was reverse transcribed and the cDNA was amplified. The primers for mLARC/CCL20 (sense 5′-TTT GCA CCT CCT CAG CCT AAG A-3′ and antisense 5′-ACC CCA GCT GTG ATC ATT TCC-3′) were designed from available sequences from GenBank. The negative control reactions had no added RNA during the RT and subsequent PCR amplification. After amplification, aliquots of the PCR reaction were size separated in a 3·0% agarose gel containing ethidium bromide and photographed.
Statistics
All results were expressed as means ± SEM. The differences between groups were evaluated by one-way analysis of variance (anova) and Fisher's post hoc test. The statistical significance was set at P < 0·05.
Results
Histological characteristics and mLARC expression in DSS-induced chronic colitis
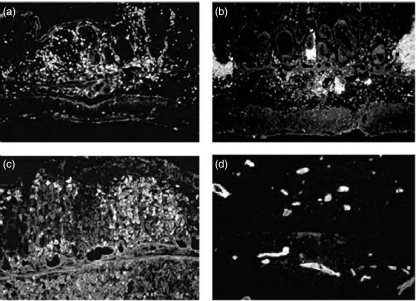
Macroscopically, mice with colitis induced by two cycles of DSS feeding exhibited shortening of colonic length and increased thickness of the colonic walls. Histologically, the lesions were characterized by many infiltrating cells, almost consisting of mononuclear cells in both the lamina propria and the submucosa. Loss of entire crypts and erosions were observed in some portions (data not shown). Immunohistochemistry revealed that the infiltrating cells consisted mainly of CD4 lymphocytes and B cells (Fig. 1a,b), expressing β7-integrin (Fig. 1c), a counter ligand of MAdCAM-1. There was also a significant increase in MAdCAM-1 expression in the mucosal and submucosal miscovessels (Fig. 1d), similar to the findings of previously reported [17].
Fig. 1.
Representative histological appearance of chronic DSS-induced colitis. (a) Immunohistochemical staining of CD4-positive cells in the colonic tissues of DSS-treated animals. (b) B cells in DSS colitis. (c) β7-positive cells in DSS colitis. (d) MAdCAM-1 positive microvessels in DSS colitis.
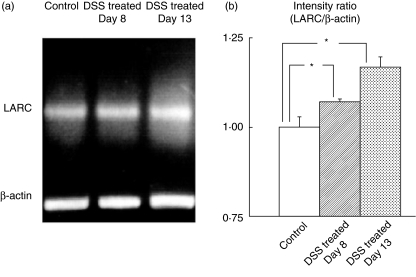
There was also a significant increase in expression of mLARC/CCL20 mRNA in colonic tissues after chronic DSS-exposure as determined by RT-PCR (Fig. 2).
Fig. 2.
Effect of DSS treatment on mRNA expression of mLARC/CCL20 in colonic tissues by RT-PCR. (a) A representative picture from one of four experiments with similar results is shown. (b) The mLARC/CCL20 mRNA abundance was normalized to control the beta-actin mRNA and is expressed as the relative increase to the control at the left. *P < 0·05 as compared with the control animals. The values are means ± SEM for six separate animals.
T and B lymphocyte migration to the colonic mucosa and submucosa: effect of DSS treatment
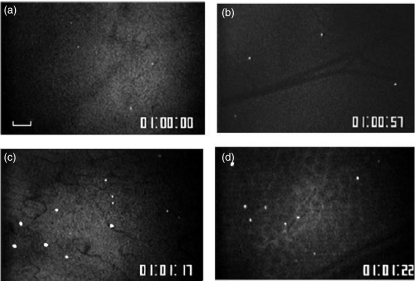
Figure 3 shows microscopic images of the distribution of sticking B lymphocytes in the colonic mucosa (Fig. 3a) and submucosa (Fig. 3b) of the control mice 60 min after infusion. Figure 4 shows the numbers of sticking T and B lymphocytes in these areas. In the control mice a small number of T and B lymphocytes adhered to the colonic mucosal microvessels and the submucosal venules. In the DSS-treated mice, however, the T and B lymphocyte adherence to the mucosal and submucosal microvessels was significantly increased (Figs 3c,d and 4). There was no significant difference in lymphocyte-endothelial adherence between the T and B cells. A comparison of the total number of lymphocytes that had entered the colonic microvessels showed no significant difference among the four groups 10 min after infusion (T-cell control mucosal side, 55·3 ± 33·5/min; T-cell control submucosal side, 66·0 ± 7·1/min; B-cell control mucosal side, 67·8 ± 28·7/min; B-cell control submucosal side, 57·2 ± 24·1/min; T-cell DSS mucosal side, 67·4 ± 31·7/min; T-cell DSS submucosal side, 65·8 ± 27·9/min; B-cell DSS mucosal side, 71·0 ± 27·9/min; B-cell DSS submucosal side, 62·7 ± 21·4/min).
Fig. 3.
Representative microscopic images of the distribution of fluorescence-labelled sticking B lymphocytes in (a) the colonic mucosa and (b) the submucosa of the control mice60 min after infusion. In the control mice, only a small number of T and B lymphocytes adhered to the colonic microvessels. On the other hand, the DSS-treated mice showing significant increases in B lymphocyte adherence to (c) the mucosal microvessels, as well as (d) the submucosal microvessels. The bar represents 100 µm.
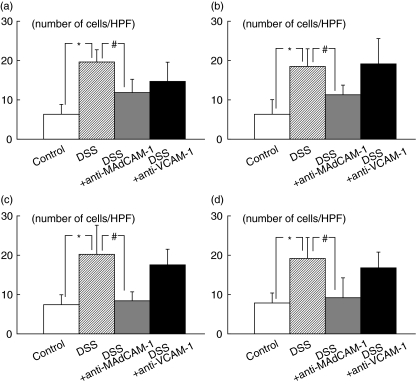
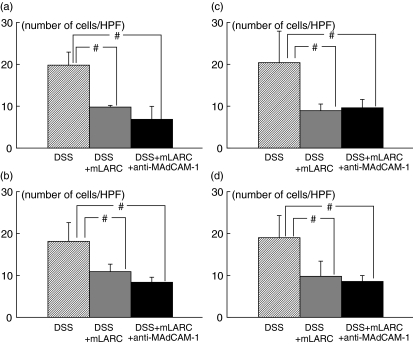
Fig. 4.
The number of T and B lymphocytes sticking to the colonic mucosal and submucosal microvessels of control and DSS-treated animals 60 min after infusion of lymphocytes, and the inhibitory effect of function-blocking of adhesion molecules (MAdCAM-1 and VCAM-1) on the sticking of T and B lymphocytes to these microvessels in DSS-treated animals. (a) T lymphocyte adherence to the colonic mucosa; (b) B lymphocyte adherence to the colonic mucosa; (c) T lymphocyte adherence to the submucosa; (d) B lymphocyte adherence to the submucosa. *P < 0·05 as compared with the control animals. #P < 0·05 as compared with the DSS-treated animals. The values are means ± SEM for six separate animals.
Effect of antibodies against adhesion molecules
Figure 4 also illustrates the effects of anti-MAdCAM-1 and anti-VCAM-1 antibodies on the sticking of T and B lymphocytes the colonic mucosal microvessels and submucosal venules of the DSS-treated mice 60 min after infusion. In these experiments the number of lymphocytes that had entered the colonic microvessels was not significantly different among the various treatment groups (data not shown). Control rat IgG treatment did not affect the numbers of adherent lymphocytes in DSS-treated mice. The increased numbers of adherent T and B lymphocytes after DSS-treatment were significantly decreased by pretreatment with mAb that blocks MAdCAM-1 for both T and B lymphocytes. On the other hand mAb that blocks VCAM-1did not significantly inhibit the DSS-induced T and B lymphocyte adherence to the colonic microvessels.
Expression of CCR6 on splenic lymphocytes and effect of mLARC-desensitization or anti-mLARC/CCL20 treatment on lymphocyte interactions in colonic microvessels
The surface expression of CCR6 was confirmed for both splenic T and splenic B lymphocytes by flow-cytometric analysis (data not shown). The desensitization of CCR6 with mLARC/CCL20 substantially inhibited the accumulation of injected lymphocytes in the colonic mucosa as well as in the colonic submucosal venules, as shown in Fig. 5. There was no significant difference in the degree of inhibition between T and B lymphocytes at both sites, although the total flux of mLARC/CCL20-treated lymphocytes through the colonic venules was, if anything, slightly increased over that in the control animals. However, as shown in Fig. 5, the desensitization with mLARC/CCL20 plus anti-MAdCAM-1 pretreatment did not show further additive effects compared with desensitization alone.
Fig. 5.
Effect of desensitization of lymphocytes with mLARC/CCL20 on the sticking of T and B lymphocytes to the colonic mucosal and submucosal microvessels in the DSS-treated animals. (a) T lymphocyte adherence to the colonic mucosa; (b) B lymphocyte adherence to the colonic mucosa; (c) T lymphocyte adherence to the submucosa; (d) B lymphocyte adherence to the submucosa. In some experiments, desensitization in combination with anti-MAdCAM-1 was performed. #P < 0·05 as compared with the DSS-treated animals. The values are means ± SEM for six separate animals.
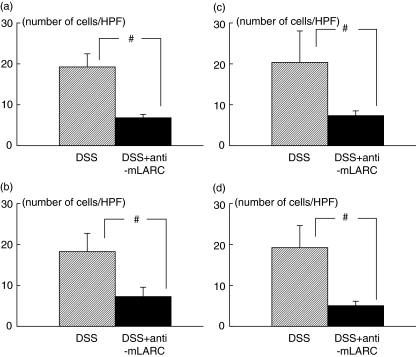
Figure 6 shows the effect of anti-mLARC/CCL20 antibody on the lymphocyte adherence in colonic microvessels of DSS colitis. Administration of monoclonal antibody to the mice significantly decreased the T and B lymphocyte adherence to colonic microvessles, which was comparable with the results of desensitization or the results of anti-MAdCAM-1 inhibition. Although there was no difference in total flux of lymphocytes into colonic venules between two groups.
Fig. 6.
The inhibitory effect of antimlARC/CCL20 antibody on the sticking of T and B lymphocytes to the colonic mucosal and submucosal microvessels in the DSS-treated animals. (a) T lymphocyte adherence to the colonic mucosa; (b) B lymphocyte adherence to the colonic mucosa; (c) T lymphocyte adherence to the submucosa; (d) B lymphocyte adherence to the submucosa. #P < 0·05 as compared with the control DSS-treated animals. The values are means ± SEM for five separate animals.
Discussion
Our data revealed that the increased expression of MAdCAM-1 in the colonic microvessels may be the major mechanism by which both T and B lymphocytes are recruited from the circulation into the inflamed tissue of DSS-induced colitis. The data are in line with those reported by Shigematsu et al. [8] who described how increased expression of MAdCAM-1, but not of VCAM-1 or intercellular adhesion molecule-1(ICAM-1), mediates the lymphocyte–endothelial cell adhesive interaction in a model of chronic colitis induced by transferring CD45RBhigh T- cells to SCID mice. There are indeed several reports indicating that antibodies against other adhesion molecules (ICAM-1 or VCAM-1) attenuate the increased leucocyte adhesion in intestinal venules [7,18,19]. In the case of ICAM-1, however, we have already reported that up-regulated ICAM-1 is not involved in T lymphocyte-endothelial adhesion in the inflamed colon [15]. Moreover, in the case of VCAM-1, most of the reports have given results obtained from a relatively acute course of inflammation, so we speculate that especially in the case of chronic colitis, MAdCAM-1, rather than VCAM-1 or ICAM-1, may play a key role in T and B lymphocyte migration.
In this study lymphocyte migration was significantly stimulated after DSS treatment in both the colonic mucosa and the submucosa. This might be related to the spatially and equally up-regulated MAdCAM-1 in the lamina propria just above the mucscularis mucosa as well as on large vessels in the submucosa in this model. We have also demonstrated that B cell migration occurred in a similar way to that of T cells in the DSS-treated mice. This is intriguing because a significant difference between T and B cell migration has been reported in the case of postcapillary venules of Peyer's patches, due to their different usage of adhesion molecules and chemokines [20]. It is known that B cells expressing β7 preferentially bind MAdCAM-1, and that these cells are memory-type, putatively activated cells [21]. In chronically inflamed colonic mucosa, B cell migration appeared to be induced by the same combination, i.e. α4β7 and MAdCAM-1, as seen for T cells.
Our in vivo migration study suggested that the mLARC/CCL20-CCR6 ligand receptor pair is largely involved in the lymphocyte–endothelial interaction in DSS-induced inflamed colonic tissues, although the exact relationship between mLARC/CCL20-CCR6 signalling and MAdCAM-1 activation is not known. Scheerens et al. [22] reported that the chemokines and receptor mRNA up-regulated in two murine models of inflammatory bowel diseases include mLARC/CCL20 and CCR6. The apparent increase in mLARC/CCL20 expression observed in the colonic tissue of DSS colitis is in accordance with these results. Although MIP-3α/CCL20 is highly expressed constitutively in Peyer's patches [23], MIP-3α/CCL20 mRNA was also expressed in intestinal tissues, and enhanced upon treatment with LPS [10]. Izadpanah et al. [24] demonstrated that MIP-3α/CCL20 mRNA and protein production were up-regulated by stimulating intestinal epithelial cells with proinflammatory cytokines. These in vitro findings are paralleled in vivo by increased expression of MIP-3α/CCL20 in cytokine- or bacteria-infected human intestinal xenografts [24], and by colonic epithelial cells in inflammatory bowel diseases [25].
Epithelium-derived MIP-3α/CCL20 may diffuse to and be presented by the vascular endothelium [26] to support the intravascular adhesion of α4β7+ memory T lymphocytes. On the other hand, MIP-3α/CCL20 may also be secreted directly from the inflamed vascular endothelium, since TNF-α has been shown to up-regulate MIP-3α/CCL20 by HUVEC [27]. Kriehuber et al. [28] demonstrated that both microvascular endothelial cells and lymph channel cells release MIP-3α/CCL20 apically upon activation. Recently, Fitzhugh et al. [29] specifically demonstrated that MIP-3α/CCL20 is produced by activated endothelial cells and CCR6 is required for memory T cells to arrest on TNF-α-activated human dermal microvascular endothelial cells in vitro under shear-stress conditions. Unlike human MIP-3α, mLARC/CCL20 is chemotactic for CCR6-expressing B cells [11]. Although the exact source of MIP-3α/CCL20 is unknown, our in vivo study suggested the possibility that it plays a key role in the directional trafficking of memory lymphocytes to the inflamed colon in inflammatory bowel diseases.
Acknowledgments
This study was supported in part by grants from the Keio University, School of Medicine, and the National Defence Medical College.
References
- 1.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimori H, Miura S, Koseki S, et al. Intravital observation of adhesion of lamina propria lymphocytes to microvessels of small intestine in mice. Gastroenterology. 2002;122:734–44. doi: 10.1053/gast.2002.31899. [DOI] [PubMed] [Google Scholar]
- 3.Souza HS, Elia CC, Spencer J, MacDonald T. Expression of lymphocyte-endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183–9. [PubMed] [Google Scholar]
- 5.Hokari R, Kato S, Matsuzaki K, et al. Involvement of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the pathogenesis of granulomatous colitis in rats. Clin Exp Immunol. 2001;126:259–65. doi: 10.1046/j.1365-2249.2001.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H. In situ expression of the cell adhesion molecule in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Laboratory Invest. 1993;59:77–85. [PubMed] [Google Scholar]
- 7.Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, Dixon MF, Axon AT. Adhesion molecules in inflammatory bowel disease. Gut. 1995;36:724–30. doi: 10.1136/gut.36.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigematsu T, Specian RD, Wolf RE, Grisham MB, Granger DN. MAdCAM mediates lymphocyte-endothelial cell adhesion in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1309–15. doi: 10.1152/ajpgi.2001.281.5.G1309. [DOI] [PubMed] [Google Scholar]
- 9.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, Imai T, Baba M, Ishikawa I, Uehira M, Nomiyama H, Yoshie O. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29:633–42. doi: 10.1002/(SICI)1521-4141(199902)29:02<633::AID-IMMU633>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–94. [PubMed] [Google Scholar]
- 12.Cook DN, Prosser DM, Forster R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–4. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 14.Miura S, Tsuzuki Y, Fukumura D, et al. Intravital demonstration of sequential migration process of lymphocyte subpopulations in rat Peyer's patches. Gastroenterology. 1995;109:1113–23. doi: 10.1016/0016-5085(95)90569-3. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe C, Miura S, Hokari R, et al. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1379–87. doi: 10.1152/ajpgi.00026.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hosoe N, Miura S, Watanabe C, et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte–endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2004;286:G458–66. doi: 10.1152/ajpgi.00167.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hokari R, Kato S, Matsuzaki K, et al. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free Radic Biol Med. 2001;31:153–63. doi: 10.1016/s0891-5849(01)00565-2. [DOI] [PubMed] [Google Scholar]
- 18.Soriano A, Salas A, Salas A, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Laboratory Invest. 2000;80:1541–51. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 19.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 20.Warnock RA, Campbell JJ, Dorf ME, Matsuzawa A, McEvoy LM, Butcher EC. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J Exp Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farstad IN, Halstensen TS, Lazarovits AI, Norstein J, Fausa O, Brandtzaeg P. Human intestinal B-cell blasts and plasma cells express the mucosal homing receptor integrin alpha 4 beta 7. Scand J Immunol. 1995;42:662–72. doi: 10.1111/j.1365-3083.1995.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 22.Scheerens H, Hessel E, de Waal-Malefyt R, Leach MW, Rennick D. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease. IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+CD45RBhigh T cells. Eur J Immunol. 2001;31:1465–74. doi: 10.1002/1521-4141(200105)31:5<1465::AID-IMMU1465>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP) -3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–94. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kangnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–9. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 25.Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are a major site of macrophage inflammatory protein 3α (MIP-3α) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–26. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 27.Hromas R, Gray PW, Chantry D, et al. Cloning and characterization of exodus, a novel beta-chemokine. Blood. 1997;89:3315–22. [PubMed] [Google Scholar]
- 28.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzhugh DJ, Naik S, Caughman SW, Hwang ST. C-C chemokine receptor 6 is essential for arrest of a subset of memory T cells on activated dermal microvascular endothelial cells under physiologic flow conditions in vitro. J Immunol. 2000;165:6677–81. doi: 10.4049/jimmunol.165.12.6677. [DOI] [PubMed] [Google Scholar]