Abstract
The incidence of mycobacterial diseases is high and the efficacy of Bacillus Calmette Guérin (BCG) is low in most areas of the world where chronic worm infections are common. However, if and how concurrent worm infections could affect immunity to mycobacterial infections has not been elucidated. In this study we investigated whether infection of mice with Schistosoma mansoni could affect the ability of the animals to control Mycobacterium bovis BCG infection and the immune response to mycobacterial antigens. BALB/c mice subclinically infected with S. mansoni were challenged with M. bovis BCG via the intravenous route. The ability of the animals to contain the replication of M. bovis BCG in their organs, lung pathology as well as the in vitro mycobacterial and worm antigen induced immune responses were evaluated. The results showed that S. mansoni coinfected mice had significantly higher levels of BCG bacilli in their organs and sustained greater lung pathology compared to Schistosoma uninfected controls. Moreover, Schistosoma infected mice show depressed mycobacterial antigen specific Th1 type responses. This is an indication that chronic worm infection could affect resistance/susceptibility to mycobacterial infections by impairing mycobacteria antigen specific Th1 type responses. This finding is potentially important in the control of TB in helminth endemic parts of the world.
Keywords: mycobacteria, helminths, immunomodulation, mice
Introduction
Tuberculosis (TB) is one of the major public health problems in the world. A report from the World Health Organization (WHO) estimates that Mycobacterium tuberculosis infection occurs in nearly a third of the world population and close to 2 million people die of the disease each year [1]. The reasons for the worsening TB problem are believed to include the HIV pandemic and the unplanned urbanization in resource poor countries of the world [2].
Most areas with high TB-associated morbidity and mortality are also characterized by high endemic prevalence of helminthic infections [3,4], but the impact of helminthiases on host response to intracellular pathogens is not clear. Actor and colleagues have shown that S. mansoni infection in the mice impairs resistance against viral infections [5]. Although the interaction between helminthes and mycobacterial infections is not well studied, it has been shown that mice immunized with PPD following induction of Th2 responses by a filarial worm Brugia malayi develop cytokine responses that were skewed toward type 2 pattern [6]. We reported that humans exposed to intestinal helminthic infections show impaired in vitro cellular responses to M. tb antigens [7]. We hypothesize that this leads to increased susceptibility to mycobacterial infections. In order to address this question further we evaluated if chronic S. mansoni infection, an infection common in our study area, would make mice more susceptible to M. bovis BCG infection.
The results showed that mice with chronic S. mansoni infection had higher bacterial loads following intravenous M. bovis BCG infection compared to Schistosoma free controls. Moreover, in vitro analysis showed that M. tb antigen specific cellular responses were significantly impaired in these animals accompanied by enhanced ConA induced Th2 type responses. This indicates that worm infections, by impairing mycobacteria antigen specific cellular responses and/or inducing a strong Th2 immune background makes the animals more susceptible to mycobacterial infections and/or disease.
Materials and methods
Animals
Female BALB/c mice were obtained from B & K Universal (Stockholm, Sweden). The animals were maintained at the animal facility of the Swedish Institute for Infectious Diseases Control (SMI) (Stockholm, Sweden). S. mansoni cercariae (Puerto Rican strain) were obtained from department of parasitology, SMI (Stockholm, Sweden). All animals were 8–10 weeks of age at the beginning of the experiments and were kept in cages with unlimited food and water supply.
The animals were divided into two groups. One group was infected with 30 infective larvae of S. mansoni and the other group was kept Schistosoma free and used as controls.
Experimental infection
S. mansoni
Mice were anaesthetized by subcutaneously injecting 0·1 ml of 8·6 mg/ml sodium pentobarbital. 100 µl of tap water containing 30 S. mansoni cercariae was placed on a shaved skin area on the abdomen and left for 20 min for active infection to occur. The presence of the infection was confirmed repeatedly by direct microscopy of stool samples during the period of the experiments.
Mycobacterium bovis BCG
M. bovis BCG, strain 1331(SSI, Copenhagen, Denmark) was obtained in lyophilized form, resuspended in PBS and 100 µl of the suspension containing 8 × 105 colony forming units (CFU) was inoculated through the lateral tail vein 8 weeks after S. mansoni infection, the time when all Schistosoma infected animals were excreting eggs.
Analysis of colony forming units
At 6, 9 and 15 weeks post BCG infection, the number of CFU of M. bovis BCG bacilli in spleens, liver and lung were evaluated as described by Jackson and colleagues [8]. Briefly, animals were sacrificed and organs were aseptically isolated and placed in sterile plastic bags containing 5 ml dilution solution (saline containing 0·05% Tween 80). The organs were homogenized using Stomacher 80 homogenizer (Turku, Finland) and serial 10-fold dilutions were plated on Middlebrook 7H11 containing 0·3 mg/ml polymyxin B and 5 mg/l amphotericin B. Plates were kept in plastic bags and incubated at 37°C and 5% CO2 until the colonies were large enough for counting (3–4 weeks). M. bovis BCG was used as a challenge organism as it is less pathogenic and easy to work with in a laboratory without P3 level facility.
Spleen cell cultures
Single cell suspensions from individual spleens from individual animals were prepared by teasing the organ loose into complete RPMI (RPMI 1640 supplemented with 10% fetal calf serum, 20 mm HEPES buffer (Sigma-Aldrich, Sweden), 2 mm l-glutamine 0·05 mm 2-mercaptoethanol (Life Technologies Paisley, UK) and 1% penicillin/streptomycin (Sigma-Aldrich Sweden) and red cells were lysed using red blood cell lysis buffer (Sigma-Aldrich, Sweden). The cell suspension was washed twice in RPMI medium and cell concentration was adjusted to 2 × 106/ml. Spleen cells were cultured at a concentration of 4 × 105/well in 96 well flat bottom Nunc culture plate in the absence or presence of 5 µg/ml concanavalin A (Sigma-Aldrich, UK), 12·5 µg/ml purified protein derivative of Mycobacterium tuberculosis (PPD, Statens Serum Institute, Copenhagen, Denmark) or 15 µg/ml soluble Schistosoma egg antigen in triplicate wells. Culture supernatants were collected at day 2 for IL-4, day 3 for IFN-γ and day 5 for IL-5 assays. These time points were chosen after studying the kinetics of the respective cytokine secretions. Schistosoma egg antigen (SEA) was prepared as described by Carter and Colley[9].
For proliferation assay, cultures were pulse labelled with 1 µci of 3H-thymidine (Amersham, France) per well and cells were harvested 18 h later onto fibre filter papers. Cultures were pulsed with tritiated thymidime at day 3 for Con A stimulation or day 5 for PPD stimulation. 3H-thymidine incorporation was determined using β liquid scintillation counter (LKB Wallac, Turku, Finland) and results were expressed as stimulation indices (counts per minute of antigen stimulated cultures/counts per minute in unstimulated cultures).
Measurement of cytokine levels by ELISA
Cytokine levels in cell culture supernatants were determined using commercially available ELISA kits (R & D Systems, London, UK) following manufacturer's instructions in duplicate wells. Concentrations of cytokines (IL-4, IL-5 and IFN-γ) in samples were calculated by a standard curve generated from recombinant cytokines (R & D systems, London, UK), and results were expressed in pg/ml. The differences in results between duplicate wells were consistently less than 10% of the mean. The sensitivity of the assay was 2 pg/ml for all cytokines measured.
Histopathological analysis
For histopathological examinations, the lung was removed and inflated, fixed in 10% buffered formalin, and were embedded in hot paraffin. The tissue was then sectioned in 5 µm thick slices and stained with haematoxilin-eosin-safranin as described elsewhere [10]. Histological changes were evaluated in a blinded fashion by at least 2 independent observers.
Statistical analysis
The student t-test was used to analyse differences between the experimental and control groups. Values of P < 0·05 were considered statistically significant.
Results
Impaired control of replication of M. bovis BCG bacilli in S. mansoni coinfected mice
At 6, 9 and 15 weeks post M. bovis BCG infection animals were sacrificed from each group and the number of CFU of BCG bacilli in the organs was determined. This experiment was repeated three times, initially with 3 animals per group and twice with 10 mice per group and in all cases the results were consistent. The results obtained from S. mansoni+ BCG coinfected group of mice were compared with Schistosoma free mice. The result showed that mice with concurrent S. mansoni infection developed significantly higher bacterial loads compared to Schistosoma free group at all the 3 time points tested (P < 0·01) and in all the 3 organs (Fig. 1a–c). In the lungs of Schistosoma free mice, bacterial loads showed slight increase from week 6–9 and then declined at week 15 whereas in the Schistosoma coinfected group, there was progressive increase from week 6 on, and at week 15 post challenge the difference between the two groups was over 15 fold (Fig. 1a). In both the liver and the spleen, there was a continuous decline in bacterial load in the Schistosoma free group, in the coinfected group however, there was an initial decline (week 6–9) which stabilized thereafter (Fig. 1b,c). In preliminary experiments, we observed that bacillary load was not significantly different between Schistosoma coinfected mice and controls at week 3 post challenge (data not shown).
Fig. 1.
Number of CFU of BCG bacilli in the (a) lungs, (b) liver and (c) spleen of S. mansoni and BCG coinfected and BCG infected mice. S. mansoni-infected mice and uninfected controls were intravenously challenged with M. bovis BCG and the number of BCG bacilli in the lung, liver and spleen were determined at weeks 6, 9 and 15 post challenge. In this representative experiment 10 animals were used per group to assess CFU at all time points. Results were means for individual mice ± standard error of the mean (SEM) *P < 0·05, **P < 0·01. This experiment was performed 3 times using 3, 10, 10 animals per group, respectively, and on each occasion the result was reproducible.
Chronic S. mansoni infection impairs proliferative and IFN-γ responses of splenocytes to a mycobacterial antigen PPD and a T cell mitogen concanavalin A (ConA)
To investigate whether the impaired ability of the animals to control BCG infection was reflected in the in vitro cellular responses to M. tb antigens, we analysed proliferative and IFN-γ responses of spleen cells in response to PPD and ConA. Spleen cell cultures from individual mice were analysed for proliferative capacity at weeks 6 and 9 post M. bovis BCG infection (Fig. 2). The background proliferation in medium alone was between 1000 and 3000 counts per minute for all cultures. The data revealed markedly lower spleen cell proliferation in response to both PPD and ConA in Schistosoma coinfected group of mice as shown in Fig. 2.
Fig. 2.
S. mansoni infection impairs proliferative responses to PPD and concanavalin A. Splenic lymphocytes isolated from S. mansoni infected mice and uninfected controls were cultured in the presence of PPD or concanavalin A. The cultures were pulse labelled with tritiated thymidine at day 3 for con A stimulated cultures and day 5 for PPD stimulated cultures and were harvested 18 h after pulse labelling. The results were expressed as stimulation indices (mean count per minute in stimulated cultures/mean count per minute in unstimulated cultures). In this representative experiment 10 animals were used in each group. (a) show responses to PPD and (b) show responses to ConA *P < 0·05, **P < 0·01. This experiment was repeated twice with consistent results.
IFN-γ analysis was done by collecting day 3 culture supernatants and analysing IFN-γ content using mouse IFN-γ ELISA kit (R & D systems, UK). The results show that PPD-induced IFN-γ responses were significantly reduced (P < 0·01) in cells from coinfected mice compared to Schistosoma free controls at both time points the test was done post M. bovis BCG challenge (weeks 6 & 9) (Fig. 3). This experiment was performed two times and the results were consistent.
Fig. 3.
S. mansoni infection significantly reduces PPD specific in vitro IFN-γ production from splenic lymphocytes. Supernatants of splenocyte cultures were assayed for IFN-γ levels using commercially available ELISA kits. The results were expressed as pg/ml and were means of duplicate wells. Ten animals were used per group in this representative experiment. (a) shows responses to PPD and (b) shows response to ConA *P < 0·05, **P < 0·01. The experiment was performed twice with comparable results.
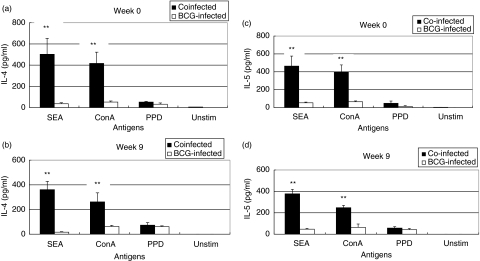
S. mansoni infected mice show enhanced Th2 cytokine responses to a T cell mitogen and soluble Schistosoma egg antigen (SEA)
To find out whether the impaired PPD and Con A induced Th1 type responses (T cell proliferation and IFN-γ production) were accompanied by dominant Th2 type responses, we examined the in vitro secretion of IL-4 and IL-5 in the two groups of animals at the time of M. bovis BCG challenge (week 0) and 9 weeks post challenge. The result shows that Schistosoma infected mice produced several fold higher IL-4 and IL-5 levels when stimulated with SEA and a T cell mitogen ConA. Nevertheless, the levels of both IL-4 and IL-5 when cells were stimulated with PPD was low in both groups of mice and was not significantly different between the two groups although slightly higher levels were observed in coinfected group of mice (Fig. 4).
Fig. 4.
S. mansoni infection induces strong Th2 responses to soluble Schistosoma egg antigen (SEA) as measured by IL-4 and IL-5 secretion. Spleenocytes from S. mansoni infected mice were cultured in the presence of SEA, PPD or concanavalin A and supernatants were assayed for IL-4 and IL-5. Eight animals per group were used for the experiment at week 0 and 10 animals per group were used for the experiment at week 9. (a,b) show IL-4 responses at weeks 0 and 9 post M. bovis BCG infection, respectively, whereas (c,d) show IL-5 responses at weeks 0 and 9 post M. bovis BCG infection **P < 0·01. This experiment was performed twice with comparable result.
S. mansoni infection enhances lung damage caused by M. bovis BCG infection
The levels of cellular accumulation and organization (granulomatous response) as well as reduction in air exchange areas caused by inflammatory responses were compared between coinfected and Schistosoma free controls at week 15 post BCG challenge. The results indicate that lungs from coinfected mice displayed significant pathological changes that involved ∼40% of the lung tissue. In the lung tissue obtained from this group of mice regions of normal lung tissue were interspersed with areas of diffuse, poorly organized inflammation as well as regions of more focused lymphocyte infiltration that lacked the organizational structure characteristics of granulomas (Fig. 5a). In contrast in mice without S. mansoni infection, the lungs were predominantly normal with mild lymphocyte infiltration and inflammation and the pathological changes involved less than 10% of the lung tissue (Fig. 5b).
Fig. 5.
Photomicrographs of lungs of mice BCG infected in the presence or absence of S. mansoni infection. (a,b) The lungs were examined at week 15-post challenge. Five consecutive sections for each of the 10 mice in each group were stained and examined. (a) is a section from mice with prior Schistosoma infection and (b) is from the group without Schistosoma infection. Magnification ×100.
Discussion
We have previously reported that deworming of helminth-exposed humans increase mycobacterial antigen specific cellular responses [7]. Whether these altered cellular responses indicated altered susceptibility to mycobacterial infections is an issue that remains to be addressed. In this report, we investigated whether chronic S. mansoni infection could alter susceptibility of BALB/c mice to BCG infection by examining bacterial load, the degree of lung pathology as well as the in vitro Th1/Th2 type responses of lymphocytes (IFN-γ secretion, T cell proliferation/IL-4, IL-5 secretion) to mycobacteria and Schistosoma egg antigens.
Here we report that mice infected with S. mansoni become more susceptible to M. bovis BCG infection and that this impaired resistance was accompanied by impaired in vitro Th1 type responses to mycobacterial antigen, PPD.This observation, if it holds true in humans may be of major public health importance as worm infections are widespread in regions of the world where there is high incidence of Mycobacterial diseases [2,11].
It has been shown in experimental animals and in clinical studies that the immune environment established by prior sensitization to helminthes influences responses to unrelated antigens [12–14]. In mice with established S. mansoni infection, immunization with sperm whale myoglobin induces antigen-specific T cells that produce Th2 cytokines, whereas uninfected animals generate Th1 type immunity [14]. Helminth infections were also reported to impair resistance against viral infections [5] and reduce cellular responses generated by vaccinations against viral pathogens [15]. Although the interaction between helminth and mycobacterial infections has not been studied, mice immunized with PPD following the induction of strong Th2 responses by filarial worm Brugia malayi were found to develop T cell cytokine responses that were polarized towards type 2 pattern [6].
The immune response to chronic S. mansoni infection in mice is generally characterized by a systemic Th2 type response at the onset of egg production with elevated production of IL-4 and IL-10 in response to parasite as well as nonparasite antigens [16, 18, 19]. However, mycobacterial infections are controlled by a Th1 type response [20–23]. Thus the elevated bacterial load in S. mansoni infected mice observed in this study could be due to inhibition of BCG induced Th1 type immunity by the established Th2 immune background caused by chronic worm infection.
To investigate this possibility we measured in vitro PPD specific proliferation and IFN-γ production of spleen cells as well as the in vitro production of Th2 cytokines in response to PPD, SEA and ConA. The results revealed that splenic T cells from mice with concomitant worm infection produce significantly lower levels of IFN-γ and exhibit impaired ability to proliferate in response to PPD as well as ConA. These were accompanied by high background response to SEA as well as mitogen-induced production of Th2 type cytokines IL-4 and IL-5 in the coinfected group of mice. This may suggest that worms increase susceptibility to mycobacterial infections by inhibiting mycobacteria antigen specific Th1 type responses and inducing a strong Th2 immune background as assessed by the strong IL-4 and IL-5 responses to a T cell mitogen ConA.
Immunity as defined by survival was not assessed since a low virulent BCG was used in this study. However, in subsequent studies we intend to use a more pathogenic organism (virulent M. tuberculosis) in an aerosol model to provide additional evidence on the impact of worm infections on subsequent immunity to mycobacterial infections.
Resistance against intracellular pathogens operates in two stages; an early nonspecific innate response and the acquired specific immunity mediated mainly by the CD4+ T cells [17,23]. During the early weeks of infection, the innate immune response is the main mechanism controlling mycobacterial growth. At the later stages however, the acquired cellular immunity becomes the dominant mechanism controlling mycobacterial infection [24].
Ferreira et al. [25] have shown that early stage responses to mycobacterial challenge can be enhanced by injection of mice with Ascaris suum extract. However, in the current study chronic infection with helminth parasite S. mansoni was associated with reduced ability to control mycobacterial infection accompanied by impaired cellular responses to mycobacteria antigen.
The differences between our findings and that of Ferreira et al. [25] may be that they evaluated the ability of mice injected with Ascaris suum extract to control mycobacterial infection during the first week of infection and our analysis was made at the later stage. This may indicate that the impact of exposure to worm antigens and/or infection with worms on the immune response to subsequent infections depends on the stage of the infection. In other words, exposure to worm antigens and/or worm infection could have variable effects on early innate and the late acquired immune responses to an infection. Indeed in a separate study we have observed (Elias et al. unpublished observation) that S. mansoni infection causes early enhancement in resistance against Mycobacterial infection, however, such effect was short lived and at later stage, the effect was negative consistent with the current report. It is worth noting that in preliminary studies we noted no significant difference between BCG infected and BCG+ S. mansoni coinfected group when assayed at three weeks after BCG infection.
The susceptibility to BCG infection induced by S. mansoni was differentially expressed in the three organs tested, being most pronounced in the lungs at later stage of infection. This suggests the possibility that there could be differences in the spatial distribution of the different antimycobacterial host effector responses and that these responses may be affected differentially by S. mansoni coinfection.
Taken together, our data show that immune response to M. bovis BCG can be affected by chronic S. mansoni infection, making the animals more permissive to M. bovis BCG replication. This supports the hypothesis that helminthic infection could have a negative effect on antimycobacterial immunity and this may have a bearing on the outcome of vaccinations against intracellular pathogens such as M. tuberculosis. This being so, the treatment of helminthes should have a role in the effort to control tuberculosis, one of the world's major infectious killer [23,26].
Acknowledgments
We greatly appreciate the assistances of Dr Yohannes Negesse and Banchiayehu Gualu in the pathology studies. The Sequella Global Tuberculosis Foundation, USA, the research department of the Swedish International Development Agency, Sida/SAREC, Sweden as well as Groschinsky foundation financially supported this work.
References
- 1.World Health Organization (WHO) Guide lines for establishing DOTS plus pilot projects for the management of multidrug resistant tuberculosis. Geneva: WHO; 2000. p. 279. [Google Scholar]
- 2.Dye C, Scheeles S, Dolin P, Pathania V, Raviglione MC. Consensus Statement. Global Burden of Tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global surveillance and Monitoring Project. J Am Med Assoc. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Chan MS. The global burden of intestinal nematode infections-fifty years on. Parasitol Today. 1997;13:438–43. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 4.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 5.Actor J, Shirai M, Kullberg M, Buller RM, Berzofsky JA, Sher A. Helminth infection results in decreased virus specific CD8+ cytotoxic T cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1994;90:948–52. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearlman E, Kazura JW, Hazlett F, Boom WH. Modulation of murine cytokine responses to mycobacterial antigens by helminth induced Th2 cell responseS. J Immunol. 1993;151:4857–64. [PubMed] [Google Scholar]
- 7.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after BCG vaccination. Clin Exp Immunol. 2001;123:219–25. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson M, Phalen SW, Lagranderie M, et al. Persistence and protective efficacy of M. tuberculosis auxotroph vaccine. Infect Immun. 1999;67:2867–73. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter CE, Colley DG. An electrophoretic analysis of S. mansoni soluble egg antigen preparation. J Parasitol. 1978;64:385–90. [PubMed] [Google Scholar]
- 10.Sherman DR, Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Smith S. Mycobacterium tuberculosis H37Rv: ∇RD1 is more virulent than M. Bovis BCG in long term murine infection. J Infect Dis. 2004;190:123–6. doi: 10.1086/421472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markus MB, Fincham JE. Mbeki and AIDS in Africa. Science. 2000;288:2131. doi: 10.1126/science.288.5474.2131d. [DOI] [PubMed] [Google Scholar]
- 12.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Down regulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, S. mansoni. J Exp Med. 1991;173:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, King CL. Helminth and BCG induced immunity in children Sensitized in utero to Filariasis and Schistosomiasis. J Immunol. 1999;162:6843–8. [PubMed] [Google Scholar]
- 14.Kullberg M, Pearce E, Heiny S, Sher A, Berzofsky JA. Infection with Schistososoma mansoni alters Th1/2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–70. [PubMed] [Google Scholar]
- 15.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levin MM, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with suppression of the interlukin-2 response to recombinant cholera toxin B sub-unit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–80. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147:2713–6. [PubMed] [Google Scholar]
- 17.Stenger S, Modlin RL. T cell mediated immunity in tuberculosis. Curr Opin Microbiol. 1999;2:89–93. doi: 10.1016/s1369-5274(99)80015-0. [DOI] [PubMed] [Google Scholar]
- 18.Ayash-Rashkovsky M, Weisman Z, Zlotnikov S, Raz E, Bentwich Z, Borkow G. Induction of Antigen-Specific Th1-Biased immune responses by plasmid DNA in Schistosoma-infected mice with a preexistent dominant Th2 immune profile. Biochem Biophys Res Commun. 2001;282:1169–76. doi: 10.1006/bbrc.2001.4698. [DOI] [PubMed] [Google Scholar]
- 19.King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, Jayaraman K, Ottesen EA, Nutman TB. Cytokine control of Parasite-specific anergy in human lymphatic filariasis: preferential induction of a regulatory T helper 2 lymphocyte subset. J Clin Invest. 1993;92:1667–73. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins HL, Kaufmann SHE. The many faces of host responses to tuberculosis. Immunology. 2001;103:1–9. doi: 10.1046/j.1365-2567.2001.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raupach B, Kaufmann SHE. Immune responses to Intracellular Bacteria. Curr Op Immunol. 2001;13:417–28. doi: 10.1016/s0952-7915(00)00236-3. [DOI] [PubMed] [Google Scholar]
- 22.Ellner JJ. The Immune Response in Human Tuberculosis-Implications for Tuberculosis Control. J Infect Dis. 1997;176:1351–9. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 23.Borkow G, Weisman Z, Leng Q, Stein M, Kalinkowich A, Wolday D, Bentwich Z. Helminths, HIV and Tuberculosis. Scand J Infect Dis. 2001;33:568–71. doi: 10.1080/00365540110026656. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida A, Koide Y, Uchijima M, Yoshida TO. Dissection of Strain difference in acquired protective immunity against Mycobacterium bovis BCG. J Immunol. 1995;155:2057–66. [PubMed] [Google Scholar]
- 25.Ferreira AP, Aarestrup FM, Bonecini-Almeida MG, Souza EE, Gomes EA, Correa JO, Teixeira HC. Effect of injection of an extract of Ascaris suum on macrophage activation during the early phase of Mycobacterium bovis BCG infection in C57Bl/6 mice. Braz J Med Biol Res. 1999;32:1429–36. doi: 10.1590/S0100-879X1999001100014. [DOI] [PubMed] [Google Scholar]
- 26.Burkow G, Bentwich Z. Eradication of helminthic infections may be essential for successful vaccination against HIV and tuberculosis. Bull World Health Organ. 2000;78:1368–9. [PMC free article] [PubMed] [Google Scholar]