Abstract
Increasing evidence has demonstrated that propylthiouracil (PTU) could induce ANCA positive vasculitis. However, our previous work has suggested that only one-fifth of the PTU-induced ANCA positive patients had clinical vasculitis and so the mechanism is not clear. Anti-endothelial cell antibodies (AECA) have been implicated in the pathogenesis of various vasculitides, including primary ANCA positive systemic vasculitis. The purpose of this study is to investigate the prevalence of AECA and their possible role in the pathogenesis of patients with PTU-induced ANCA positive vasculitis. Sera from 11 patients with PTU-induced ANCA positive vasculitis at both active and quiescent phases, and sera from 10 patients with PTU-induced ANCA but without clinical vasculitis, were studied. Sera from 30 healthy blood donors were collected as normal controls. Soluble proteins from 1% Triton-100 extracted in vitro cultured human umbilical vein endothelial cells were used as antigens and an immunoblotting technique was performed to determine the presence of AECA, and their specific target antigens were identified. In patients with PTU-induced ANCA positive vasculitis, 10 of the 11 patients in an active phase of disease were serum IgG-AECA positive and six protein bands of endothelial antigens could be blotted (61 kD, 69 kD, 77 kD, 85 kD, 91 kD and 97 kD). However, in the quiescent phase, seven of the 10 positive sera turned negative. None of the ANCA positive but vasculitis negative patients or normal controls were AECA positive. In conclusion, AECA could be found in sera from patients with PTU-induced ANCA positive vasculitis and were associated more closely with vasculitic disease activity.
Keywords: anti-endothelial cell antibodies (AECA), antineutrophil cytoplasmic antibodies (ANCA), propylthiouracil (PTU), vasculitis
Introduction
Systemic vasculitides consist of a group of diseases which have common pathological characteristics, such as inflammation and fibrinoid necrosis of the blood vessel wall. Among them, antineutrophil cytoplasmic antibodies (ANCA) are important serological diagnostic markers for primary systemic small vasculitic disorders such as Wegener's granulomatosis (WG), microscopic polyangiitis (MPA) and Churg–Strauss syndrome (CSS). Recently, propylthiouracil (PTU)-induced ANCA positive vasculitis has been paid much attention [1–6]. The mechanism of this disease is unclear. Our previous work suggested that over 20% of patients with hyperthyroidism taking PTU were serum ANCA positive, but only one-fifth of the PTU-induced ANCA positive patients had clinical vasculitis [7,8]. For patients with PTU-induced ANCA positive vasculitis, serum ANCA could remain positive in remission for a long time [7,8]. Therefore, it was reasonable to speculate that factors other than ANCA might be also associated with active vasculitic lesions in patients with PTU-induced vasculitis. In vasculitides, it was suggested that endothelium was not only the target of injury, but also an active participant of vasculitic damage [9]. Anti-endothelial cell antibodies (AECA) have been described in various autoimmune and vasculitic disorders [10–18]. They have been implicated in the pathogenesis of vascular injury common to these disorders. The aim of the current study was to investigate the prevalence of AECAs and their possible association with disease activity in patients with PTU-induced ANCA positive vasculitis.
Materials and methods
Patients and sera
Sera were collected from 11 patients with PTU-induced ANCA positive vasculitis at both active and remission phases in our hospital from 1999 to 2003 as the disease group; one patient was male and 10 were female, with an average age of 34·2 ± 4·9 (17–57) years. Details of their clinical, laboratory and pathological parameters are listed in Table 1. The average Birmingham Vasculitis Activity Scores (BVAS) were 12·5 ± 6·6. The interval time between active and remission phase was 28 ± 5·6 days. All the patients withdrew PTU immediately and were treated with corticosteroid and cyclophosphamide.
Table 1.
Clinical and pathological data of patients with PTU-induced ANCA positive vasculitis
| No. | Sex | Age (years) | Interval (days) | Involvement organs | BVAS | IIF assay | Antigen-specific ELISA | Kidney pathology | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | 75 | K, L, J, No, Sk, A | 20 | P | MPO, LF, CG | CrGN, FSNGN | S, P, MP, PE | HDD and KT |
| 2 | M | 34 | 2000 | K, L, J, M, Ey, A | 12 | P, C | MPO, PR3, HLE, LF, CG, AZU | Minor lesion | S, P, CTX | CR |
| 3 | F | 17 | 300 | K, L, J, M, Sk, A | 20 | P | MPO, HLE, LF, CG, AZU | Minor lesion | S | CR |
| 4 | F | 23 | 360 | K, L, J, Sk, A | 16 | P, C | MPO | CrGN | S, P, MP, CTX | CR |
| 5 | F | 45 | 720 | M, Ea, A | 4 | P, C | MPO, PR3, LF, AZU | None | S | CR |
| 6 | F | 28 | 1800 | K, M, A | 13 | P | MPO, HLE, CG, AZU | FSNGN | S, MP, P, CTX | CR |
| 7 | F | 17 | 2400 | K, Sk, A | 14 | P | MPO | IgA-N | S, P | CR |
| 8 | F | 21 | 360 | K, L, J, M, Sk, A | 20 | P | MPO | MN(I) FSNGN | S, P, MP, CTX, PE | CR |
| 9 | F | 52 | 1800 | J, M, Sk, Ey | 6 | P | MPO | None | S | CR |
| 10 | F | 30 | 1080 | K, J, Ea, Sk, Ey | 12 | P | MPO, HLE, LF, CG, AZU | Minor lesion | S | CR |
| 11 | F | 52 | 660 | J | 1 | P | MPO, PR3, HLE, LF, CG, AZU | None | S | CR |
M: male, F: female. Interval: interval between taking PTU and onset of vasculitis. K: kidney; L: lung; J: joint; N: nose; Sk: skin; A: anaemia; M: muscle; Ey: eye Ea: ear. FSNGN: focal segmental necrotizing glomerulonephritis; CrGN: cresentic glomerulonephritis. IgA-N: IgA nephropathy; MN: membranous nephropathy. S: stop PTU; P: oral predisone; MP: methylpredisone impulse; CTX: oral cyclophosphamide; PE: plasma exchange; HDD: haemodialysis dependent; KT: kidney transplantation; CR: complete remission.
Sera from 10 patients with PTU-induced positive ANCA but without clinical vasculitis were obtained as disease controls; nine of the 10 sera were p-ANCA positive and one serum was c-ANCA positive. Only one serum recognized myeloperoxidase (MPO), one serum recognized proteinase 3 (PR3), six sera recognized lactoferrin (LF) and three sera recognized human leucocyte elastase (HLE) in antigen-specific enzyme-linked immunosorbent assay (ELISA). Sera from 30 healthy donors were collected as normal controls. All sera were stored at −30°C until use.
Methods
Preparation of primary human umbilical vein endothelial cells (HUVEC)
Endothelial cells were harvested from human umbilical veins by collagenase (0·1%, Gibco, Invitrogen Corporation, NY, USA) digestion and plated in culture flasks in M199 medium (Gibco) supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 20% fetal calf serum (Gibco), 6·25 µ/ml heparin, 2·38 mg/ml HEPES and 20 µg/ml endothelium cell growth factor (ECGF, Roche, Mannheim, Germany). Cultures were maintained in humidified atmosphere of 5% CO2 at 37°C and the culture medium was changed thrice a week. After 5–7 days, cell monolayers were treated with 0·05% trypsin and 0·02% EDTA in Ca+-Mg++-free Hanks's balanced salt solution and cells were resuspended in complete medium. Subcultures were performed approximately every 5–7 days at the ratio of 1 : 2–3 [19]. The cells were identified by typical endothelial cell morphology with phase contrast microscopy, von Willebrand factor and Weibel–Palade bodies, which were observed by indirect immunofluorescence and immunohistochemistry with rabbit-antihuman vWF antibody (ZYMED) and electron microscopy, respectively. Cells were used for antigen extraction between the third and fifth passages.
Preparation of soluble proteins from HUVEC
Primary HUVEC were trypsinized from the flasks and washed twice with ice-cold phosphate buffered saline (PBS), counted and lysed (108 cells/ml) by 1% Triton X-100 (Sigma) with 1·0 mmol/l phenylmethylsulphonyl fluoride (PMSF) on ice for 40 min with gentle agitation. After centrifugation at 4°C at 15000 g/min for 15 min, the supernatant was collected as soluble proteins [19–21].
Western blot analysis to detect AECA
Soluble proteins from HUVEC were electrophoresed in reducing conditions on sodium dodecylsulphate (SDS) gradient of polyacrylamide gel (20 mA, 60 min). The proteins were then transferred to nitrocellulose (NC) membrane (Schleicher & Schuell, NH, USA) by electrophoretic semidry blotting system (Amersham Pharmacia, NJ, USA) at 0·8 mA/cm2 for 70 min. The NC membrane was cut into strips which were blocked at room temperature for 30 min with 2% skimmed milk in 10 mmol/l Tris/HCl buffer with 0·1% Tween-20 (TBST). Patients’ sera and control sera were diluted at 1 : 50 in TBST with 2% skimmed milk and incubated for 4 h at room temperature in gentle agitation, followed by three washes with TBST for 10 min each time. The strips were then incubated with phosphatase labelled affinity purified goat antihuman IgG (Sigma, St Louis, MO, USA) diluted at 1 : 6000 with TBST with 2% skimmed milk for 1 h at room temperature using gentle agitation. After three washes with TBST (10 min each time), the reaction was revealed by addition of appropriate substrate: nitroblue tetrazoleum (NBT) and 5 bromo−4 chloro−3 indolyl phosphate (BCIP) (Sigma). The reaction was stopped after 5–15 min with distilled water [22].
Statistical analysis
χ2 analysis was used to compare the AECA prevalences in patients with and without individual clinical parameters. Statistical analysis was performed using the SPSS (SPSS Inc. Chicago, IL, USA) version 10·0 statistical analysis program. A P-value of less than 0·05 was considered to be statistically significant.
Results
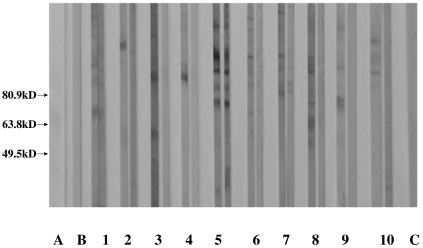
In patients with PTU-induced ANCA positive vasculitis, 10 of the 11 patients (91%) in active phase were IgG-AECA positive by Western blot analysis and six endothelial antigens at 61 kD, 69 kD, 77 kD, 85 kD, 91 kD and 97 kD could be recognized (Fig. 1), and some sera could blot two or more bands simultaneously. However, no significant correlation between AECA recongnizing different bands and clinical, laboratory and pathological parameters could be found.
Fig. 1.
Immunoblotting analysis of AECAs’ changes of the 10 positive sera from active phase to remission phase in patients with PTU-induced ANCA positive vasculitis. Line A: molecular marker; line B: blank control (PBS); lines 1–10: sera from the 10 positive patients (as nos 1–10 in Table 1) in every pair of strips; left: sera at active phase, right: sera at remission phase. Among them, lines 2–4, 6, 8–10 turned negative at remission phase. Line C: normal control.
Serum AECA from seven of the above 10 positive patients were not detectable at remission stage (Fig. 1). In sera from patients with PTU-induced ANCA positive but without vasculitis and sera from normal controls, none was serum AECA positive.
Discussion
PTU-induced ANCA positive vasculitis has been paid much attention recently [1–6]. The pathogenesis of this disease remains obscure [23,24]. It was speculated that the mechanism of ANCA production might be different between PTU-induced ANCA positive vasculitis and primary ANCA positive vasculitis. In the latter, the majority of ANCA recognized only a single target antigen such as MPO or PR3, while PTU-induced ANCA were polyclonal B cell activation, and multiple ANCA target antigens could be recognized by one serum and the target antigens included MPO, PR3, HLE, LF, bactericidal/permeability-increasing protein (BPI), cathepsin G (CG) and azurocidin (AZU) [2,7,8]. Increasing evidence has suggested that ANCA might play an important role in the pathogenesis of primary small vasculitis [9,25–37]. However, our previous work demonstrated clearly that the majority of PTU-induced ANCA positive patients did not show clinical evidence of vasculitis [7,8]. Sera from most patients with PTU-induced vasculitis had ANCA antigen specificities for MPO and/or PR3 compared with that of patients without vasculitis [7,8] indicated that antibodies to MPO and PR3 might be risk factors for patients with PTU-induced ANCA to develop clinical vasculitis. Our further study revealed that anti-MPO antibodies from patients with PTU-induced vasculitis might recognize more restricted epitopes on MPO molecules compared with anti-MPO antibodies from patients with primary vasculitis [38]. The roles played by IgG subclasses and affinity of PTU-induced anti-MPO antibodies in the pathogenesis of PTU-induced vasculitis are being currently investigated in our laboratory. However, some patients with PTU-induced anti-MPO antibodies had no evidence of clinical vasculitis, therefore factors other than ANCA might also be implicated in the development of vasculitis.
Endothelial cell damage is an important phenomenon in the early course of vasculitis. In recent years, there have seen significant advances in the understanding of inflammation and interaction between the vascular endothelium, mediators and immune effector cells. This has helped to elucidate further those specific processes relevant to vasculitis which result in endothelial cell damage. In WG and MPA, the evidence favours an autoimmune inflammatory response characterized by specific mediators in which the endothelium is both a target and an active participant. Among them, AECA, a heterogeneous group of antibodies quite distinct from the ANCA family, have been detected in variety of diseases which share a varying degree of vessel wall damage and might be pathogenic in inducing autoimmune vascular disease, but there has been no report about AECA in PTU-induced ANCA positive vasculitis. In patients with primary ANCA-associated vasculitis it is difficult to obtain sera before the onset of clinical vasculitis. However, PTU-induced ANCA positive patients with and without clinical vasculitis provide ideal human models to study possible factors associated with the development of vasculitis other than ANCA.
In the current study, we found that the majority (91%) of patients with PTU-induced ANCA positive vasculitis in active phase were IgG-AECA positive by Western blot analysis and six endothelial antigens at 61 kD, 69 kD, 77 kD, 85 kD, 91 kD and 97 kD could be recognized. Del Papa et al. [39] reported that the majority of patients with WG displayed a constant precipitation pattern of five proteins (25, 68, 125, 155 and 180 kD). Our previous work also suggested that 45 of 72 (62·5%) patients with MPA in active phase were IgG-AECA positive by Western blot analysis and seven endothelial antigens at 65 kD, 72 kD, 77 kD, 88 kD, 97 kD, 111 kD and 120 kD could be recognized; in patients with WG, 64% (32/50) of patients had AECA and their target antigens included 61 kD, 65 kD, 69 kD, 77 kD, 83 kD, 88 kD, 97 kD, 105 kD, 113 kD and 120 kD, respectively [40]. Therefore, most AECA antigens from both primary ANCA-associated small vasculitis and PTU-induced ANCA positive vasculitis might be overlapped. Another interesting finding in our current study was that seven of the 10 positive sera in active phase with PTU-induced ANCA positive vasculitis turned negative in remission phase, suggesting that AECA might be correlated with vasculitic disease activities. Our results support the finding by Gobel et al. [41] and del Papa et al. [42] in primary vasculitis. A more important finding in the current study was that none of the patients with PTU-induced ANCA but without clinical vasculitis were AECA positive. These indicated that AECA might be a serological marker for the development of vasculitis and a close association with vasculitic disease activity. However, whether the target antigens blotted by patients’ sera were specific to endothelial cells needs to be investigated further using other cell types.
Increasing evidence suggests that AECA could induce endothelium damage. AECA could bind to endothelial cells and to display complement-dependent or antibody-dependent cellular cytotoxicity. However, a recent study revealed that AECA did not appear to support complement-mediated cytotoxicity (CDC), but a proportion could support antibody-dependent cellular cytotoxicity (ADCC), suggesting that they might contribute to vascular injury [43,44]. AECA could also up-regulate the expression of adhesion molecules (E-selectin, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) and to induce the secretion of cytokine and chemokine [interleukin (IL-6, IL-1, IL-8 and monocyte chemoattractant protein (MCP-1)], which could cause leucocyte recruitment and adhesion. AECA could also stimulate the expression of NFκB [45,46]. In turn, these cytokines could enhance the binding ability of AECA to endothelium. In primary ANCA-associated vasculitis, these adhesion molecules could enhance polymorphonuclear leucocytes (PMNs) to combine with endothelial cells and exaggerate inflammatory reaction. Some cytokines, such as granulocyte-macrophage colony stimulating factor (GM-CSF), could enhance the expression of PR3 on the surface of PMNs and thus PR3 could react with ANCA [47]. Therefore, it was speculated that AECA might play an important role in the development of clinical vasculitis.
In summary, the current study revealed that AECA, recognizing a variety of antigens on endothelial cells, could be found in sera from patients with PTU-induced ANCA positive vasculitis and might be associated with vasculitic disease activities. Further characterization of the putative antigens is needed to understand better their pathophysiological role.
References
- 1.Dolman KM, Gans RO, Vervaat TJ, et al. Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet. 1993;342:651–2. doi: 10.1016/0140-6736(93)91761-a. [DOI] [PubMed] [Google Scholar]
- 2.Xu XD, Zhao MH, Zhang YK, Guo XH, Wang HY. Clinicopathological characteristics of propylthiouracil-induced antineutrophil cytoplasmic antibody-positive vasculitis and their target antigens: a report of 4 cases and literature review. Zhonghua Nei Ke Za Zhi. 2002;41:404–7. [PubMed] [Google Scholar]
- 3.Choi HK, Merkel P, Walker AM, Niles JL. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43:405–13. doi: 10.1002/1529-0131(200002)43:2<405::AID-ANR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Sato H, Hattori M, Fujieda M, et al. High prevalence of antineutrophil cytoplasmic antibody positivity in childhood onset Graves’ disease treated with propylthiouracil. J Clin Endocrinol Metab. 2000;85:4270–3. doi: 10.1210/jcem.85.11.7000. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi K, Sata M, Machiya J, et al. Antineutrophil cytoplasmic antibody positive alveolar haemorrhage during propylthiouracil therapy for hyperthyroidism. Respirology. 2003;8:532–5. doi: 10.1046/j.1440-1843.2003.00499.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs EM, Hartkamp A, Kaasjager HA. PTU-associated cutaneous vasculitis with ANCA anti-MPO and anti-PR3 antibodies. Neth J Med. 2003;61:296–9. [PubMed] [Google Scholar]
- 7.Guo XH, Zhao MH, Gao Y, Wang SF, Gao Y. Antineutrophil cytoplasmic antibody associated vasculitis induced by antithyroid agents. Nat Med J China. 2003;83:932–5. [PubMed] [Google Scholar]
- 8.Gao Y, Zhao MH, Guo X-H, Xin G, Gao Y, Wang H-Y. Antineutrophil cytoplasmic antibody (ANCA) associated vasculitis induced by antithyroid agents. Endocrinol Res. 2004;30:205–13. [Google Scholar]
- 9.Cid MC, Segarra M, Garcia-Martinez A, Hernandez-Rodriguez J. Endothelial cells, antineutrophil cytoplasmic antibodies, and cytokines in the pathogenesis of systemic vasculitis. Curr Rheumatol Rep. 2004;6:184–94. doi: 10.1007/s11926-004-0067-3. [DOI] [PubMed] [Google Scholar]
- 10.Healy CM, Carvalho D, Pearson JD, Thornhill MH. Raised anti-endothelial cell autoantibodies (AECA), but not anti-neutrophil cytoplasmic autoantibodies (ANCA), in recurrent oral ulceration. modulation of AECA binding by tumour necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma) Clin Exp Immunol. 1996;106:523–8. doi: 10.1046/j.1365-2249.1996.d01-877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujieda M, Oishi N, Kurashige T. Antibodies to endothelial cells in Kawasaki disease lyse endothelial cells without cytokine pretreatment. Clin Exp Immunol. 1997;107:120–6. doi: 10.1046/j.1365-2249.1997.d01-894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triolo G, Triolo G, Accardo-Palumbo A, et al. IgG anti-endothelial cell antibodies (AECA) in type I diabetes mellitus; induction of adhesion molecule expression in cultured endothelial cells. Clin Exp Immunol. 1998;111:491–6. doi: 10.1046/j.1365-2249.1998.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Cruz DP, Keser G, Direskeneli H, Khamashta MA, Hughes GR. Anti-endothelial cell antibodies in systemic vasculitis and systemic lupus erythematosus (SLE): effects of heat inactivation on binding and specificity. Clin Exp Immunol. 1999;115:567–70. doi: 10.1046/j.1365-2249.1999.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihn H, Sato S, Fujimoto M, et al. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosis. Clin Exp Immunol. 2000;119:203–9. doi: 10.1046/j.1365-2249.2000.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bordron A, Revelen R, D’Arbonneau F, et al. Functional heterogeneity of anti-endothelial cell antibodies. Clin Exp Immunol. 2001;124:492–501. doi: 10.1046/j.1365-2249.2001.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunebaum E, Blank M, Cohen S, et al. The role of anti-endothelial cell antibodies in Kawasaki disease in vitro and in vivo studies. Clin Exp Immunol. 2002;130:233–40. doi: 10.1046/j.1365-2249.2002.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YH, Wang SJ, Chuang YH, Lin YT, Chiang BL. The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-alpha treatment in children with Henoch–Schonlein purpura. Clin Exp Immunol. 2002;130:352–7. doi: 10.1046/j.1365-2249.2002.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripathy NK, Sinha N, Nityanand S. Anti-annexin V antibodies in Takayasu's arteritis: prevalence and relationship with disease activity. Clin Exp Immunol. 2003;134:360–4. doi: 10.1046/j.1365-2249.2003.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissman AM. Solubilization of cellular proteins. In: John E, Coligan ADA, Kruisbeek M, et al., editors. Current protocols in immunology. London: Greene Publishing Associated and Wiley-Interscience; 1991. pp. 8.1.1–8 1.9. [Google Scholar]
- 20.Wood NL, Schook LB, Studer EJ, et al. Biochemical characterization of human vascular endothelial cell monocyte antigens defined by monoclonal antibodies. Transplantation. 1988;45:787–92. doi: 10.1097/00007890-198804000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Lee KH, Chung HS, Kim HS, et al. Human alpha-enolase from endothelial cell as a target antigen of anti-endothelial cell antibody in Behçet's disease. Arthritis Rheum. 2003;48:2025–35. doi: 10.1002/art.11074. [DOI] [PubMed] [Google Scholar]
- 22.Revelen R, D’Arbonneau F, Guillevin L, Bordron A, Youinou P, Dueymes M. Comparison of cell-ELISA, flow cytometry and Western blotting for the detection of antiendothelial cell antibodies. Clin Exp Rheumatol. 2002;20:19–26. [PubMed] [Google Scholar]
- 23.Wada N, Mukai M, Kohno M, Notoya A, Ito T, Yoshioka N. Prevalence of serum anti-myeloperoxidase antineutrophil cytoplasmic antibodies (MPO-ANCA) in patients with Graves’ disease treated with propylthiouracil and thiamazole. Endocrinol J. 2002;49:329–34. doi: 10.1507/endocrj.49.329. [DOI] [PubMed] [Google Scholar]
- 24.Noh JY, Asari T, Hamada N, et al. Frequency of appearance of myeloperoxidase–antineutrophil cytoplasmic antibody (MPO-ANCA) in Graves’ disease patients treated with propylthiouracil and the relationship between MPO-ANCA and clinical manifestations. Clin Endocrinol (Oxf) 2001;54:651–4. doi: 10.1046/j.1365-2265.2001.01282.x. [DOI] [PubMed] [Google Scholar]
- 25.Haubitz M, Gerlach M, Kruse HJ, Brunkhorst R. Endothelial tissue factor stimulation by proteinase 3 and elastase. Clin Exp Immunol. 2001;126:584–8. doi: 10.1046/j.1365-2249.2001.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidebach W, Viana VS, Leon EP, et al. C-ANCA-positive IgG fraction from patients with Wegener's granulomatosis induces lung vasculitis in rats. Clin Exp Immunol. 2002;129:54–60. doi: 10.1046/j.1365-2249.2002.01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland M, Takada K, Okumoto T, et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol. 2002;129:183–90. doi: 10.1046/j.1365-2249.2002.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Geld YM, Tool AT, Videler J, et al. Interference of PR3-ANCA with the enzymatic activity of PR3: differences in patients during active disease or remission of Wegener's granulomatosis. Clin Exp Immunol. 2002;129:562–70. doi: 10.1046/j.1365-2249.2002.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlsson S, Wieslander J, Segelmark M. Increased circulating levels of proteinase 3 in patients with anti-neutrophilic cytoplasmic autoantibodies-associated systemic vasculitis in remission. Clin Exp Immunol. 2003;131:528–35. doi: 10.1046/j.1365-2249.2003.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clayton AR, Savage CO. Production of antineutrophil cytoplasm antibodies derived from circulating B cells in patients with systemic vasculitis. Clin Exp Immunol. 2003;132:174–9. doi: 10.1046/j.1365-2249.2003.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patry YC, Nachman PH, Audrain MA, Falk RJ, Meflah K, Esnault VL. Difference in antigenic determinant profiles between human and rat myeloperoxidase. Clin Exp Immunol. 2003;132:505–8. doi: 10.1046/j.1365-2249.2003.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day CJ, Hewins P, Savage CO. New developments in the pathogenesis of ANCA-associated vasculitis. Clin Exp Rheumatol. 2003;21(Suppl. 6):S35–48. [PubMed] [Google Scholar]
- 33.Selga D, Segelmark M, Wieslander J, Gunnarsson L, Hellmark T. Epitope mapping of anti-PR3 antibodies using chimeric human/mouse PR3 recombinant proteins. Clin Exp Immunol. 2004;135:164–72. doi: 10.1111/j.1365-2249.2004.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreiber A, Luft FC, Kettritz R. Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int. 2004;65:2172–83. doi: 10.1111/j.1523-1755.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 35.Heeringa P, Cohen Tervaert JW. Pathophysiology of ANCA-associated vasculitides: are ANCA really pathogenic? Kidney Int. 2004;65:1564–7. doi: 10.1111/j.1523-1755.2004.05412.x. [DOI] [PubMed] [Google Scholar]
- 36.Hewins P, Williams JM, Wakelam MJ, Savage CO. Activation of Syk in neutrophils by antineutrophil cytoplasm antibodies occurs via Fcgamma receptors and CD18. J Am Soc Nephrol. 2004;15:796–808. doi: 10.1097/01.asn.0000113241.98702.77. [DOI] [PubMed] [Google Scholar]
- 37.Reumaux D, Duthilleul P, Roos D. Pathogenesis of diseases associated with antineutrophil cytoplasm autoantibodies. Hum Immunol. 2004;65:1–12. doi: 10.1016/j.humimm.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Ye H, Zhao MH, Gao Y, et al. Anti-myeloperoxidase antibodies in sera from patients with propylthiouracil induced vasculitis might recognize restricted epitopes on myeloperoxidase molecule. Clin Exp Immunol. 2004;138:179–82. doi: 10.1111/j.1365-2249.2004.02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Papa N, Conforti G, Gambini D, et al. Characterization of the endothelial surface proteins recognized by anti-endothelial antibodies in primary and secondary autoimmune vasculitis. Clin Immunol Immunopathol. 1994;70:211–6. doi: 10.1006/clin.1994.1031. [DOI] [PubMed] [Google Scholar]
- 40.Yu F, Zhao MH, Zhang YK, Zhang Y, Wang HY. The significance of anti-endothelial cell antibodies in patients with ANCA associated systemic vasculitis and their target antigens. Chin J Nephrol. 2004;20(Suppl.):11–5. [Google Scholar]
- 41.Gobel U, Eichhorn BG, Kettritz R, et al. Disease activity and autoantibodies to endothelial cell in patients with Wegener's granulomatosis. Am J Kidney Dis. 1996;28:186–94. doi: 10.1016/s0272-6386(96)90300-5. [DOI] [PubMed] [Google Scholar]
- 42.Del Papa N, Guidali L, Sironi M, et al. Anti-endothelial cell IgG antibodies from patients with Wegener's granulomatosis bind to human endothelial cells in vitro and induce adhesion molecule expression and cytokine secretion. Arthritis Rheum. 1996;39:758–66. doi: 10.1002/art.1780390507. [DOI] [PubMed] [Google Scholar]
- 43.Savage COS, Pottinger BE. Vascular damage in Wegener's granulomatosis and microscopic polyarteritis presence of anti-endothelial cell antibodies and their relation to anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1991;85:14–19. doi: 10.1111/j.1365-2249.1991.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer O, Kaiser P, Haim T, et al. Anti-vascular endothelial cell antibodies (AECA): comparison of two assay methods and clinical applications. Rev Rhum Engl Ed. 1995;62:737–47. [PubMed] [Google Scholar]
- 45.Del Papa N, Meroni PL, Barcellini W, et al. Antibodies to endothelial cells in primary vasculitides mediate in vitro endothelial cytotoxicity in the presence of normal peripheral blood mononuclear cells. Clin Immunol Immunopathol. 1992;63:267–74. doi: 10.1016/0090-1229(92)90232-d. [DOI] [PubMed] [Google Scholar]
- 46.Blank M, Krause I, Goldkorn T, et al. Monoclonal anti-endothelial cell antibodies from a patient with Takayasu arteritis activate endothelial cells from large vessels. Arthritis Rheum. 1999;42:1421–32. doi: 10.1002/1529-0131(199907)42:7<1421::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 47.Hellmich B, Csernok E, Trabandt A, Gross WL, Ernst M. Effect of G-CSF and GM-CSF on surface expression of MPO and PR3 on PMN in vitro. Clin Exp Immunol. 2000;120(Suppl. 1):1–79. doi: 10.1046/j.1365-2249.2000.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]