Abstract
Dendritic cells (DCs) play a key role in the induction and regulation of antigen-specific immunity. Studies have shown that, similar to infection, cellular necrosis can stimulate DC maturation. However, the ability of necrotic cell death to modulate DC cytokine secretion has yet to be explored. We investigated the regulation of interleukin (IL)-12 secretion by human DCs in response to tumour cell necrosis in an in vitro culture model. Two human tumour cell lines (K562 and JAr) were induced to undergo necrosis using heat injury and repeated cycles of freezing and thawing. Both types of tumour cells tested in this study, when injured, induced secretion of monomeric IL-12p40 by monocyte-derived DCs. Furthermore, priming DCs with necrotic cells augmented IL-12p70 secretion significantly in conjunction with CD40 cross-linking. This was physiologically relevant because cell death-pulsed DCs were more potent than non-pulsed DCs at stimulating T cells to proliferate and secrete interferon (IFN)-γ. The Toll-like receptor 4 (TLR4) played a role in mediating the DC response to heat-killed, but not freeze/thaw-killed necrotic cells. For both methods of injury, proteins contributed to the effect of necrosis on dendritic cells, whereas DNA was involved in the effect of freeze/thawed cells only. These findings indicate that necrotic tumour cell death is not sufficient to induce bioactive IL-12p70, the Th1 promoting cytokine, but acts to augment its secretion via the CD40/CD40L pathway. The results also highlight that the mode of cell death may determine the mechanism of dendritic cell stimulation.
Keywords: Dendritic cells, cytokines, interleukins, apoptosis, toll-like receptors, human studies
Introduction
Dendritic cells (DCs) are innate immune cells which play an important role in initiating adaptive immune responses [1]. They are distributed widely in non-lymphoid tissues, where they exist in an immature state. When they are exposed to pathogens or inflammatory stimuli, DCs mature, up-regulate co-stimulatory molecules and acquire the capacity to secrete immunomodulatory cytokines. Several factors may induce DC maturation and activation including microorganisms, bacterial and viral products and cytokines. There is growing evidence that necrotic cell death can also serve this function. For example, in a mouse model, necrotic but not apoptotic cells and tissues up-regulated co-stimulatory molecules in vitro and in vivo[2]. A study of the response of human DCs in vitro demonstrated that necrotic death of a number of tumour cell lines, but not primary cells in culture, also up-regulated co-stimulatory molecules on DCs [3]. However, the ability of necrosis to stimulate cytokine secretion by DCs has not been investigated. We hypothesized that interleukin (IL)-12 could play a critical role in the response of DCs to necrosis because of its well-known ability to promote Th1 responses, the type of cell-mediated immunity responsible for monitoring the intracellular environment.
IL-12 is a heterodimeric cytokine (p35 and p40 subunits) produced by DCs upon activation and plays a critical role in the differentiation of Th1 cells [4,5]. Both subunits must be formed in the same cell to generate the biologically active heterodimeric IL-12p70 [4]. DCs produce IL-12p70 when their CD40 interacts with CD40 ligand (CD40L) on activated T cells [4]. Both microbial and T cell-derived stimuli can synergize to induce production of high levels of IL-12 p70 [6]. Furthermore, inflammatory cytokines such as IL-1β and type I interferon (IFN) released early during an immune response were found to be potent co-factors for CD40L-mediated IL-12p70 production by DCs [7].
In this study, we tested the hypothesis that products of tumour cell necrosis would induce IL-12 secretion by DCs and synergize with CD40 ligation for the regulation of this important Th1-promoting cytokine. We used two tumour cell lines of separate lineage, and tested the effect of products of their necrotic death on the ability of human monocyte-derived DCs to secrete IL-12.
Material and methods
Generation of dendritic cells
Peripheral blood was obtained from normal donors in heparinized Vacutainer tubes or from a single donor buffy coat (North London Blood Bank at Colindale, London, UK). DCs were prepared as described previously [8]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by sedimentation through Histopaque (Sigma, Dorset, UK) and allowed to adhere to tissue culture plastic (96-well plates, Greiner, Gloucester, UK) for 2 h. The non-adherent cells were removed. The adherent cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 1000 U/ml penicillin, 10 mg/ml streptomycin, 250 µg/ml amphotericin B and 20 µg/ml gentamycin (Sigma, Dorset, UK). Adherent PBMCs were cultured for 7 days in the presence of IL-4 at a concentration of 25 ng/ml (2 U/ng, First Link, West Midland, UK) and granulocyte macrophage-colony stimulating factor (GM-CSF) at a concentration of 50 ng/ml (10 U/ng, Schering-Plough, Hertfordshire, UK). Cytokines were added on days 2, 4 and 6. All DCs used in experiments were at day 7 of culture.
Cell lines
K562, a myelogenous leukaemia cell line that lacks human leucocyte antigen (HLA) molecules [9,10], was a kind gift from Dr N. Imami (Department of Immunology, Chelsea and Westminster Hospital, London, UK). These cells were cultured in supplemented RPMI-1640 medium and passaged every 2 days. JAr, a human choriocarcinomata cell line [11,12], was a kind gift from Dr D. Sooranna (Department of Fetal and Maternal Medicine, Chelsea and Westminster Hospital, London, UK). JAr cells do not express any HLA molecules [13], and the mRNA of TNF or IL-1 was either absent or weakly expressed [14]. The cells were cultured as monolayers in supplemented RPMI-1640 medium and harvested when confluent using trypsin/ethylenediamine tetra-acetic acid (EDTA) (0·5/0·2 g/l, Sigma, Dorset, UK). On average, cells were diluted 1 : 4–1 : 6 twice a week.
P3×3BII is a mouse myeloma cell line transfected with human CD40L [15] (a kind gift from Dr D. Graf, MRC, Hammersmith Hospital, London, UK). They were cultured at a concentration of 1 × 105/ml in supplemented RPMI-1640 and passaged twice a week. All cell lines were tested for mycoplasma by nested polymerase chain reaction (PCR) (Takara, Shiga, Japan) and confirmed to be mycoplasma free.
Cellular injury
Cellular injury was induced by two methods. With heat treatment, cells were incubated at 63°C for 30 min. Freeze–thaw killing involved snap-freezing cells in liquid nitrogen and thawing at 37°C. This process was repeated six times. Cell viability was checked by trypan blue (1%, Sigma, Dorset, UK) before culture to confirm that more than 90% of cells were dead. To confirm that the mode of cell death after the above forms of injury was indeed necrosis, cells were stained with annexin V and propidium iodide after exposing them to injury. The assay was performed according to the manufacturer's recommendation (Oncogene Research Products, Boston, USA). Briefly, cells were adjusted to 106/ml, 0·5 ml of cell suspension was transferred to a microcentrifuge tube and stained with 10 µl media reagent binding and 1·2 µl of annexin V. After 15-min incubation, cells were centrifuged at 200 g and resuspended in a cold binding buffer and 10 µl propidium iodide. Stained cells were examined immediately after staining by FACSCalibur flowcytometer and data analysed by cellquest software (Becton Dickinson, Oxford, UK). Cell death by thermal treatment or repeated cycles of freezing and thawing induced necrosis of the two types of tumour cells tested (data not shown).
Co-culture of DCs with cell lines
K562 and JAr cells (3 × 105/ml, injured and non-injured) were added to DCs at a concentration of 3 × 105/ml on day 7 of DC culture, in a 96-well tissue culture plate (Greiner). DCs were stimulated with the proinflammatory cytokines tumour necrosis factor (TNF)-α and IL-1β at a concentration of 30 ng/ml each (First Link Ltd, Brierley Hill, UK). Non-injured tumour cells were used as negative controls. It was not possible to use fixed-tumour cells as negative controls because they were stimulatory for DCs [16,17] (data not shown). Co-cultures were incubated at 37°C for 48 h in a humidified incubator containing 5% CO2 in air. In some experiments DCs were cultured with dead cells (heat-killed and freeze/thaw-killed cells) at a final concentration of 3 × 105/ml, with CD40L-transfected cells at a final concentration of 1·5 × 105/ml or with both dead cells and CD40L-transfected cell lines simultaneously. After 48 h, supernatants were collected for IL-12p40, IL-12p70 and IL-10 measurement by enzyme-linked immunosorbent assay (ELISA) (see below). In some experiments, K562 cells that have been exposed to cell death were fractionated by centrifugation to test the ability of particulate and soluble fractions to stimulate DCs. With heat injury, cells still maintained their integrity (not ruptured) and hence were fractionated by centrifugation at relatively low centrifugal force (1500 r.p.m. for 10 min). In contrast, repeated cycles of freeze and thaw resulted in complete disruption of the cell membrane and cells appeared completely fragmented, and hence a relatively higher speed (10 000 r.p.m. for 10 min) was needed to separate the particulate from the soluble fraction. After fractionation, the soluble fraction was added to DCs directly in 96-well tissue culture plates, while the particulate fraction was resuspended in fresh CM-10 before adding it to DCs.
Effect of injured K562 cells on DC differentiation from monocytes
PBMCs were cultured at a concentration of 1 × 106/ml in 24-well tissue culture plates (Greiner) and allowed to adhere for 2 h. The non-adherent cells were removed and adherent monocytes were resuspended in supplemented RPMI-1640 medium. Non-injured or heat-killed K562 cells were added to monocytes at a 1 : 1 ratio every day from day 0 to day 5 in different wells of monocyte culture. Monocytes were allowed to interact with K562 cells for 48 h before harvesting the culture supernatant to measure IL-12p40. IL-4 (25 ng/ml) and GM-CSF (50 ng/ml) were added on days 0, 2 and 4 of the differentiation culture.
Enzymatic digestion of dead cells by DNase or trypsin
Particulate fraction of dead cells (killed by heat-injury or freeze/thaw cycles) were treated with DNase (Sigma Aldrich, Dorset, UK) at a concentration of 200 µg/ml or with trypsin at a concentration of 0·5 mg/ml for 1 h at 37°C to allow for the digestion of the particulate fraction of dead cells. In experiments where trypsin was used, the particulate fraction was prepared in serum-free condition. After 1 h, trypsin activity was stopped by addition of serum. Trypsin- and DNase-digested dead cells were then cultured with DCs in 96-well tissue culture plates (Greiner). After 48 h of co-culture, supernatants were collected for IL-12 measurement by ELISA (see below).
Blocking of Toll-like receptor-4 (TLR4) on DCs
In these experiments, DCs were preincubated in 96-well tissue culture plates with functional grade antihuman TLR4 blocking antibody (clone HTA125) or its isotype-matched control (IgG2a) (both from eBioscience, San Diego, CA, USA) at a final concentration of 20 µg/ml. After 45 min, the particulate fractions of heat-killed or freeze/thaw-killed cells were added to DCs at a final concentration of 3 × 105/ml. DCs were also cultured with the particulate fraction of these cells in the absence of the blocking antibody and in the presence of the isotype-matched control. After 48-h incubation, supernatants were collected for IL-12 measurement by ELISA.
Immunofluorescence
Cells were harvested 48 h after appropriate incubation, washed and stained on ice for 20 min for the expression of co-stimulatory molecules. The antibodies were R-phycoerythrin (PE)-conjugated mouse monoclonal anti-CD86 antibodies (FUN-1), fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal anti-CD80 (BB1) and anti-CD86 (FUN-1). Isotype-matched monoclonal antibody control was mouse IgG1 (DAK-G01). All antibodies were purchased from Becton Dickinson, Oxford, UK.
Determination of IL-12, IL-10 and IFN-γ production
Cytokine production from the stimulated and the unstimulated cells was measured in culture supernatants collected after 48 h by ELISA. Briefly, NUNC-maxisorb 96-well plates (Gibco Life Technologies, Paisley, UK) were coated with purified antihuman IL-12p40 (clone C8·3) or purified antihuman IL-12p70 (clone 20C2) or rat antihuman IL-10 monoclonal antibody (clone JES3–9D7) and incubated overnight at 4°C. For measurement of IFN, plates were coated with mouse antihuman IFN-γ monoclonal antibody (clone NIB42). Cytokine binding was then detected with biotinylated-mouse monoclonal antihuman IL-12 (p40/p70) detection antibody (clone C8·6) or rat antihuman IL-10 clone JES3–12G8) or mouse antihuman IFN-γ (clone 4S.B3), followed by horseradish peroxidase-conjugated avidin (Vector Laboratories, Peterborough, UK) and a substrate solution containing 150 mg 2,2-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid, pH 4·5, mixed with H2O2 at a final concentration of 0·03%). Cytokine standards and antibodies were purchased from Pharmingen, San Diego, CA, USA.
T cell proliferation and IFN-γ production in the allogeneic mixed leukocyte reaction (MLR)
Necrotic or injured K562 cells were added to day 7 immature DCs. After 48 h of co-culture, the DCs were assayed for their T cell-stimulatory capacity in the MLR. DCs previously exposed to necrotic or non-injured K562 cells were titrated into round-bottomed 96-well plates containing the non-adherent population of allogeneic PBMCs (5 × 105/ml), and the cultures were incubated for 6 days. Cultures were then pulsed with [3H]-thymidine (Amersham International, Amersham, UK) for the final 18 h. Cells were harvested and T cell proliferation was measured by scintillation counting. In a parallel set of wells, the culture supernatants were collected and assayed for IFN-γ production by ELISA (see above). Assays were performed in triplicate.
Statistical analysis
Each experiment was repeated at least five times on different donors unless stated otherwise. Two repeats of experiments showing DC activation in response to cell death from the same donor showed similar results. For all experiments, statistical analysis for experimental repeats was performed using the non-parametric Wilcoxon paired-sample test to compare DCs response before and after stimulation. Differences were considered significant if P < 0·05.
Results
A series of in vitro experiments were performed to study the effect of injury to the HLA-negative tumour cell lines (K562 and JAr) on the ability of DCs to secrete IL-12. Human blood monocyte-derived DCs were co-cultured with these tumour cell lines after induction of tumour cell necrosis by heat injury or repeated cycles of freezing and thawing.
Cell death of tumour cell lines stimulated DCs to secrete IL-12p40 and up-regulate co-stimulatory molecules
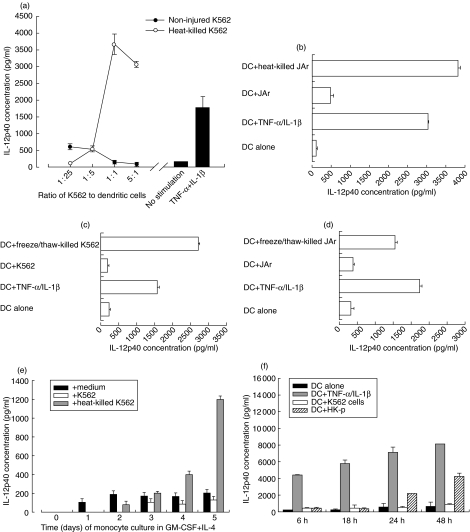
DCs were derived from monocytes by culture with GM-CSF and IL-4, as described in Materials and methods. After 7 days, heat-killed K562 cells were added. Controls were DCs co-cultured with non-injured K562 cells, a mixture of TNF-α and IL-1β, or medium only. DC maturation was assessed 48 h after co-culture by measuring the secretion of IL-12 in the culture supernatants and the up-regulation of the co-stimulatory molecules (CD80 and CD86) (as per Materials and methods). Heat-killed K562 cells stimulated DCs to secrete IL-12p40 in a dose-dependent manner (Fig. 1 a), whereas non-injured K562 cells did not, at any point in time over 48 h of co-culture (Fig, 1a,f). The highest level of DCs stimulation was observed when the ratio of K562 cells to DCs was 1 : 1 (Fig. 1a). Furthermore, heat-killed but not non-injured K562 cells induced DCs to up-regulate the surface expression of the co-stimulatory molecules CD80 and CD86 (data not shown). Such maturation was equivalent to that induced when DCs were cultured in the presence of TNF-α and IL-1β.
Fig. 1.
Necrotic, but not intact, tumour cells K562 and JAr stimulate DCs to secrete IL-12p40. Monocyte-derived DCs (3 × 105/ml) were co-cultured with heat-killed (a and b), freeze/thaw-killed (c and d) or non-injured K562 or JAr cells (3 × 105/ml except in (a) when K562 cells were added to DCs at varying ratios as shown), and IL-12p40 concentration in the culture supernatants was measured (Materials and methods). Heat-killed K562 cells were added to monocytes at different stages of differentiation into DCs (e). Non-injured K562 cells do not stimulate secretion of IL-12p40 by dendritic cells over 48 h (f). Compared with non-injured cells, killed K562 cells stimulated significantly more IL-12p40 secretion, both when (a) heat (at a 1 : 1 ratio, P = 0·008) and (c) freezing/thawing (P = 0·043 in three donors) were used to induce necrosis. Similarly, compared with non-injured cells, killed JAr cells stimulated significantly more IL-12p40 secretion, both when (b) heat (P = 0·005) and (d) freezing/thawing (P = 0·011) were used to induce necrosis.
This observation was confirmed by repeating the same type of experiment using the other tumour cell line, namely the choriocarcinoma cell line JAr (see Materials and methods). Heat-killed but not intact JAr cells stimulated DCs to secrete IL-12p40 in the culture supernatants (Fig. 1b), and up-regulated their co-stimulatory molecules CD80 and CD86 (data not shown).
Induction of cellular necrosis by repeated cycles of freezing and thawing of the K562 cells (Fig. 1c) and JAr cells (Fig. 1d) also had similar stimulatory effects on DCs. DCs cultured with K562 cells killed by freezing and thawing were stimulated to secrete IL-12p40, as were cells cultured with TNF-α and IL-1β. The levels of IL-12p40 produced were negligible when DCs were co-cultured with non-injured cells. Similarly, non-injured K562 cells did not stimulate DCs to up-regulate the surface expression of CD80 and CD86 molecules, whereas DCs cultured with K562 or JAr cells killed by freezing and thawing up-regulated the expression of the co-stimulatory molecules CD80 and CD86 (data not shown). Injured K562 and JAr cells did not stimulate undifferentiated monocytes to secrete IL-12. However, injured K562 and JAr cells stimulated IL-12p40 secretion by monocytes as early as day 2 of their differentiation into DCs. The amount of IL-12p40 secretion increased gradually as monocytes differentiated to immature DCs (Fig. 1e). Taken together these results indicate that, regardless of the form of cell injury and the type of tumour cell, necrotic cells stimulate DCs to secrete IL-12p40.
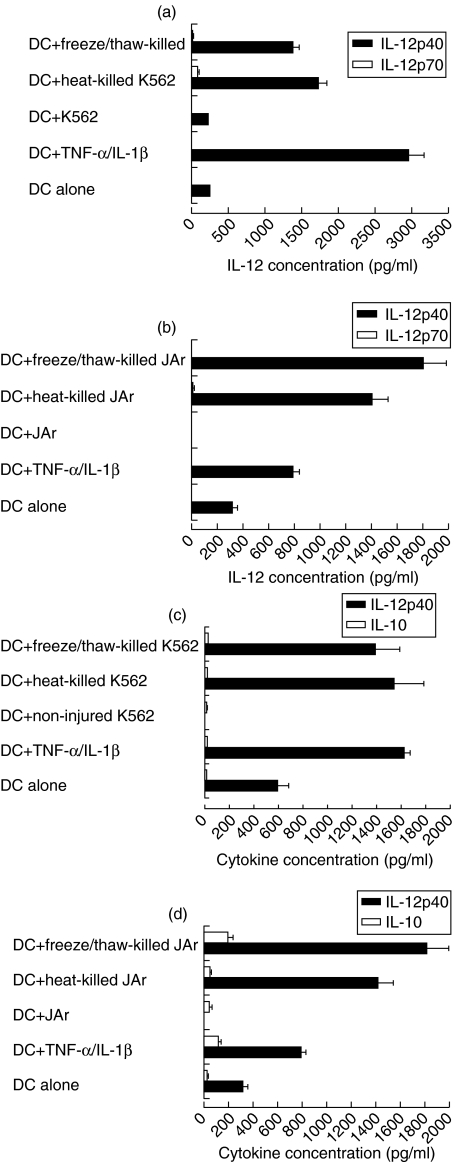
Necrotic cells did not stimulate DCs to secrete the biologically active IL-12 p70 heterodimer
Having established that cell death stimulated the secretion of IL-12 p40 subunit from dendritic cells, it was necessary to test whether the secretion of the biologically active form of this cytokine, IL-12p70, was also induced by necrotic cell death. Monocyte-derived DCs were cultured alone, in the presence of TNF-α and IL-1β, non-injured, heat-killed or freeze/thaw-killed tumour cell lines (K562, Fig. 2a and JAr, Fig. 2b). From 18 independent experiments performed with DCs from different donors, the level of IL-12p70 ranged from undetectable to 603 pg/ml (mean = 96 pg/ml, s.d. = 137) when DCs were cultured alone, and ranged from undetectable to 5765 pg/ml (mean = 400 pg/ml, s.d. = 70) when DCs were stimulated with TNF-α and IL-1β. However, there was no statistically significant difference between the two experimental conditions (P = 0·333). DCs did not secrete IL-12p70 when cultured with non-injured K562 cells, whereas a small amount of IL-12p70 was detected with injury, not exceeding 185 pg/ml (mean = 20 pg/ml, s.d. = 53) for heat injury, and not exceeding 90 pg/ml (mean = 4 pg/ml, s.d. = 10) for freeze/thaw injury. This small amount of IL-12p70 was observed in some but not all the experiments (four of 13 for heat-injury and two of five for freeze/thaw injury). Figure 2a shows one representative experiment. Similar results were obtained with the other tumour cell line, JAr (Fig. 2b).
Fig. 2.
Necrotic K562 and JAr cells consistently induce DCs to secrete IL-12p40, but do not induce IL-12p70 (a and b) or IL-10 (c and d) secretion. Monocyte-derived DCs (3 × 105/ml) were cultured with TNF-α/IL-1β, heat-killed, freeze/thaw-killed or non-injured K562 (a and c) or JAr cells (b and d). Both K562 and JAr cells were cultured at a density of 3 × 105 cells/ml.
We concluded that, unlike IL-12p40, DC secretion of IL-12p70 was not significantly induced in response to stimulation by necrotic cell death. Furthermore, IL-10, a potent inhibitor of the expression of many proinflammatory cytokines including IL-12 [18,19], was measured in supernatants of DC-injured cell co-cultures. Results showed that DCs exposed to injured cells did not induce the secretion of IL-10 compared with DCs cultured alone or with non-injured cells (K562, Fig. 2c and JAr, Fig. 2d).
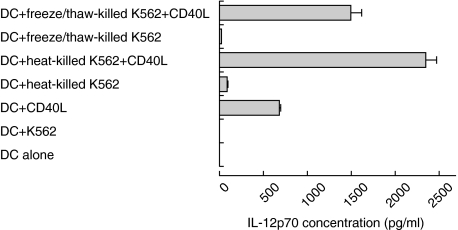
Necrotic cell death augmented IL-12p70 production from DCs in response to CD40 cross-linking
To investigate the possibility that cell death might synergize with CD40 ligation for the induction of high level of bioactive IL-12p70 heterodimer we focused on one tumour cell line, K562. Monocyte-derived DCs were cultured with CD40L-transfected cells at a ratio of 2 : 1 with or without killed cells. Supernatants were collected after 48 h of culture for measurement of IL-12 p40 and p70. Unlike with the p40 subunit, IL-12p70 was not secreted in response to killed cells, but high levels of IL-12p70 were detected when DCs were stimulated with CD40L-transfected cell lines. Furthermore, IL-12p70 secretion by DCs was significantly increased when both killed cells and CD40 ligation were used (Fig. 3). These results show that cell death and CD40L signals synergize to induce the secretion of high levels of IL-12p70 by DCs.
Fig. 3.
Necrotic K562 cells enhance IL-12p70 secretion by DCs only in response to CD40 cross-linking. Monocyte-derived DCs were cultured with heat-killed or freeze/thaw killed K562 cells in the presence or absence of CD40L transfected cells (1·5 × 105/ml), or with CD40L-transfected cells alone. This figure depicts representative data from one donor. Error bars represent duplicates of culture. In six experiments with different donors, IL-12p70 secretion in the presence of injured cells and CD40 ligation was significantly enhanced for heat-killed K562 cells (P = 0·04), for freeze/thaw-killed K562 cells (P = 0·028) compared with CD40 ligation alone.
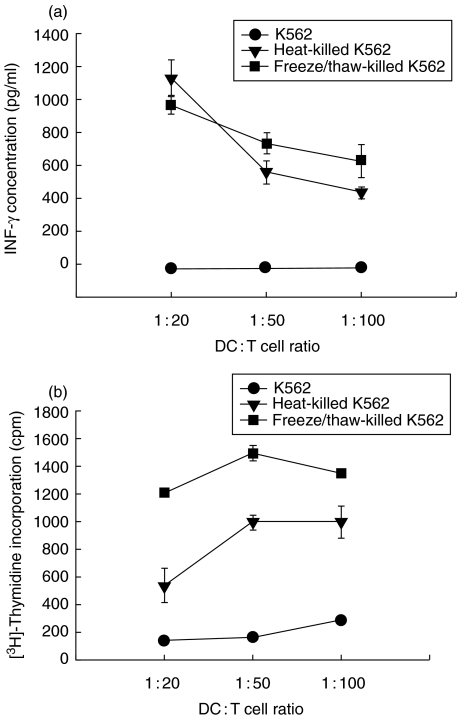
Necrotic cell death enhanced the ability of DCs to stimulate IFN-γ secretion and proliferation by allogeneic T cells
We investigated the ability of DCs exposed to injured cells to induce the secretion of IFN-γ by allogeneic T cells. DCs co-cultured with injured K562 cells induced IFN-γ secretion by T cells. Maximum IFN-γ production was seen when DCs were co-cultured with T cells at a ratio of 1 : 20 (Fig. 4a). Similarly, T cell proliferation was induced by DCs previously exposed to injured cells but not to non-injured cells (Fig. 4b). These experiments are consistent with the notion that cell death-mediated induction of IL-12 plays an important role in the ability of DCs to stimulate T cells to secrete IFN-γ.
Fig. 4.
Necrotic K562 cells enhanced the ability of DCs to stimulate the secretion of IFN-γ (P = 0·01, ratio 1 : 50 for both forms of injury) and the proliferation (P = 0·001, ratio 1 : 50 for both forms of injury) of allogeneic T cells. Killed K562 cells (3 × 105/ml) were co-cultured with DCs (3 × 105/ml) at a 1 : 1 ratio. After 48 h, DCs were titrated into tissue culture plates containing allogeneic PBMCs (5 × 105/ml) and the cultures were incubated for 6 days. Supernatants were harvested and IFN-γ was measured by ELISA (a). Cultures were pulsed with [3H]thymidine for the final 18 h (b). T cells alone gave counts <200 c.p.m. Results are representative of two experiments. Values shown represent the mean of triplicate wells.
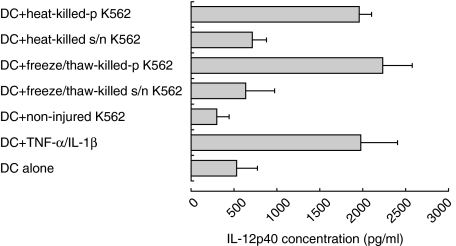
Secretion of IL-12p40 by DCs in response to necrotic K562 cells was mediated by their particulate fraction
For both forms of necrotic cell death (heat-killing and repeated cycles of freezing and thawing), the particulate fraction of dead cells activated DCs and stimulated them to secrete IL-12p40. Less IL-12p40 was secreted in response to the soluble fraction of dead cells (Fig. 5). These experiments indicate that the majority of immunostimulatory components which activated DCs are in the particulate (non-soluble) fraction of necrotic K562 cells.
Fig. 5.
Particulate fractions derived from killed K562 cells stimulate DCs to secrete IL-12p40. Monocyte-derived DCs were cultured for 48 h with non-injured, killed K562 cells, or with the particulate (p) or soluble (s/n) fractions of heat or freeze/thaw-killed cells (Materials and methods). Error bars represent duplicates of culture. In five experiments with different donors, particulate fractions of injured cells stimulated more IL-12p40 secretion than their soluble fractions (for heat-killed K562 cells P = 0·04, for freeze/thaw-killed K562 cells P = 0·043).
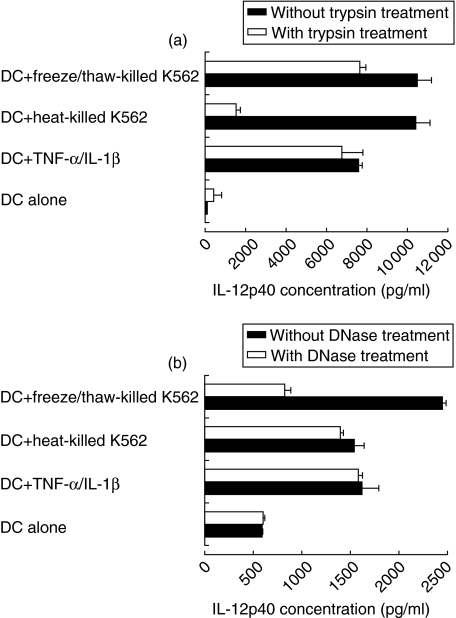
Both protein and DNA of necrotic cells contributed to IL-12p40 secretion by DCs
To identify the factors responsible for IL-12p40 secretion by DCs, these particulate fractions of necrotic cells were digested with either trypsin or DNase for 1 h, then added to DC culture for 48 h. DCs cultured in the presence of TNF-α and IL-1β were treated similarly with trypsin (inactivated by the addition of serum to the medium) or DNase and used as a control for the ability of DCs to respond to activating stimuli in the presence of these enzymes. IL-12p40 secretion by DCs in response to the particulate fraction of killed K562 cells was reduced by 80% with trypsin but not with DNase digestion (Fig. 6a,b). These results suggested that proteins rather than DNA played an important role in IL-12 secretion by DCs in response to necrotic cell death induced by thermal injury. On the other hand, when cells were killed by repeated cycles of freezing and thawing, both DNA and cellular proteins contribute to IL-12 secretion by DCs in response to necrotic cells. Digestion of the particulate fraction of freeze/thaw-killed K562 cells with trypsin reduced its ability to stimulate the secretion of IL-12p40 from DCs by 20% (Fig. 6a). This effect was not as profound as in the case of heat injury-induced death. A more pronounced reduction (reaching 75%) in the secretion of IL-12p40 by DCs was observed when freeze/thaw-killed K562 cells were pretreated with DNase before adding them to DCs (Fig. 6b). Diminished IL-12p40 secretion by DCs in response to digested dead cells was not due to a direct effect of these enzymes on DCs. The level of IL-12p40 secreted by DCs in response to TNF-α and IL-1β was not affected by the presence of trypsin or DNase. These results suggest that different methods of necrosis might stimulate IL-12 secretion by DCs through different sets of macromolecules. While heat-induced cellular death seemed to stimulate IL-12 mainly through proteins, freeze/thaw-induced cell killing might involve DNA-dependent IL-12 secretion.
Fig. 6.
Trypsin or DNase digestion of necrotic K562 cells reduced secretion of IL-12p40 by DCs. Monocyte-derived DCs were cultured with killed K562 cells, trypsin-digested (a) or DNase-digested (b) killed cells. Error bars represent duplicates of culture. In five experiments with different donors, trypsin-digestion of particulate fractions of injured cells significantly reduced IL-12p40 secretion (for heat-killed K562 cells P = 0·028, for freeze/thaw-killed K562 cells P = 0·043). DNase-digestion significantly reduced IL-12p40 secretion only with freeze/thaw-killed K562 cells (P = 0·04) but not with heat-killed cells (P = 0·06).
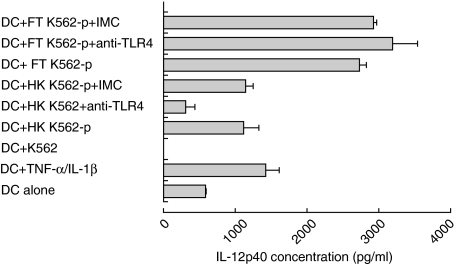
Role of TLR4 on DCs in IL-12p40 secretion in response to necrotic K562 cells
The aim of this set of experiments was to investigate whether TLR4 might contribute to the activation of DCs by necrotic K562 cell death. TLR4 has been suggested as a receptor for endogenous signals which could be derived from necrotic cells [20]. In this study, this receptor was blocked by incubating monocyte-derived DCs with anti-TLR4 antibody or its isotype-matched control (IMC) for 1 h before adding the particulate fraction of killed K562 cells (Materials and methods). Blocking of TLR4 resulted in a significant reduction of IL-12p40 secretion by DCs stimulated with the particulate fraction of heat-killed K562 cells. In contrast, blocking TLR4 on DCs did not result in the reduction of IL-12p40 secretion by DCs stimulated by particulate fraction of freeze/thaw-killed K562 cells (Fig. 7). The addition of IMC antibody did not affect the secretion of IL-12p40 from DCs in response to heat-killed or freeze/thaw-killed K562 cells. The experiment was repeated on five different donors to confirm these findings. IL-12p40 secretion was reduced significantly when TLR4 was blocked on DCs stimulated with heat-killed (P = 0·043), but not with freeze/thaw-killed K562 cells (P = 0·893).
Fig. 7.
Blocking of TLR4 on DC significantly reduced the secretion of IL-12p40 by DC when stimulated with heat-killed (P = 0·043 in five experiments) but not freeze/thaw-killed K562 cells (P = 0·893 in five experiments). Monocyte-derived DC were co-cultured for 48 h with non-injured K562 cells, particulate fraction of heat-killed (HK K562-p) or of freeze/thaw-killed (FT K562-p) K562 cells in the presence of anti-TLR4 antibody or its isotype-matched control (IMC). Error bars represent duplicates of culture.
Discussion
These results demonstrate that necrotic cell death of tumour cells stimulate DCs to secrete IL-12 p40 subunit but not the biologically active heterodimer IL-12p70. However, DCs primed with necrotic cells secrete more IL-12p70 upon CD40 ligation. In many cell lines, the expression of IL-12p40 transcripts correlates with the ability of these cells to produce the heterodimeric IL-12p70, whereas mRNA transcript of the other subunit, IL-12p35, was shown to be expressed constitutively [21,22]. Other reports demonstrate that a low level of p35 mRNA is expressed constitutively in freshly prepared human peripheral blood monocytes [23]. These findings lead to the assumption that production of IL-12p40 is representative of the biologically active IL-12p70. However, free monomeric IL-12p40 (measured in this study) has no biological activity [24,25]. Indeed, bioactive heterodimeric IL-12p70 production by human lipopolysaccharide (LPS)-activated monocytes is determined by the expression of IL-12p35 [26]. Furthermore, our results show that IL-10 is not secreted by DCs in response to injured cells. IL-10 is a potent inhibitor of IL-12, probably by blocking transcription of both p35 and p40 encoding genes [18,19]. Therefore, it is unlikely that IL-10 has a role in regulating IL-12 secretion by DCs under our experimental conditions.
The secretion of IL-12p70 by murine DCs in vivo can be mediated by CD40 ligation only in the presence of innate signals initiated by microbial stimuli [6]. In the present study, we have shown that products of necrotic cell death prime DCs for the secretion of high concentration of IL-12 p70 in response to CD40 ligation. Unlike murine DCs, human DCs secrete some IL-12p70 upon CD40 ligation. However, IL-12p70 production was significantly enhanced when the signal from CD40 ligation was combined with the presence of necrotic cells. This shows that necrosis is not sufficient to induce IL-12p70, but may promote Th1 responses by augmenting its secretion via the CD40/CD40L pathway. These results may imply that the expression of IL-12p35 subunit in DCs, which can be constitutively expressed in other cell types [21,22], may well be more tightly controlled than the expression of IL-12p40. This observation is supported by a recent study on the ability of Leishmania to prime CD40L-induced IL-12p70 [27]. In this work, reverse transcription-polymerase chain reaction (RT-PCR) analysis confirmed that the production of IL-12p70 from Leishmania-primed DCs in response to CD40 ligation was limited by the expression of IL-12p35 mRNA. These findings and ours suggest that the production of high levels of bioactive IL-12 by DCs is dependent on two signals: an innate stimulus (microbes or necrosis) that primarily induces IL-12 p40, and a signal through CD40, which is essential for the induction of the IL-12 p35.
In addition to IL-12p40 release, the ability of necrosis to augment the production of IL-12p70 by DCs in response to CD40 engagement could result from direct interaction with other intracellular factors which may control the IL-12 p35 gene. Another possibility is that this enhancement is mediated indirectly through the release of proinflammatory cytokines from cell death-activated DCs, or the injured tumour cells themselves. For example, IL-1β secreted by monocyte-derived DCs in response to inflammatory stimuli would synergize with CD40L for IL-12p70 release from DCs [28].
In this study, we confirmed that necrotic cell death stimulated the up-regulation of co-stimulatory molecules (CD80 and CD86), as published previously [3]. Our findings are consistent with the danger model [29] and the injury hypothesis [30]. These models postulate that the crucial decision to respond or not to respond within the immune system is based (at least partly) on the presence or absence of injury to ‘self’. The latter provides signals for DCs to mature, up-regulate co-stimulatory molecules and initiate an immune response.
The nature of the molecules derived from necrotic cells which stimulate IL-12p40 secretion by DCs is not yet elucidated fully. In this study, we demonstrate that digestion of necrotic cells with trypsin decreased the stimulatory effect of necrotic K562 cells killed by either heat or freezing and thawing. The reduction was more pronounced with heat-induced necrosis compared with freezing and thawing, suggesting that a non-proteinacious factor may contribute to DC activation by the release of intracellular components when cells are exposed to repeated cycles of freezing and thawing. In contrast to heat-induced necrosis, treatment of products of freeze/thaw-induced necrotic K562 cells with DNase decreased the secretion of IL-12 from DCs. These data are consistent with the possibility that proteins and DNA might play a role in DC activation by necrotic cells, perhaps depending on the original mechanism of inducing cell death.
Stress proteins have already been proposed by a number of studies as endogenous factors released from necrotic cells which can stimulate immune responses. Human heat-shock protein 60 (hsp60) can stimulate a monocyte cell line to secrete TNF-α and synthesize mRNA for IL-12 [31]. In mice, hsp60 might activate monocytes via TLR4 [20]. Similarly, hsp70 can induce monocyte secretion of IL-1β, IL-6 and TNF-α[32]. Another stress protein, namely gp96, can bind to DCs via the CD91 receptor [33], which appears to be a common receptor for a number of heat shock proteins [34]. The availability of such receptors for heat shock proteins on antigen-presenting cells makes stress proteins attractive candidates for signals derived from dead cells. Our finding that TLR4 played a role in DC activation by necrotic cell death induced by heat (but not freeze/thaw) is consistent with a role for hsp60. TLR2 is another receptor that can sense hsp60 [35]. This may explain reduction of IL-12p40 secretion by heat-killed K562 cells when TLR4 only was blocked.
Another candidate protein derived from necrotic cells which may contribute to IL-12p40 secretion is high mobility group box 1 chromosomal protein (HMGB1). It is composed of 215 amino acids, binds to DNA, stabilizes nucleosomes and allows DNA bending which facilitates gene transcription. Recent studies identified this protein as a critical factor connecting necrotic cell death to inflammation [36,37]. Apoptotic cells failed to release this protein, even after undergoing secondary necrosis. Li and coworkers showed recently that necrotic cells released a nuclear factor (identical to HMGB1) that interacted with macrophages via Toll-like receptor 2 (TLR2) and induced genes involved in inflammation [38]. In addition, other proteins, such as inflammatory cytokines, may be released de novo following necrosis and contribute to DC stimulation.
Cellular DNA might be released undigested by rapid non-physiological cell death, and may contribute to IL-12p40 secretion by DCs. In an in vitro study, murine fibroblasts osmotically or mechanically injured were able to induce maturation of bone marrow-derived DCs. This effect was reduced in the presence of both DNase and proteinase K enzymes, suggesting a role for both cellular protein and DNA in DC maturation [39]. This is similar to our findings that both proteins and DNA from necrotic cells may induce IL-12 depending on the method of injury. Eukaryotic genomes are not rich in the immunostimulatory CpG motifs compared with prokaryotic DNA [40]. Furthermore, eukaryotic CpG DNA is predominantly present in non-immunostimulatory methylated form, which serves as a mechanism for repression of gene transcription [41]. It is tempting to speculate that eukaryotic DNA, at least in tumour cells, may contain immunostimulatory motifs similar to prokaryotic CpG DNA. Furthermore, patterns of CpG methylation may be different in tumour cell lines [42]. Monocyte-derived DCs do not express TLR9, the known receptor for CpG motifs, but eukaryotic DNA recognition could well be mediated by other TLRs. Alternatively, multimeric forms of different TLRs might contribute to the process of eukaryotic DNA recognition. In a murine system, intact double-stranded DNA released from necrotic cells activated DCs, but CpG methylation did not inhibit this activation [39]. Modification of DNA by antineoplastic agents simulated DCs [43]. Furthermore, the method of cell death is a crucial determinant of the nature of DNA damage and hence the mechanism of DC activation. However, unlike with alkylating agents, DCs exposed to freeze/thaw-killed tumour cells did not stimulate T cell proliferation [43]. This is in contrast to our study, where both heat- and freeze/thaw-killed cells primed DCs to stimulate T cell proliferation and secretion of IFN-γ compared with untreated tumour cells. This difference might be due to differences in the method of induction of cellular freeze/thaw injury and the type of cell used. Sauter et al. reported that extensive necrosis (exposing cells to not less than four cycles of freeze and thaw) was required to release DC maturation factors and to stimulate the secretion of IFN-γ from T cells [3].
The observation that non-physiological cell death stimulated DCs to secrete IL-12 supports the notion that the immune system can distinguish between injured and intact self. That cell death primes DCs to release high levels of IL-12p70 optimally in response to CD40 ligation ensures that this crucial proinflammatory cytokine is secreted only at the DC–T cell interface. This tight regulation would protect the host from harmful autoreactive or allergic immune responses. In addition, these data are relevant for DC-based vaccinations used for the induction of antitumour immunity. Several studies have shown that vaccination with DCs pulsed with tumour cell lysates could induce significant antitumour CTL responses and antitumour immunity [44,45]. However, our results indicate variable degrees of success. Analysis of effects of products of these killed tumour cells, particularly with different methods of injury, on the functional state of DCs and their ability to induce IL-12 by DCs is a crucial step for the rational optimization of immunotherapy. Furthermore, our data may be useful for the rational development of novel strategies for vaccinations against tumours through controlling methods of inducing cell death.
Acknowledgments
We would like to thank Catherine Hargan for help with IL-12 assays, Mohamed El-Kalaawy for help with collecting blood, Richard Hooper for statistical advice, and the following colleagues for critical reading of the manuscript: Dr N. Shaun, B. Thomas and Dr Steve Patterson. This work was supported by grants from the Joint Research Committee of the Chelsea and Westminster Hospital (MAAI, FNG, DJW), the Peel Medical Trust (MAAI) and a scholarship from the Egyptian Culture Bureau (HK).
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 3.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 6.Schulz O, Edwards DA, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Luft T, Luetjens P, Hochrein H, et al. IFN-alpha enhances CD40 ligand-mediated activation of immature monocyte-derived dendritic cells. Int Immunol. 2002;14:367–80. doi: 10.1093/intimm/14.4.367. [DOI] [PubMed] [Google Scholar]
- 8.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koeffler HP, Golde DW. Human myeloid leukemia cell lines: a review. Blood. 1980;56:344–50. [PubMed] [Google Scholar]
- 10.Andersson LC, Nilsson K, Gahmberg CG. K562 – a human erythroleukemic cell line. Int J Cancer. 1979;23:143–7. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 11.Kohler PO, Bridson WE. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab. 1971;32:683–7. doi: 10.1210/jcem-32-5-683. [DOI] [PubMed] [Google Scholar]
- 12.Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28:1231–6. [PubMed] [Google Scholar]
- 13.Kovats S, Main EK, Librach C, et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–3. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 14.Bennett WA, Lagoo-Deenadayalan S, Brackin MN, Hale E, Cowan BD. Cytokine expression by models of human trophoblast as assessed by a semiquantitative reverse transcription-polymerase chain reaction technique. Am J Reprod Immunol. 1996;36:285–94. doi: 10.1111/j.1600-0897.1996.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Graf D, Korthauer U, Mages HW, Senger G, Kroczek RA. Cloning of TRAP, a ligand for CD40 on human T cells. Eur J Immunol. 1992;22:3191–4. doi: 10.1002/eji.1830221226. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Ohno T. Protective antitumor immunity induced by fixed tumor cells in combination with adjuvant in a murine hepatoma model. Cancer Lett. 2003;202:153–9. doi: 10.1016/s0304-3835(03)00479-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu SQ, Saijo K, Todoroki T, Ohno T. Induction of human autologous cytotoxic T lymphocytes on formalin-fixed and paraffin-embedded tumour sections. Nat Med. 1995;1:267–71. doi: 10.1038/nm0395-267. [DOI] [PubMed] [Google Scholar]
- 18.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–44. [PubMed] [Google Scholar]
- 19.D'Andrea A, Aste-Amezaga M, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 21.Scheicher C, Mehlig M, Dienes HP, Reske K. Uptake of microparticle-adsorbed protein antigen by bone marrow-derived dendritic cells results in up-regulation of interleukin-1 alpha and interleukin-12 p40/p35 and triggers prolonged, efficient antigen presentation. Eur J Immunol. 1995;25:1566–72. doi: 10.1002/eji.1830250615. [DOI] [PubMed] [Google Scholar]
- 22.Bost KL, Clements JD. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1995;63:1076–83. doi: 10.1128/iai.63.3.1076-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 24.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 25.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snijders A, Hilkens CM, van der Pouw Kraan TC, et al. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–12. [PubMed] [Google Scholar]
- 27.McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luft T, Jefford M, Luetjens P, et al. IL-1 beta enhances CD40 ligand-mediated cytokine secretion by human dendritic cells (DC): a mechanism for T cell-independent DC activation. J Immunol. 2002;168:713–22. doi: 10.4049/jimmunol.168.2.713. [DOI] [PubMed] [Google Scholar]
- 29.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim MA, Chain BM, Katz DR. The injured cell: the role of the dendritic cell system as a sentinel receptor pathway. Immunol Today. 1995;16:181–6. doi: 10.1016/0167-5699(95)80118-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–19. [PubMed] [Google Scholar]
- 32.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 33.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 34.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 35.Vabulas RM, Ahmad-Nejad P, Da Costa C, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 36.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 37.Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Carpio DF, Zheng Y, et al. An essential role of the nf-kappab/toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–35. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 39.Ishii KJ, Suzuki K, Coban C, et al. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602–7. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 40.Pisetsky DS. Immune activation by bacterial DNA. A new genetic code. Immunity. 1996;5:303–10. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 41.Chan MF, Liang G, Jones PA. Relationship between transcription and DNA methylation. Curr Top Microbiol Immunol. 2000;249:75–86. doi: 10.1007/978-3-642-59696-4_5. [DOI] [PubMed] [Google Scholar]
- 42.Robertson KD, Jones PA. DNA methylation: past, present and future directions. Carcinogenesis. 2000;21:461–7. doi: 10.1093/carcin/21.3.461. [DOI] [PubMed] [Google Scholar]
- 43.Rad AN, Pollara G, Sohaib SM, et al. The differential influence of allogeneic tumor cell death via DNA damage on dendritic cell maturation and antigen presentation. Cancer Res. 2003;63:5143–50. [PubMed] [Google Scholar]
- 44.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482–7. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu K, Fields RC, Giedlin M, Mule JJ. Systemic administration of interleukin 2 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines. Proc Natl Acad Sci USA. 1999;96:2268–73. doi: 10.1073/pnas.96.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]