Abstract
Invasive aspergillosis (IA) is a major cause of infection-related mortality in patients with haematological malignancies, especially in recipients of haematopoietic stem cell transplants. We have prepared overlapping pentadecapeptides (11-aa overlap with previous peptide) spanning the entire 427-aa coding region of the Aspergillus allergen, Asp f16 shown previously in mice to induce Th1-type cell responses in vivo and in humans to induce proliferative and cytotoxic CD4+ T cell responses. Mature dendritic cells (DC) pulsed with a complete pool of peptides were used to generate T cell lines. Two lines from HLA-B*3501+ donors were found to be strongly cytotoxic to autologous Asp f16-peptide pool- and Aspergillus culture extract-pulsed targets after 4–5 weekly primings. Cytotoxic T lymphocyte (CTL) culture supernatant killed Aspergillus conidia, and cells directly killed Aspergillus hyphae. Cytotoxic activity and interferon (IFN)-γ production were mediated exclusively by CD8+ T cells in response to pool-pulsed targets. Interleukin (IL)-4 production was not detected. CTL activity was restricted by HLA-B*3501 and based on peptide prediction programmes was most probably directed to YFKYTAAAL (YFK), LPLCSAQTW (LPL) and GTRFPQTPM (GTR) in one donor, while only LPL was recognized by CTL from the second donor. Pool-pulsed B*3503+ BLCL but not B*3502+ or B*3508+ BLCL presented peptide to donor no. 1. B*3503+ BLCL presented YFK and to a lesser extent GTR, but not peptide LPL. Our data show that in addition to our previously identified Class II restricted peptide response, DC pulsed with a pentadecapeptide pool from Asp f16 are capable of inducing polyclonal, HLA-Class I-restricted, Aspergillus-specific T cells that may be capable of conferring immunity to IA.
Keywords: Aspergillus fumigatus, CTL, immunotherapy, pentadecapeptides
Introduction
Immune deficiency is a common side effect of haematological malignancy and is especially severe during periods of dose-intense chemotherapy or radiotherapy for the treatment of the disease or as preparation for haematopoietic stem cell transplant (HSCT). Immune deficiency can be even more prolonged in patients who suffer from graft-versus-host disease (GVHD) secondarily to allogeneic HSCT. Invasive aspergillosis (IA), characterized by hyphal invasion and destruction of pulmonary tissue, is a major cause of infection-related mortality in patients during these periods of extreme immune deficiency [1,2]. Although neutropenia is a leading predisposing factor for IA, impaired T cell immunity has been associated significantly with a higher risk of infection, particularly late infection in HSCT patients with GVHD [2–7]. Evidence from murine models and from patients with allergic bronchopulmonary aspergillosis (ABPA) shows that T cell immunity plays a central role in this disease and that T-helper 1 (Th1) responses may be essential in the prevention and control of infection [8–12].
It is possible to induce protective immunity against Aspergillus infection by immunizing mice with antigen in the form of crude extracts from mycelia, spores and whole culture filtrates [12–14]. These preparations contain non-antigenic components including carbohydrates, proteins, nucleic acids and even toxins in addition to the relevant antigens [15–17]. Mycelia extracts of organisms grown in serum free medium contain as many as 52 distinct proteins [18]. Currently, 23 recombinant A. fumigatus allergens have been identified and cloned based on reactivity to IgG and/or IgE antibodies in serum from patients with ABPA (IUIS official list of allergens: http://www.allergen.org). Some of these recombinant allergens are strongly homologous to human proteins. Other allergens induce primarily Th2 immune responses or antibody responses that do not protect the organism from infection, thus are not antigens that would be useful to target for the development of immunotherapeutic approaches to IA [19–23]. However, the Asp f16 allergen was found not only to induce IgE responses, but also vigorous T cell proliferative responses that were highest in patients with ABPA [24]. Furthermore, in mice local delivery of Asp f16 allergen in the presence of CpG oligodeoxynucleotides as adjuvants resulted in the functional maturation and activation of airway dendritic cells (DC) capable of inducing a protective Th1 type T cell response to infection with Aspergillus conidia that was similar to that induced by crude culture filtrates [12].
Our own and a number of other laboratories have focused on means by which antigen-specific T cell immunity to infectious agents such as A. fumigatus might be augmented during periods when HSCT patients are most susceptible to infections, such as early after transplant or secondary to treatment for acute or chronic GVHD [25–27]. Transfer of donor-derived T cell clones [28] or T cell lines [29] for the prevention or treatment of cytomegalovirus (CMV) and Epstein–Barr virus infection post-HSCT has proved to be effective. While these antiviral protective responses are predominately mediated by CD8+ T cells, antigen-specific CD4+ T cells are also required for an effective in vivo response [30]. To date, similar approaches to prevent or treat fungal infections have not been reported due, at least in part, to lack of well-defined purified antigens that might induce protective T cell responses [17]. Given the promising preclinical results using Asp f16 our effort to develop an immunotherapeutic approach to prevent or treat Aspergillus infection has now focused on this antigen. We have used a series of 104 overlapping pentadecapeptides [11-amino acid (aa) overlap] spanning the entire 427-aa coding region of Asp f16 and have shown strong proliferative T cell responses by peripheral blood mononuclear cells (PBMC) from normal donors when peptides are presented on autologous DC [31]. Through repeated priming of donor T cells with peptide-pool pulsed autologous DC, we went on to identify a single peptide sequence, WSIDGAVVR, from Asp f16 that induced a potent Th1 response by CD4+ T cells in one of these normal donors. These T cells produced interferon (IFN)-γ and directly mediated cytotoxicity to peptide-pulsed targets that was restricted by the HLA-DRB1-*0301 allele of the donor. This T cell line also recognized targets pulsed with crude antigen extracts of Aspergillus cultures; supernatant from the CTL line was directly cytotoxic to Aspergillus conidia and the CTL directly killed Aspergillus hyphae. In this report we continue our studies of the Asp f16 allergen by generating CTL lines in two additional normal donors using an identical approach. Unlike the CD4+ T cell response of the previous donor, both donors made a CD8+ T cell response restricted by the same HLA Class I allele, B*3501. One donor recognized a single peptide while the other recognized this peptide plus two others each restricted by HLA-B*3501.
Materials and methods
Study participants and HLA-typing
Non-mobilized apheresis products were obtained from healthy individuals after written informed consent under research protocols approved by the Medical College of Wisconsin and Froedtert Hospital Investigational Review Boards. Sequence-based HLA typing was performed on cells from all donors by the Immunogenetics Laboratory, Blood Center of South-eastern Wisconsin, Milwaukee, WI, USA.
Overlapping pentadecapeptides
Overlapping 15 amino acid (aa) pentadecapeptides (n = 104) spanning the entire 427 aa sequence of the Asp f16 allergen were synthesized and analysed by NMI Technologietransfer GmbH, Reutlingen, Germany. Each pentadecapeptide overlaps the previous peptide by 11-aa. Peptides were prepared in single use aliquots and stored frozen at −70°C as described previously [31]. A complete pool containing all 104 peptides, each at 1 µg/ml was prepared, along with 21 small pools arranged in a matrix (10 vertical and 11 horizontal) consisting of four to 11 pentadecapeptides at 2 µg/ml of each peptide. Smaller pools together with single peptides were used to identify the specificity of the CTL.
Generation and peptide pulsing of monocyte (Mo)-derived DC
Generation of immature blood Mo-derived DC was performed as described previously [27], with minor modification [31]. Instead of the more standard 5–6-day culture period in medium containing granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin (IL)-4 to generate immature DC, we pulsed immature DC generated after only 2–3 days in culture (fast-DC) with Asp f16-peptide pool complete (PPC) for 6 h, then matured the DC for 2 days with a mixture of 10 ng/ml IL-1β, 1000 U/ml IL-6, 10 ng/ml tumour necrosis factor (TNF)-α (all from R&D Systems, Inc., Minneapolis, MN, USA) and 1 µg/ml prostaglandin (PG)-E2 (Sigma-Aldrich Corp, St Louis, MO, USA) before using them to prime proliferative and CTL responses.
Priming of polyclonal Aspergillus-CTL effectors
Asp f16-CTL effectors were generated according to our published method [27] by co-culture of mature, irradiated (25 Gy), PPC-pulsed fast-DC with autologous lymphocytes at ratio 1 : 10, respectively, in the presence of 500 pg/ml IL-12 (R&D Systems) in RPMI complete medium (RPMI-1640 with 25 m m HEPES, 2 mm l-glutamine, BioWhittaker, Walkersville, MD, USA) with 10% pooled human AB serum (HS, Labquip Ltd, Niagara Falls, NY, USA). Cultures were fed every third day with half-fresh RPMI complete medium−10% HS and 200 IU/ml IL-2 (Chiron, Emeryville, CA, USA). The cultures were restimulated every 7 days and cell density was adjusted to 106 cells/ml and maintained at this concentration throughout the culture period.
51Cr-release assay
Lytic activity of Asp f16-effectors was measured by a 4-h 51Cr-release assay as described previously [27]. Targets included autologous DC or PHA blasts and partially HLA-matched B lymphoblastoid cell lines (BLCL). Additional targets such as the natural killer (NK)-sensitive cell line K562 (American Type Culture Collection, Manassas, VA, USA) were also used. Targets were either unpulsed, or pulsed with A. fumigatus commercial culture filtrate antigen (Asp f-CF, Greer Laboratories Inc., Lenoir, NC, USA) (50 µg/ml), Asp f16-PPC (1 µg/ml each peptide), one of the small peptide pools (2 µg/ml each peptide) or with single peptides (2 µg/ml) by incubation with antigen in serum-free RPMI complete medium for 1 h at 37°C prior to labelling and washing. HLA-typed BLCL were either homozygous cell lines used in the 10th International Histocompatibility workshop, or were BLCL prepared under experimental protocols from patients or donors undergoing allogeneic HSCT. Consent was obtained at the time of sample collection for use of patient and donor BLCL for other experimental protocols. Spontaneous release was measured using medium with targets (no effectors) and maximum release was measured using 0·5% Triton X-100 (Sigma-Aldrich Corp) with targets only. Specific lysis was calculated as:
T cell subset separation
The CTL line from donor RD0401 was fractionated by positive selection of CD4+ T cells using Miltenyi CD4-conjugated beads (Miltenyi Biotec, Auburn, CA, USA) and a magnetic cell sorter (MACS). The purity of the separation was assessed by FACS analysis and the cytolytic activity of the CD4-enriched and CD4-depleted fraction was assessed separately as indicated above using HLA-matched non- and Asp f16-PPC-pulsed BLCL as targets.
Conidiacidal assay
A. fumigatus conidia were cultured, harvested and labelled with a fluorescent molecular stain (FUN-1, Molecular Probes, Eugene, OR, USA) as described by Balajee et al. [32]. CTL were restimulated 24 h prior to the assay. FUN-1-stained live conidia (106) were incubated overnight in fresh RPMI culture medium or in CTL culture medium (1 ml) and then examined under a fluorescence microscope. Live and heat-inactivated (85°C for 30 min) FUN-1-labelled conidia in medium alone were used as positive and negative controls, respectively. Metabolically active conidia accumulate orange fluorescence in vacuoles, while dormant and dead conidia stain green.
Hyphae killing assay
Hyphae killing was determined by using a tetrazolium dye XTT modified from a method described by Meshulam et al. [33], as described previously [31]. Briefly, Aspergillus conidia were germinated at 45°C for 16 h to form hyphae. Asp f16-specific CTL or CMVpp65-specific CTL were added to hyphae [effector : hyphae (E : H) ratio of 3 : 1] and incubated at 37°C for 2 h (with continued mixing) in the presence or absence of Asp f16-PPC or pp65 peptide mix (Jerini Peptide Technologies, Berlin, Germany). Controls included tubes containing live hyphae, CTL and 10% formaldehyde killed hyphae alone. Lysis was stopped by addition of 1 ml ice-cold distilled water, the tubes mixed, then centrifuged at 4°C for 10 min at 3000 g prior to the addition of 400 µl of freshly prepared XTT (0·5 mg/ml, Sigma) containing coenzyme Q (40 µg/ml, Sigma). After 1 h incubation at 37°C with continued mixing, 100 µl supernatant was transferred to a flat-bottomed 96-well plate and absorption at 450 nm was determined with 96-well plate reader (Packard, Meriden, CT, USA). Absorption of each well at 650 nm was also obtained to control non-specific absorption. The percentage of fungal cell damage was defined by the following equation:
Quantification of T cells producing IFN-γ in response to Asp f16-pentadecapetides
IFN-γ production was assessed by a method modified from Koehne et al. [34] using the FastImmune Intracellular Cytokine Detection Kits (BD Biosciences, San Jose, CA, USA) as described previously [31]. Tubes containing effectors either unstimulated or stimulated with staphylococcal enterotoxin B superantigen (SEB) were added as negative and positive controls, respectively. Staining and flow analysis were performed according to the manufacture's recommendations.
Immunophenotyping
Approximately 0·25 × 106 cells/tube were immunophenotyped using a four-colour direct panel including FITC-, PE-, PerCP- and APC-conjugated MoAb to: CD3, CD4, CD8, CD14, CD19, CD45, CD56, CD69, CD107a, IFN-γ, IL-4 and T cell receptor (TCR)-γ/δ. Non-specific binding was monitored using isotypic controls. All antibodies were obtained from Becton-Dickinson, Hialeah, FL, USA. The stained cells were acquired on a FACSCalibur flow cytometer and were analysed from list mode data using the FCAP software package.
Statistics
Statistical analysis was performed with unpaired t-test, for comparison between two groups and one-way anova (and non-parametric) test, for comparison between more than two groups using graphpad prism version 3·0 for Macintosh (GraphPad software, San Diego, CA, USA). P-values of <0·05, <0·01 and <0·001 were considered statistically significant, highly significant and very highly significant, respectively.
Results
Induction of Aspergillus-specific CD8+ cytotoxic T cell responses by Asp f16-PPC pulsed fast-DC
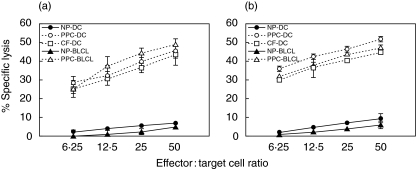
Mature fast-DC pulsed with Asp f16-PPC were used to prime CTL responses from three donors, cells from each of whom also proliferated in response to PPC-pulsed DC [31]. A CD4+ CTL line generated from one of these donors has been described previously [31]. Additional CTL lines were obtained from the other two donors. Both lines were found to be strongly cytotoxic and lysed autologous Asp f16-PPC- and Aspergillus culture extract-pulsed DC and autologous or HLA-matched BLCL targets to the same extent (P > 0·05, one-way analysis of variance) after 4–5-weekly primings (Fig. 1). The cytolytic activity towards Asp f16-PPC-BLCL targets of the line from donor RD0401 increased steadily from 49·1 ± 3·0% (E : T ratio = 50 : 1) at week 4 to 63·0 ± 2·3% by week 7, whereas the lytic activity of the line from donor RD0309 increased from 46·8 ± 1·4% at week 5 to 68·5 ± 1·1% at week 7. The effectors did not show any significant lytic activity to autologous non-pulsed DC, non-pulsed autologous or HLA-matched BLCL (Fig. 1), autologous PHA blasts (not shown) or culture-filtrate pulsed BLCL under the conditions described in Methods for target cell pulsing (not shown). Moreover, no NK-mediated cytotoxicity was seen as determined by using K562 target cells (not shown).
Fig. 1.
Lytic activity of the generated Asp f16-specific T cells. Asp f16-CTL from donor RD0401 (a) and RD0309 (b) were generated by four or five stimulations, respectively, with autologous, mature, fast-DC, pulsed with Asp f16-PPC. Cytolytic activity was assessed 5 days after the last priming using autologous Asp f16-PPC- and CF-pulsed DC or Asp f16-PPC-pulsed HLA-matched (a) or autologous (b) BLCL as targets. Non-pulsed DC and non-pulsed BLCL targets were used as controls. Data are presented as the mean of triplicate samples ± s.d. Asp f16-effectors lysed CF- and PPC-pulsed targets to a similar extent but not non-pulsed targets (P > 0·05, one-way analysis of variance). Less than 5% lytic activity was seen using NK-sensitive cell line K562 and autologous PHA blasts as targets in the assay (not shown). NP: Non-pulsed, PPC: complete overlapping pentadecapeptides pool, CF: commercial Aspergillus culture-filtrate antigen.
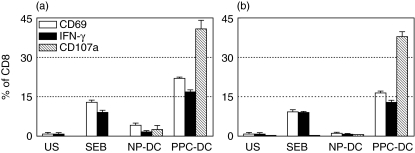
The Asp f16-effectors from donor RD0401 were predominately CD3+/CD4+, but the minor CD3+/CD8+ population increased steadily from 5·2 ± 0·5% of the total cells in the culture at week 3 to 19·0 ± 0·6% at week 7. To assess the phenotype of the cells mediating CTL activity, CD4+ and CD8+ effectors were separated from the Asp f16 bulk-culture. Cytolytic activity of the separated CD4+ (purity = 99·7%) and CD8+ (purity = 99·9%) cells was assessed using HLA-matched non- and Asp f16-PPC-pulsed BLCL as targets. CD8+ cells specifically lysed PPC-pulsed BLCL (65% at 50 : 1), and there was no significant cytolytic activity by CD4+ cells (<5% at 50 : 1). To further support the role of CD8 + T cells in the Aspergillus-specific CTL response we performed FACS-based assays for cell activation, IFN-γ production and degranulation of cytolytic granules in response to Asp f16-PPC-pulsed targets. CD8+ cells, but not CD4+ cells, increased significantly (P < 0·001, unpaired t-test) CD69 expression (22·1 ± 0·5%) and IFN-γ production (16·8 ± 0·9%) in response to PPC-pulsed autologous DC compared to NP-DC. A high level of CD107a expression was induced exclusively on CD8+ cells (41·1 ± 3·3% of CD8+ cells) in response to Asp f16-PPC-pulsed autologous DC indicating degranulation (Fig. 2a). The phenotype of the CTL line from donor RD0309 at week 5 was divided more evenly between CD4+ and CD8+ T cells with 51·4% CD3+/CD4+ and 49·4% CD3+/CD8+. As for donor RD0401, exposure to Asp f16-PPC pulsed targets stimulated exclusively the CD3+/CD8+ population, with 12·7% of CD8+ T cells producing IFN-γ, 16·4% co-expressing CD69 and 37·7% co-expressing CD107a (Fig. 2b). All these results indicated that Aspergillus-specific cytotoxic CD8+ T cells were generated by priming with PPC-pulsed fast-DC in both donors.
Fig. 2.
IFN-γ production and degranulation by Asp f16-specific CTL. CD8+ effectors from donor RD0401 (a) and RD0309 (b) were activated (white bars), produced IFN-γ (black bars) and expressed CD107a (hatched bars) in response to PPC-pulsed autologous DC compared to NP-DC. Data are shown as the mean ± s.d. of replicate staining tubes. SEB was used as a positive control and unstimulated CTL were used as a negative control. NP: non-pulsed, PPC: complete overlapping pentadecapeptides pool, US: unstimulated.
Identification of Asp f16-epitopes recognized by the CTL effectors
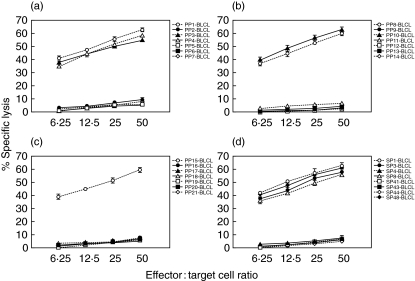
To define the epitope(s) inducing the Asp f16-specific response, we screened 21 smaller pools containing four to 11 pentadecapeptides using cytolytic assays. The CTL line from donor RD0401 showed lytic activity to targets pulsed with six of the smaller pools, PP1, PP3, PP4, PP8, PP11 and PP15 (Fig. 3a–c). We then screened the eight single peptides (SP1, SP3, SP4, SP8, SP41, SP43, SP44 and SP48) shared by the pools. No cytolytic activity was found in response to SP8, SP41, SP43 and SP44, whereas the cytolytic activity in response to SP1, SP3, SP4 or SP48 was not significantly different from the response to the entire pool (P > 0·05, one-way analysis of variance) (Fig. 3d). We additionally screened the CTL line against HLA-matched targets pulsed with pentadecapeptides SP2, SP5, SP47 and SP49 and found no lytic activity (Table 1), therefore we could narrow one epitope contained in SP1 to the sequence MYFKYTAAALAA. SP3 and SP4 shared the sequence VLPLCSAQTWS, making this a sequence that probably contains a second epitope and a third epitope present in SP48 is probably GTRFPQTPM (Table 1). The CTL line from donor RD0309 was screened in an identical manner but was found to react only to PP3, PP4 and PP11 and reacted to the single peptides, SP3 and SP4 shared by these pools, but not to SP2 or SP5, indicating recognition of the shared sequence VLPLCSAQTWS (Table 1).
Fig. 3.
Peptide specificity of the Asp f16 specific CTL. The Asp f16-specific CTL line from donor RD0401 was screened in a 51Cr-release assay against HLA-matched BLCL pulsed with 21 smaller pools each containing 4–11 pentadecapeptides. Cytotoxicity was seen to six smaller pools, PP1, PP3, PP4, PP8, PP11 and PP15 (a,b,c) and specificity could be narrowed by screening single peptides shared by the pools to four candidate peptides, SP1, SP3-SP4 and SP48 (d). Data are presented as mean value of triplicate samples ± s.d. PP: peptide pool, SP: single peptide.
Table 1.
Asp f16 epitopes.
| SP no. | Sequence | % Lytic activity RD0401 | % Lytic activity RD0309 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | MYFK | YTAA | ALAA | VLP | 63·4 ± 2·5 | n.d. | |||
| 2 | YTAA | ALAA | VLPL | CSA | 7·4 ± 0·6 | 5·2 ± 2·6 | |||
| First epitope is YFKYTAAAL (position 2 → 10) | |||||||||
| 2 | YTAA | ALAA | VLPL | CSA | 7·4 ± 0·6 | 5·2 ± 2·6 | |||
| 3 | ALAA | VLPL | CSAQ | TWS | 58·4 ± 2·7 | 63·3 ± 5·8 | |||
| 4 | VLPL | CSAQ | TWSK | CNP | 56·5 ± 0·9 | 64·9 ± 4·6 | |||
| 5 | CSAQ | TWSK | CNPL | AET | 6·9 ± 0·6 | 9·8 ± 1·0 | |||
| Second epitope is LPLCSAQTW (position 14 → 22) | |||||||||
| 47 | TYND | AKGG | TRFP | QTP | 6·3 ± 0·1 | n.d. | |||
| 48 | AKGG | TRFP | QTPM | RLR | 61·7 ± 0·8 | n.d. | |||
| 49 | TRFP | QTPM | RLRL | AAG | 5·2 ± 1·2 | n.d. | |||
| Third epitope is GTRFPQTPM (position 192 → 200) | |||||||||
Asp f16-CTL were screened in 51Cr-release assays to HLA-matched BLCL targets (RD0401) or autologous BLCL (RD0309) pulsed with single peptides shared by the smaller pools which were recognized (indicated in bold) and by flanking peptides as listed in the table. Data are presented as the percentage specific lysis ± s.d., at an E : T ratio of 50 : 1. The CTL line from RD0401 lysed targets pulsed with SP1, SP3, SP4 and SP48 but not targets pulsed with SP2, SP5, SP47 and SP49. Therefore one epitope is contained in SP1, a second epitope is contained in the sequence VLPLCSAQTWS, shared between SP3 and SP4 and is also the epitope recognized by CTL from donor RD0309, while a third epitope is contained in SP48. A database search of peptides likely to be restricted to B*3501 identified the three epitopes as YFKYTAAAL (position 2–10), LPLCSAQTW (position 14–22) and GTRFPQTPM (position 192–200). SP: Single peptide.
The HLA type of the CTL donor RD0401was determined by DNA sequencing and is shown in the legend to Table 2. Asp f16-PPC pulsed BLCL matched with the effectors for one or more HLA alleles were used in cytotoxicity assays to identify the restricting elements for cytolytic activity to Asp f16. BLCL from bone marrow (BM) donors 1 and 2 together covered all the HLA alleles of donor RD0401 and shared only HLA-B*3501 with each other (Table 2). Asp f16-CTL lysed to exactly the same extent PPC-BLCL from BM 1 and BM 2 donors (P > 0·05, unpaired t-test). In addition, Asp f16-effectors were cytotoxic for two additional PPC-pulsed B*3501+ targets but not for PPC-pulsed B*3501– targets (Table 2). In a similar fashion the CTL line from donor RD0309 (also HLA-B*3501+) was screened using Asp f16-PPC-pulsed BLCL from partially HLA-matched donors and similarly lysed only targets sharing HLA-B*3501 with the donor. These results indicated that Asp f16-epitopes were presented by HLA*B3501 for both donors. A database search of peptides likely to be restricted by HLA*B3501 identified the probable epitope in SP1 recognized by RD0401 as YFKYTAAAL (position 2–10), the epitope shared by SP3 and SP4 as LPLCSAQTW (position 14–22) recognized by both donors, and confirmed the epitope in SP48 identified through pool screening as GTRFPQTPM (position 192–200) recognized by RD0401, as shown in Table 1. Together these results show that DC pulsed with a pentadecapeptide pool from Asp f16 are capable of inducing polyclonal, HLA-Class I restricted, Aspergillus-specific CTL directed to multiple peptides contained within the pool.
Table 2.
HLA restriction pattern of Asp f16-specific effectors.
| PPC-BLCL | HLA shared with RD0401 | % Lysis RD0401 | HLA shared with RD0309 | % Lysis RD0309 |
|---|---|---|---|---|
| RD0309 | DRB1*0401, DQ*0301, DQ*0501, A*0301, B*3501, DRB1*0101, DQ*0501 | 60·8 ± 1·0 | A*0201, A*0301, B*3501, B*4402, C*0401, C*0501, DRB1*0101, DRB4*0101 | 60·8 ± 2·8 |
| BM Don #1 | A*0301, A*1101, B*1801, B*3501, C*0704 | 61·4 ± 1·4 | n.a. | n.d. |
| BM Don #2 | B*3501, C*0304, DRB1*0101, DRB1*0301 | 61·9 ± 1·2 | B*3501, DRB1*0101, DQ*0501 | 61·5 ± 2·5 |
| L0081785 | A*0301, B*1801, DRB1*0301, DQ*0201, DRB3*0202 | 10·2 ± 1·1 | A*0301, C*0501 | 3·4 ± 0·5 |
| BM Don #3 | A*0301, DRB3*0202 | 7·9 ± 1·2 | n.a. | |
| RD0307 | A*1101, B*3501, DQ*0501, DRB3*0202 | 62·3 ± 0·6 | B*3501, C*0401, DQ*0501 | 60·6 ± 1·5 |
| TISI | DRB3*0202 | 8·8 ± 2·3 | NA | |
| BM Don #4 | n.a. | n.d. | A*0301, DQ*0501 | 5·3 ± 1·0 |
| BM Don #5 | n.a. | n.d. | A*0201, DRB1*0401 | 8·3 ± 0·9 |
| BM Don #6 | n.a. | n.d. | A*0201, B*4402, C*0501 | 0·3 ± 1·0 |
| BM Don #7 | n.a. | n.d. | A*0301, C*0401, DRB4*0101 | 7·8 ± 1·1 |
| RD0402 | n.a. | n.d. | DQ*0301 | 0·8 ± 1·2 |
The full HLA typing of donor RD0401 is A*0301, A*1101, B*1801, B*3501, C*0304, C*0704, DRB1*0101, DRB1*0301, DQ*0201, DQ*0501, DRB3*0202. Typing for donor RD0309 is shown in the table. The BLCL from donor RD0401 was not established at the time of the experiment. However, BM donors 1 and 2 together share all the HLA alleles of RD0401 but together shared only HLA-B*3501. Data are shown as the mean ± s.d. percentage specific lysis of Asp f16-PPC-pulsed targets at an E : T ratio of 50 : 1. Only targets that were B*3501+ were lysed by either donor CTL line.
Cross-reaction of Asp f16-effectors with other HLA*B35 BLCL
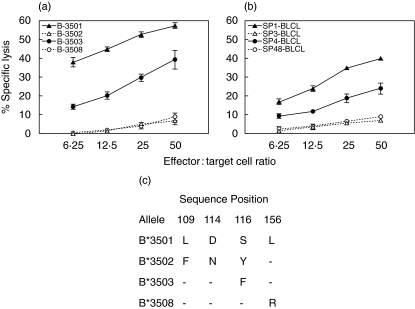
To assess whether the epitopes are presented by multiple subtypes of HLA-B35, CTL from RD0401 were tested with peptide pulsed BLCL targets from B*3502+, B*3503+ and B*3508+ donors in the cytotoxicity assay. At least two different donors from each HLA-B35 subtype were analysed. Asp f16-CTL from RD0401 mediated significant lysis of PPC-pulsed B*3503+ BLCL, although to a somewhat lesser degree than to HLA*B3501+ targets, but failed to lyse PPC presented on B*3502+ or B*3508+ BLCL, as shown in Fig. 4a. The cross-reactivity of Asp f16-effectors with B*3503+ BLCL pulsed with either SP1, SP3, SP4 or SP48 was tested to define the shared epitopes presented by HLA-B*3503. Asp f16-effectors were able to lyse B*3503+ BLCL pulsed with SP1 (40·0 ± 1·0, E : T ratio = 50 : 1) and SP48 (24·1 ± 2·9, E : T ratio = 50 : 1) but did not lyse B*3503+ BLCL pulsed with either SP3 or SP4 (Fig. 4b). These data indicate that B*3503+ BLCL presented the epitope enclosed in SP1 (YFKYTAAAL) and to a lesser extent the epitope enclosed in SP48 (GTRFPQTPM) but not epitope LPLCSAQTW enclosed in SP3 and SP4 to Asp f16-specific HLA-B*3501+ CTL. The sequence differences between the HLA-B35 alleles tested is shown in Fig. 4c.
Fig. 4.
Cross-reaction of HLA-B3501 restricted Asp f16-effectors with other HLA-B35 alleles. Asp f16-CTL from donor RD0401 lysed PPC-pulsed B*3503+ BLCL but not B*3502+, or B*3508+ BLCL (a). B*3503+ BLCL presented epitope contained in SP1 (YFKYTAAAL) and to a lesser extent the epitope contained in SP48 (GTRFPQTPM) but not peptide LPLCSAQTW contained in SP3 and SP4 (b). Data are presented as mean value of triplicate samples ± s.d. At least two different donors from each HLA-B35 subtype are analysed in this experiment. (c) Sequence differences between the HLA-B35 alleles tested. A dash indicates identity with the B*3501 allele at the indicated position. PPC: complete overlapping pentadecapeptides pool, SP: single peptide.
Direct killing of Aspergillus by Asp f16-specific CTL
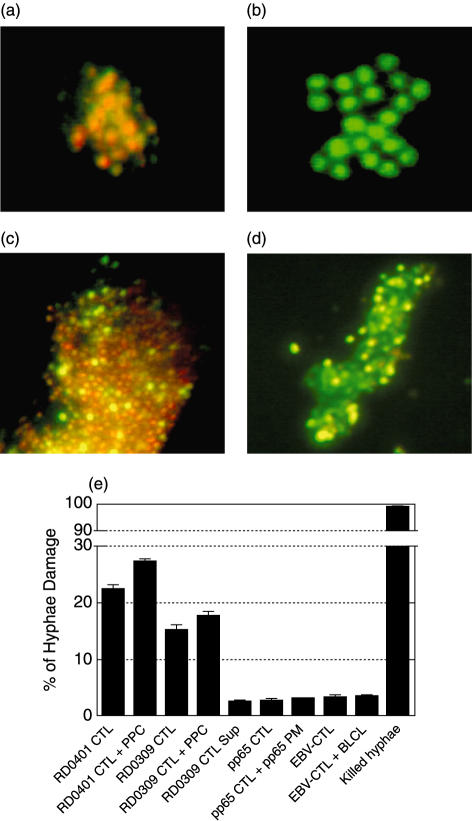
We have shown previously that CD4+ Asp f16-specific effectors were able to kill Aspergillus directly using two assays, one for conidia and the second for hyphae killing [31]. The CD8+ CTL from donor RD0401 were tested similarly in a FUN-1 conidiacidal assay. In this assay metabolically active live conidia (positive control) accumulate orange fluorescence in vacuoles (Fig. 5a), whereas dormant, heat-killed conidia (negative control) stain green (Fig. 5b). FUN-1-labelled Aspergillus conidia cultured overnight in fresh culture medium (Fig. 5c) stained orange but labelled conidia incubated in CTL culture supernatant (Fig. 5d) became inactive and stained green, indicating that Asp f16-effector culture-supernatant contains substances that killed fresh Aspergillus conidia. Because lymphocytes take up FUN-1 cells could not be added directly in this assay. We determined the direct killing effect of the CTL by co-culture with Aspergillus hyphae in an XTT dye reduction assay (Fig. 5e). CTL from RD0401 resulted in 22·4 ± 0·9% killing that increased to 27·3 ± 0·6% killing when Asp f16-PPC was added to the culture, while CTL from donor RD0309 were somewhat less potent, showing 15·2 ± 1·6% lysis without and 17·8 ± 1·4% lysis with added peptide. In contrast, two control CTL lines, one generated by priming with DC pulsed with a pool of peptides from CMV-pp65 and the second primed with autologous EBV transformed BLCL, failed to result in any significant hyphae killing without or with added antigen, even though these lines exhibited potent killing of their respective virus-infected targets (32·4 ± 4·3% for the CMV-pp65-specific CTL and 47·0 ± 0·5% for the EBV-CTL at an E : T of 25 : 1). Culture medium from peptide pool activated donor RD0309 CTL also resulted in background lysis of Aspergillus hyphae, as can be seen in Fig. 5e. Formalin-killed hyphae showed ∼99% killing while hyphae in fresh medium alone were used as a negative control to blank the enzyme-linked immunosorbent assay (ELISA) reader. These data suggest that, like CD4+ effectors, CD8+ CTL generated by using PPC-pulsed fast-DC not only kill peptide-pulsed targets but also have activity directly against the fungus.
Fig. 5.
Conidiacidal and hyphae damage assay. In the FUN-1 conidiacidal assay metabolically active live conidia (positive control) accumulate orange fluorescence in vacuoles (a), whereas dormant, heat-killed conidia (negative control) stain green (b). FUN-1-labelled Aspergillus conidia cultured overnight in fresh culture medium (c) stained orange but labelled conidia cultured in supernatant from the donor RD0401 CTL-culture (d) became inactive and stained green. In XTT hyphae damage assay (e) Asp f16-CTL from donor RD0401 resulted in 22·4 ± 0·9% killing (effector : hyphae ratio of 3 : 1) that increased to 27·3 ± 0·6% killing when Asp f16-PPC was added to the culture, whereas CTL from donor RD0309 resulted in 15·2 ± 1·6% lysis in the absence and 17·8 ± 1·4% in the presence of Asp f16-PPC. Control CTL lines included a line specific for CMV pp65 in the absence or presence of added peptide mix containing overlapping pentadecapeptides spanning the coding region of pp65 (pp65-PM) and an Epstein–Barr virus- specific CTL line (EBV-CTL) in the absence and presence of the autologous BLCL against which it was raised. CTL activity of the control lines in standard 51Cr release assays was 32·4 ± 4·3% and 47·0 ± 0·5%, respectively, at an E : T ratio of 25 : 1 to virus infected targets. Culture supernatant from donor RD0309 CTL resulted in only background killing. Formalin killed hyphae showed ∼99% killing. Hyphae in medium alone were used to blank the ELISA reader. Data are presented as mean value ± s.d. of triplicate assays.
Discussion
The methodology to generate Aspergillus-specific Th1 T cell responses from healthy donors by using mature, autologous, fast, Mo-derived DC pulsed with a complete pentadecapeptide pool (PPC) of Asp f16 has been described previously by us [31]. We found that PBMC from five of five donors tested proliferated strongly in response to Asp f16-PPC-pulsed DC. A HLA-Class II DRB1*0301 restricted CD4+ CTL line was generated from one donor that was specific for a single peptide, WSIDGAVVR. IFN-γ-producing precursors could be detected in the unstimulated peripheral blood from each of seven DRB1*0301+ donors in response to peptide pool, and response to the single peptide containing WSIDGAVVR was similar to the complete pool for five of these donors, indicating that peptide WSIDGAVVR may be recognized widely. Here we describe T cell lines from two additional donors, RD0401 and RD0309 that were strongly and specifically cytotoxic to autologous Asp f16-PPC- and Aspergillus culture extract-pulsed targets. Unlike our previously described donor, CTL activity and IFN-γ production by both lines was exclusively from CD3+CD8+ T cells even though both lines were a mixture of CD4+ and CD8+ T cells. Similar to the previously described line, there was no IL-4 production by CD4+ T cells indicative of a Th2 response. Asp f16-PPC-pulsed BLCL induced a high level of CD107a on CD8+ T cells with little or no expression on the CD4+ T cells in both lines (Fig. 2). CD107a (also known as lysosomal-associated membrane protein-1) is a vesicle membrane protein that becomes transiently mobilized to the cell surface during the release of cytotoxic mediators such as perforin and granzymes of cytotoxic T cells [35]. The use of CD107a mobilization in flow cytometric analysis as a marker of degranulation in antigen specific CTL has been described recently [36].
CD8+ CTL usually recognize peptide fragments from eight to 11 aa residues in length bound to MHC class I molecules [37], although longer peptides may also be recognized [38,39]. Synthetic, overlapping pentadecapeptides that span regions encoding viral antigens have been used for the identification of T cell epitopes [40–43]. Overlapping pentadecapeptides (each peptide overlaps the preceding peptide by 11-aa) are of an optimal size for the generation of T cell responses to both the shorter Class I-binding peptides and to the longer 11–13-aa peptides that bind to Class II molecules [41]. The use of pentadecapeptide pools to prime epitope-specific T cells is dependent upon adequate processing and presentation by DC of smaller immunogenic peptides derived from the pentadecapeptides [44]. Specific epitopes were identified in CTL assays using 21 smaller pools containing four to 11 pentadecapeptides. The CTL line from donor RD0401 reacted to six smaller pools (PP1, PP3, PP4, PP8, PP11 and PP15) and specificity could be narrowed by screening individual peptides shared by the pools to four candidate peptides (SP1, SP3, SP4, SP48). The CTL line from donor RD0309 reacted only with three smaller pools, PP3, PP4 and PP11, sharing two of the same candidate peptides (SP3 and SP4). Through examination of the amino acid sequences in these peptides and in the adjacent peptides that were not reactive (see Table 1) we could narrow the candidate sequences for the first epitope recognized by CTL from RD0401 to MYFKYTAAALAA. The second epitope recognized by both cell lines was contained within VLPLCSAQTWS and the third also recognized only by RD0401 to narrowed to GTRFPQTRM. Once HLA restriction was determined to be by HLA-B*3501 for both donors, a database [45] search identified the probable sequences as aa 2–10 YFKYTAAAL (YFK), aa 14–22 LPLCSAQTW (LPL) and aa 192–200 GTRFPQTPM (GTR).
Each of the identified epitopes induced similar reactivity by the RD0401 line when the individual pentadecapeptide containing these sequences were pulsed onto B*3501+ targets. RD0401 CTL cross-reacted with Asp f16-PPC-pulsed B*3503+ BLCL but not B*3502+, or B*3508+ BLCL and this cross-reactivity was primarily to peptide YFK and to a lesser extent GTR, but not to peptide LPL. B*3502 differs from B*3501 at 3 aa while B*3503 and B*3508 differ at just one amino acid, position 116 and position 156, respectively, as shown in Fig. 4[46]. Position 116 lies in the floor of the peptide binding groove while position 156 is on the exterior surface of the α-helix in a position that is likely to contact the TCR. B*3501 and B*3503 share leucine, a highly hydrophobic amino acid (hydrophobicity index of 3·8) at position 156, which is replaced by arginine, a much larger amino acid that is only partly hydrophobic (hydrophobicity index of −4·5) in B*3508, a difference that may well affect interaction with TCR. While B*3502 shares leucine 156 with B*3501 it contains two disparities, positions 114 and 116, that both lie in the floor of the peptide-binding groove and may affect the ability of the peptide to bind to the MHC molecule and one disparity at position 109 that lies in the α-helix that may affect presentation [46]. The serine to phenylalanine difference between B*3501 and B*3503 at position 116 appears to permit binding of peptides YFK and GTR but not peptide LPL. Asp f16 shares sequence homology with a probable membrane protein from Saccharomyces cerevisiae in the region from 99 to 214 and 31% identity with the hybrid endo-β-1,3–1,4 gulcanase in the regions between aa 73–204, an area containing peptide GTR [24]. However, a search for matching peptides using the Protein Information Resource programme revealed that each of the three identified peptides was limited to A. fumigatus[47].
The manner by which Th1-type T cells protect against fungal infection is not fully understood. Inhaled Aspergillus conidia invade the respiratory tract and attach more readily to epithelium damaged from the transplant preparative regimen, infection or by GVHD and its treatment than in healthy individuals [48]. Cytokines produced by Th1-type T cells such as IL-2, IFN-γ and IL-12 may activate secondarily pulmonary macrophages and granulocytes that serve as a first line of defence against infection [2,49]. Direct contact with activated T cells has been shown to inhibit the ability of germinating Aspergillus conidia to adhere to surfaces, which may limit infection [50]. The role for a CD8+ T cell response to A. fumigatus or other fungal infection is less clear. CD8+ T cells have been demonstrated to inhibit directly the growth of Candida hyphae and may thus limit the spread of the infection [51] and CD8+ T cells produce high levels of IFN-γ after antigen encounter, and therefore may act more like Th1-type CD4+ T cells as opposed to Th2-type suppressor T cells. Both CD8+ T cell lines in this report and our CD4+ Class II-restricted Asp f16-specific T cell line were able to directly kill hyphae to a similar extent (18–27%) at the same 3 : 1 E : T ratio. Hyphae killing was not seen when culture supernatant from peptide primed CTL in the absence of cells was used in the assay, suggesting that the secreted molecules involved in conidia killing were not adequate alone to kill hyphae and may require the presence of the Asp f16-specific CTL. The increased killing seen when Asp f16-specific effectors were pulsed with peptide may reflect increased activation and secretion of lytic molecules (granzyme, perforin) induced by the peptides. Further experiments are planned to examine CD107a expression secondary to heat-killed conidia and hyphae exposure in the absence of added peptides to determine if the CTL lytic machinery is directly activated by fungus. While the role of antigen-specific CD8+ T cells in the immunotherapy or prevention of herpes virus infections in HSCT patients has been demonstrated [29,52], the benefit of CD8+ CTL immunotherapy in the setting of fungal infection remains to be determined. Finding two unrelated donors each responding to the same HLA-B*3501-restricted peptide (LPL), and the identification of two additional B*3501-restricted peptides that can be cross-presented by other HLA-B*35 subtypes, suggests a functional role of memory CD8+ T cell responses towards A. fumigatus in healthy donors. Indeed, even though peptide WSIDGAVVR appeared to be recognized by most HLA-DRB1*0301+ individuals donor RD0401, who is DRB1*0301+, failed to respond to this peptide in an ELISPOT assay of fresh PBMC (data not shown) and instead made a polyclonal CD8+ CTL response after repeated primings. Probably both antigen-specific CD4+ and CD8+ T cells will be needed to maintain a protective A. fumigatus specific immune response in vivo [30,53]. Our results indicate that priming with Asp f16 PPC-pulsed fast-DC can induce either a CD4+ or a CD8+ T cell response and, at least thus far, in the absence of a Th2-type response characteristic of other allergens purified from Aspergillus [54]. To determine further the biological relevance of Asp f16-specific T cell responses we plan to measure T cell precursor frequencies to peptide-pool pulsed autologous DC in ABPA patients, patients with allergies to Aspergillus, and in transplant patients during and following Aspergillus infection to determine if higher frequencies are associated with response to treatment.
In conclusion, our data demonstrate further the potential usefulness of Asp f16 as an immunogen to induce protective immune responses to prevent or treat IA in severely immunocompromised patients. The methodology described here, utilizing autologous Asp f16 pentadecapeptide pool pulsed-DC also permits the rapid generation of polyclonal Th1-type CD4+ or CD8+ Aspergillus-specific T cell lines that recognize multiple epitopes on the immunizing antigen. Such T cells could be generated from individuals even when epitopes and HLA restriction are not already identified and could therefore be applied to patients of any HLA type capable of responding to Asp f16 peptides. Clinical trials to determine the safety and potential usefulness of Asp f16-specific T cell lines in preventing IA are warranted.
Acknowledgments
The authors thank Dr Guenther Koehne (Rush University, Chicago) for helpful discussion on the preparation of the pentadecapeptides, Dr Tom Ellis (Blood Center of South-eastern Wisconsin) for allele level HLA typing of the apheresis donors used in these experiments and Candace Krepel (Medical College of Wisconsin) for use of her laboratory for the growing and preparation of Aspergillus conidia. This work was supported in part by a grant from the Medical College of Wisconsin Cancer Center.
References
- 1.Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–66. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 2.Wiederhold NP, Lewis RE, Kontoyiannis DP. Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy. 2003;23:1592–610. doi: 10.1592/phco.23.15.1592.31965. [DOI] [PubMed] [Google Scholar]
- 3.Ribaud P, Chastang C, Latge JP, et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis. 1999;28:322–30. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 4.Hebart H, Bollinger C, Fisch P, et al. Analysis of T cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–8. doi: 10.1182/blood-2002-01-0265. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–66. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 6.De La Rosa GR, Champlin RE, Kontoyiannis DP. Risk factors for the development of invasive fungal infections in allogeneic blood and marrow transplant recipients. Transpl Infect Dis. 2002;4:3–9. doi: 10.1034/j.1399-3062.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 8.Romani L. The T cell response against fungal infections. Curr Opin Immunol. 1997;9:484–90. doi: 10.1016/s0952-7915(97)80099-4. [DOI] [PubMed] [Google Scholar]
- 9.Cenci E, Perito S, Enssle KH, et al. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–70. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenci E, Mencacci A, Del Sero G, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180:1957–68. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 11.Grazziutti ML, Rex JH, Cowart RE, Anaissie EJ, Ford A, Savary CA. Aspergillus fumigatus conidia induce a Th1-type cytokine response. J Infect Dis. 1997;176:1579–83. doi: 10.1086/514157. [DOI] [PubMed] [Google Scholar]
- 12.Bozza S, Gaziano R, Lipford GB, et al. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002;4:1281–90. doi: 10.1016/s1286-4579(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 13.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol. 2000;165:381–8. doi: 10.4049/jimmunol.165.1.381. [DOI] [PubMed] [Google Scholar]
- 14.Bozza S, Perruccio K, Montagnoli C, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102:3807–14. doi: 10.1182/blood-2003-03-0748. [DOI] [PubMed] [Google Scholar]
- 15.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–50. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurup VP, Kumar A. Immunodiagnosis of aspergillosis. Clin Microbiol Rev. 1991;4:439–56. doi: 10.1128/cmr.4.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee B, Kurup VP. Molecular biology of Aspergillus allergens. Front Biosci. 2003;8:S128–39. doi: 10.2741/982. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Chaparas SD. Characterization of antigens from Aspergillus fumigatus. I. Preparation of antigens from organisms grown in completely synthetic medium. Am Rev Respir Dis. 1978;118:547–51. doi: 10.1164/arrd.1978.118.3.547. [DOI] [PubMed] [Google Scholar]
- 19.Crameri R. Recombinant Aspergillus fumigatus allergens: from the nucleotide sequences to clinical applications. Int Arch Allergy Immunol. 1998;115:99–114. doi: 10.1159/000023889. [DOI] [PubMed] [Google Scholar]
- 20.Crameri R, Faith A, Hemmann S, et al. Humoral and cell-mediated autoimmunity in allergy to Aspergillus fumigatus. J Exp Med. 1996;184:265–70. doi: 10.1084/jem.184.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutsen AP, Mueller KR, Levine AD, Chouhan B, Hutcheson PS, Slavin RG. Asp fI CD4+ TH2-like T cell lines in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1994;94:215–21. doi: 10.1016/0091-6749(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 22.Kurup VP, Banerjee B, Hemmann S, Greenberger PA, Blaser K, Crameri R. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy. 2000;30:988–93. doi: 10.1046/j.1365-2222.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 23.Kurup VP, Xia JQ, Crameri R, et al. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin Immunol. 2001;98:327–36. doi: 10.1006/clim.2000.4993. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee B, Kurup VP, Greenberger PA, Johnson BD, Fink JN. Cloning and expression of Aspergillus fumigatus allergen Asp f16 mediating both humoral and cell-mediated immunity in allergic bronchopulmonary aspergillosis (ABPA) Clin Exp Allergy. 2001;31:761–70. doi: 10.1046/j.1365-2222.2001.01076.x. [DOI] [PubMed] [Google Scholar]
- 25.Einsele H, Hebart H. Cellular immunity to viral and fungal antigens after stem cell transplantation. Curr Opin Hematol. 2002;9:485–9. doi: 10.1097/00062752-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Einsele H. Antigen-specific T cells for the treatment of infections after transplantation. Hematol J. 2003;4:10–17. doi: 10.1038/sj.thj.6200213. [DOI] [PubMed] [Google Scholar]
- 27.Ramadan G, Konings S, Kurup VP, Keever-Taylor CA. Generation of Aspergillus- and CMV-specific T cell responses using autologous fast DC. Cytotherapy. 2004;6:223–34. doi: 10.1080/14653240410006040. [DOI] [PubMed] [Google Scholar]
- 28.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–41. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 29.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 30.Riddell SR, Greenberg PD. T cell therapy of cytomegalovirus and human immunodeficiency virus infection. J Antimicrob Chemother. 2000;45(Suppl. T3):35–43. doi: 10.1093/jac/45.suppl_4.35. [DOI] [PubMed] [Google Scholar]
- 31.Ramadan G, Davies B, Kurup V, Keever-Taylor C. Generation of Th1 T cell responses directed to a HLA Class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;139:257–67. doi: 10.1111/j.1365-2249.2005.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balajee SA, Marr KA. Conidial viability assay for rapid susceptibility testing of Aspergillus species. J Clin Microbiol. 2002;40:2741–5. doi: 10.1128/JCM.40.8.2741-2745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) J Infect Dis. 1995;172:1153–6. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 34.Koehne G, Smith KM, Ferguson TL, et al. Quantitation, selection, and functional characterization of Epstein–Barr virus-specific and alloreactive T cells detected by intracellular interferon-gamma production and growth of cytotoxic precursors. Blood. 2002;99:1730–40. doi: 10.1182/blood.v99.5.1730. [DOI] [PubMed] [Google Scholar]
- 35.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 36.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Meth. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 37.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–6. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 38.Bertoletti A, Chisari FV, Penna A, et al. Definition of a minimal optimal cytotoxic T cell epitope within the hepatitis B virus nucleocapsid protein. J Virol. 1993;67:2376–80. doi: 10.1128/jvi.67.4.2376-2380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauly T, Elbers K, Konig M, Lengsfeld T, Saalmuller A, Thiel HJ. Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J Gen Virol. 1995;76:3039–49. doi: 10.1099/0022-1317-76-12-3039. [DOI] [PubMed] [Google Scholar]
- 40.Kern F, Faulhaber N, Frommel C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–82. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Maecker HT, Dunn HS, Suni MA, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Meth. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 42.Armengol E, Wiesmuller KH, Wienhold D, et al. Identification of T cell epitopes in the structural and non-structural proteins of classical swine fever virus. J Gen Virol. 2002;83:551–60. doi: 10.1099/0022-1317-83-3-551. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi D, Williams RY, O'Reilly RJ, Koehne G. Generation of cytomegalovirus (CMV)-specific T lymphocytes using protein-spanning pools of pp65-derived pentadecapeptides for adoptive immunotherapy. Blood. doi: 10.1182/blood-2003-05-1433. Prepublished October 28, 2004; DOI 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 44.Kessler BM, Glas R, Ploegh HL. MHC class I antigen processing regulated by cytosolic proteolysis-short cuts that alter peptide generation. Mol Immunol. 2002;39:171–9. doi: 10.1016/s0161-5890(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 45.Sathiamurthy M, Hickman HD, Cavett JW, et al. Population of the HLA ligand database. Tissue Antigens. 2003;61:12–19. doi: 10.1034/j.1399-0039.2003.610102.x. [DOI] [PubMed] [Google Scholar]
- 46.Satz ML, Fernandez-Vina M, Theiler GC, et al. Allelic heterogeneity of HLA-B35 subtypes in different populations as assessed by DNA typing. Tissue Antigens. 1995;46:196–203. doi: 10.1111/j.1399-0039.1995.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu CH, Yeh LS, Huang H, et al. The protein information resource. Nucl Acids Res. 2003;31:345–7. doi: 10.1093/nar/gkg040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–9. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- 49.Clemons KV, Calich VL, Burger E, et al. Pathogenesis I. Interactions of host cells and fungi. Med Mycol. 2000;38(Suppl. 1):99–111. [PubMed] [Google Scholar]
- 50.Martins MD, Rodriguez LJ, Savary CA, et al. Activated lymphocytes reduce adherence of Aspergillus fumigatus. Med Mycol. 1998;36:281–9. [PubMed] [Google Scholar]
- 51.Beno DW, Stover AG, Mathews HL. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J Immunol. 1995;154:5273–81. [PubMed] [Google Scholar]
- 52.Riddell SR, Walter BA, Gilbert MJ, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplant. 1994;14(Suppl. 4):S78–84. [PubMed] [Google Scholar]
- 53.Sun JC, Williams MA, Bevan MJ. CD4(+) T cells are required for the maintenance, not programming, of memory CD8(+) T cells after acute infection. Nat Immunol. 2004;5:927–33. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurup VP. Fungal allergens. Curr Allergy Asthma Rep. 2003;3:416–23. doi: 10.1007/s11882-003-0078-6. [DOI] [PubMed] [Google Scholar]