Abstract
The aetiology of Crohn's disease (CD) remains unknown. Since SAMP1/Yit mice have been reported to develop CD-like spontaneous enteric inflammation, such mice have been studied as an animal model of CD. In this study, using this model we examined T lymphocyte migration in microvessels of intestinal mucosa in vivo and the expression of adhesion molecules by immunohistochemistry. Fluorescence-labelled T lymphocytes isolated from AKR/J (control) mice were injected into the tail veins of recipient mice, and T lymphocyte migration in the postcapillary venules of Peyer's patches, submucosal microvessels, and villus capillaries of the terminal ileum was monitored using an intravital microscope. Adhesion of T lymphocytes was significantly increased in 35 week old SAMP1/Yit mice compared with that in AKR/J or 15 week old SAMP1/Yit mice. Immunohistochemical study showed increased infiltration of CD4, CD8 and β7-integrin-positive cells and increased expression of MAdCAM-1 and VCAM-1 in the terminal ileum of SAMP1/Yit mice. Antibodies against MAdCAM-1 and VCAM-1 significantly inhibited adhesion of T lymphocytes to microvessels of the terminal ileum, and anti-MAdCAM-1 antibody showed stronger suppressive effect than the anti-VCAM-1 antibody. Periodical administration of anti-MAdCAM-1 antibody twice a week for 7 weeks significantly ameliorated ileitis of SAMP1/Yit mice, but submucosal hypertrophy was not significantly suppressed. Anti-VCAM-1 antibody treatment failed to show significant resolution of ileitis. In addition, anti-MAdCAM-1 antibody treatment also attenuated established ileitis. The results demonstrate that, although MAdCAM-1 and VCAM-1 play an important role in T lymphocyte–endothelial cell interactions in SAMP1/Yit mice, MAdCAM-1 may be a more appropriate target for therapeutic modulation of chronic ileitis.
Keywords: SAMP1/Yit, T lymphocyte, MAdCAM-1, VCAM-1
Introduction
Crohn's disease (CD) is one of the chronic inflammatory bowel disease and the aetiology of its disease remains unknown [1]. CD is characterized by transmural inflammation, fistulas and granulomas formation randomly distributed throughout the gastrointestinal tract [2]. Although genetic, environmental and immunologic factors have been proposed and investigated, the precise pathogenic mechanisms remain unclear [1,3–5]. During the active phase of CD, various proinflammatory cytokines are released within the gut mucosal compartment [6]. Recent clinical studies have reported a dramatic improvement in CD patients treated with a TNF-alpha-neutralizing antibody [7,8]. These findings suggest that intervening with the acute inflammatory response may be beneficial in the treatment of CD.
Senescence accelerated mice (SAM) were derived from AKR/J mice established by Takeda et al. [9]. The SAMP1/Yit strain, the subline of the SAMP1 strain, is a unique murine model of ileitis, which spontaneously develops chronic ileitis with virtually 100% penetrance after 30 week of age under specific pathogen free condition [10–12]. In the SAMP1/Yit model, discontinuous transmural inflammation, presence of formation of granulomas, and muscular hypertrophy are observed [10]. Furthermore, Rivera-Nieves et al. [13] reported that the SAMP1/YitFc substrain developed perianal disease with ulceration and fistulae. These histological features of human Crohn's disease are not shared with most previous animal models of inflammatory bowel disease. As the previous reports, genetic and environmental factors related to ileitis in SAMP1/Yit mice [14,15]. Kosiewicz et al. [16] have shown that transfer of the sorted CD4 subset from SAMP1/Yit strain to severe combined immunodeficient (SCID) mice induced intestinal inflammatory disease. These studies suggest that an immune imbalance in the target organ may be the major cause of development of the organ specific inflammatory disease. They have also reported that pathogenic Th1-type T cells from SAMP1/Yit mice can mediate a Crohn's like disease in an adoptive transfer model through a mechanism that may require TNF production [13,16,17]. On the other hand, Burns et al. [18] have reported that adhesion molecules which have been implicated in T cell homing and neutrophil trafficking also appear to play a key role in the initiation of ileitis in SAMP1/Yit mice.
Recirculation of lymphocytes from blood to lymphoid tissue is generally noted as a key phenomenon in immunological surveillance [19]. Lymphocyte homing from blood to lymphoid tissue and inflammatory sites depends on the interaction between lymphocytes and high endothelial venules. This interaction contains multistep theory mediated by selectins, integrins, and immunoglobulin superfamily adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) [20,21]. The inhibition of VCAM-1 in LPS-induced enterocolitis ameliorates inflammation [22]. The homing of lymphocytes appears to be organ specific, and lymphocyte homing to the Peyer's patches and mucosal sites is thought to be regulated by α4β7-integrin and its counter ligand mucosal addressin cell adhesion molecule-1 (MAdCAM-1) [23,24]. MAdCAM-1 is an immunoglobulin superfamily adhesion molecule that has been implicated in the specific recruitment of lymphocytes to the sites of inflammation in the gut [25,26]. Immunohistochemical methods have revealed that MAdCAM-1 is up-regulated in the inflamed colonic mucosa of humans with ulcerative colitis or Crohn's disease [25].
Although there have been several studies on lymphocyte migration in vivo and on interaction of lymphocytes with endothelial cells of venules in the intestinal mucosa [27–30], there are very few reports that directly access lymphocyte–endothelial cell interactions in the microvasculature of inflamed intestine of SAMP1/Yit mice. In this study, using this model we examined T lymphocyte migration in microvessels of intestinal mucosa in vivo and whether the inhibition of adhesion molecules lead to the suppression of spontaneous ileitis in SAMP1/Yit mice.
Methods
Animals
SAMP1/Yit mice were kindly provided by Yakult Central Institute for Microbiological Research, Tokyo, Japan and were maintained in an animal colony at National Defence Medical College (NDMC), Saitama, Japan. Control AKR/J mice were purchased from Japan Clea Co., Tokyo, Japan and were maintained in an animal colony at NDMC. The care and use of laboratory animals were in accordance with the guideline for laboratory animals in NDMC.
Immunohistochemistry
Localization and expression of CD4, CD8, β7-integrin, MAdCAM-1 and VCAM-1 in the intestinal mucosa were assessed by immunohistochemistry using the labelled streptavidin biotin method. The terminal ileum of 35 weeks-old SAMP1/Yit mice and age matched AKR/J mice were removed and fixed in PLP (periodate, lysine-paraformaldehyde) solution, and were vertically embedded in OCT (optimum cutting temperature) compound (Miles Inc., Elkhart, IN, USA) before being frozen in dry ice and acetone. Well-orientated cryostat sections of 6 µm in thickness were transferred to poly L-lysine(PLL)-coated slides and air dried for 1 h at room temperature. After they were washed in PBS containing 1% Triton X (LKB-Produkter, Bromma, Sweden) for 5 min, sections were incubated in 10% normal goat serum in PBS. Monoclonal antibodies used in this study were follows: anti-mouse CD4 antibody (RM4-5; BD PharMingen, San Diego, CA, USA), anti-mouse CD8 antibody (53/6·7; BD PharMingen), anti-mouse β7-integrin antibody (FIB27; BD PharMingen), anti-mouse MAdCAM-1 antibody (MECA-367; BD PharMingen), anti-mouse VCAM-1 antibody (MVCAM429; BD PharMingen). Isotype matched IgG was used as controls.They were diluted 50–100 times with PBS and layered on the section overnight at 4°C. Sections were incubated with second antibody, biotinylated anti-rat IgG class antibody (BD PharMingen) for 1 h at room temperature. Then, sections were incubated with FITC-conjugated streptavidin (Streptavidin-fluorescein) (Amersham Biosciences, Backinghamshire, UK) for 30 min at room temperature. Rinsing with PBS was preformed among each step. A cover slip was applied using glycerol jelly. These sections were observed under a fluorescent microscope (BX60, Olympus, Tokyo, Japan). The infiltrating cells were expressed as the numbers of CD4, CD8 and β7-integrin-positive cells per millimeter of muscularis mucosa.
Isolation of T lymphocytes and labelling with carboxyfluorescein diacetate succinimidyl ester
AKR/J mouse spleen and mesenteric lymph nodes (mlN) was isolated after a midline incision and crashed with slide glasses. Pellets were incubated with DNase (Roche, Mannheim, Germany) for 10 min, washed twice with phosphate buffered saline (PBS), and haemolysed with NH3Cl-Tris buffer. Thereafter, T lymphocytes were isolated by T cell isolation column (CL101, Cedarlane, Ontario, Canada). The expression of α4-integrin and α4β7-integrin on isolated T cells from spleen and mlN of AKR/J was examined by a fluorescence-activated cell sorter (FACS Calibur™, Becton-Dickinson, Mountain View, CA, USA) using rat anti-mouse PE-conjugated anti-mouse α4-integrin MAb (9C10: BD PharMingen, USA) and PE-conjugated anti-mouse α4β7-integrin MAb (DATK32: BD PharMingen). There was no significant difference between T cells from spleen and mlN in the expression of α4- or α4β7-integrin. Because T cells from young (15 weeks) and old (35 weeks) mice did not differ in expression of these molecules, we used T cells from AKR/J mice age of 15–21 weeks in the following migration studies. Carboxyfluorescein diacetate succinimidyl ester (CFDSE: Molecular Probes, Eugene, OR, USA) was dissolved in dimethylsulphoxide at 15·6 m m, divided into a small aliquots (300 µl), and stored at −20°C until the experiments. T lymphocytes (1 × 107) in 1 ml of PBS were incubated with CFDSE solution for 15 min at 37°C and washed twice by PBS.
Animal preparation for intravital observation
For migration studies, 35 or 15 week-old SAMP1/Yit mice and 35 week-old control AKR/J mice were anaesthetized with 50 mg/kg pentobarbital sodium, and their abdomen was opened with a midline incision. 1–3 cm of ileal segments ending at the ileocecal valve were chosen for observation and placed on wet cotton. The intestine was kept warm and moist by continuous superfusion with PBS warmed to 37°C. Each side of suitable ileal segment was ligated avoiding the damage for microcirculation and PBS was injected into the closed segment using a 30 gauge needle. The behaviour of T lymphocytes in postcapillary venules (PCVs) of Peyer's patches and submucosal venules was observed from the serosal side by intravital microscope. In the same animals, the migration in mucosal villus microvessels was also observed from the mucosal surface after opening the intestinal lumen along its antimesenteric side. The behaviour of CFDSE-labelled T lymphocytes in PCVs of Peyer's patches, submucosal venules, and mucosal villus microvessels of small intestine were visualized on the monitor through a SIT system by a fluorescence microscope (BX51WI, Olympus, Tokyo, Japan) with a contrast enhancing unit (C-2400–08, Hamamatsu Photonics, Shizuoka, Japan) and a ×10 ultraviolet-fluorite objective lens (Fluor, Nikon, Tokyo, Japan) according to a previously described method [22,27], and recorded on digital videotape with a high-speed video recording system (WV-DR7, SONY, Tokyo, Japan).
Administration of monoclonal antibodies
In some experiments, 2 mg/kg rat anti-mouse MAdCAM-1 monoclonal antibodies (MAbs) (MECA369; BD PharMingen) or anti-VCAM-1 MAbs (MVCAM429; BD PharMingen) were administered via tail vein 30 min prior to CFDSE-labelled T lymphocyte administration and compared with isotype-matched rat IgG (BD PharMingen).
Analysis of T lymphocytes dynamics
The number of adherent T lymphocytes was determined off-line by the digital videotaped images. T lymphocytes adhering to the vascular walls without movement and remaining stationary for a period of >30 s were defined as sticking T lymphocytes. We counted the numbers of sticking T lymphocytes in the field using × 10 objective lens at 10-min intervals up to 60 min after the CFDSE labelled T lymphocytes administration.
Effects of periodical administration of anti-MAdCAM-1 and anti-VCAM-1 on ileitis of SAMP1/Yit mice (prevention study)
We next examined whether periodical administration of anti-MAdCAM-1 MAb and VCAM-1 MAb ameliorates ileitis of SAMP1/Yit mice. 2 mg/kg of anti-MAdCAM-1, VCAM-1 or isotype-matched IgG was administered i.p to SAMP1/Yit mice every other day from the 14th week to 21st week. Mice were sacrificed in the 21st week, and body weight, small intestinal length, villus height, submucosal thickness, and the number of infiltrating immune cells were determined. As the infiltrated immune cells, the expression of CD4, CD8 and β7-integrin was immunohistochemically evaluated using the labelled streptavidin biotin method as described above.
Effects of periodical administration of anti-MAdCAM-1 on established ileitis of SAMP1/Yit mice age of 28 weeks (treatment study)
We next examined whether periodical administration of anti-MAdCAM-1 MAb ameliorates ileitis of SAMP1/Yit mice age of 28 weeks, when all mice has established ileitis. Two mg/kg of anti-MAdCAM-1, or isotype-matched IgG was administered i.p to SAMP1/Yit mice every other day from the week 28 to week 35. Mice were sacrificed at week 35, and villus height, submucosal thickness, and the number of infiltrating immune cells were determined. As the infiltrated immune cells, the expression of CD4, CD8 and β7-integrin was immunohistochemically evaluated using the labelled streptavidin biotin method as described above.
Statistics
All results were expressed as means ± SE of 4–5 mice. For the comparison of adhesion and the number of infiltrating cells, the mean values were statistically evaluated by a Mann–Whitney U-test. Statistical significance was defined as P < 0·05.
Results
Immunohistochemistry for CD4, CD8, β7-integrin, VCAM-1 and MAdCAM-1
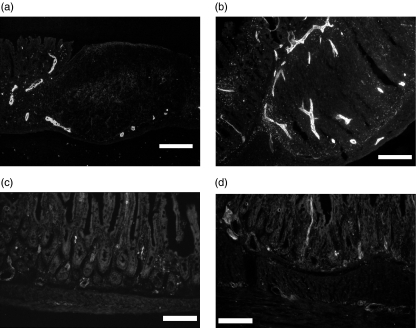
From immunohistochemical analysis on ileal mucosa, the number of infiltrating cells in the lamina propria of SAMP1/Yit mice was significantly greater than that of AKR/J mice (CD4-positive cells: AKR/J versus SAMP: 299 ± 36 versus 420 ± 48, P = 0·042, CD8-positive cells: AKR/J versus SAMP: 169 ± 30 versus 552 ± 99, P = 0·002, β7-integrin-positive cells: AKR/J versus SAMP: 125 ± 13 versus 237 ± 32, P = 0·002). These data are consistent with the previously reported papers [10,16]. MAdCAM-1-positive vessels were observed in the lamina propria of AKR/J mice (Fig. 1a). On the other hand, in the lamina propria of SAMP1/Yit mice, the number of MAdCAM-1-positive vessels was significantly greater than that in AKR/J mice at three sites (Fig. 1b). As shown in Fig. 1c, few vessels were VCAM-1-positive in AKR/J mice. VCAM-1-positive vessels were also increased in the ileal mucosa of SAMP1/Yit mice, dominantly in submucosal venules (Fig. 1d).
Fig. 1.
Immunohistochemical study of MAdCAM-1 and VCAM-1 expression in the intestinal mucosa. (a) MAdCAM-1 expression of AKR/J mice on the vessel near Peyer's patches (b) MAdCAM-1 expression of SAMP1/Yit mice demonstrating the increased MAdCAM-1 expression (c) VCAM-1 expression of AKR/J mice was weak in the lamina propria. (d) VCAM-1 positive vessels of SAMP-1/Yit mice were increased mainly in the submucosal area compared with those in AKR/J mice. Bar: 100 µm (objective lens × 20).
T lymphocyte adherence in the ileal mucosa of AKR/J and SAMP1/Yit mice
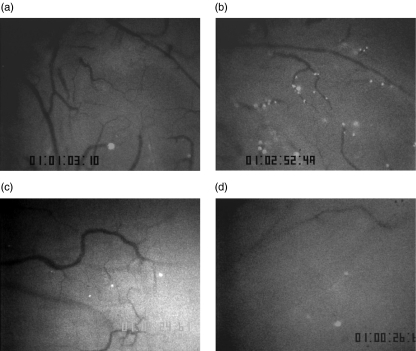
Figure 2 shows representative microscopic images of the distribution of fluorescence-labelled adherent T lymphocytes in PCVs of Peyer's patches of 35-weeks-old SAMP1/Yit mice and AKR/J mice at 60 min after labelled T lymphocyte infusion. In control AKR/J mice, there were few adhered T lymphocytes in PCVs of Peyer's patches (Fig. 2a). On the other hand, there were more adhered T lymphocytes in SAMP1/Yit mice than those in AKR/J mice (Fig. 2b).
Fig. 2.
Representative photographs of adherence of CFDSE-labelled T lymphocytes in PCVs of Peyer's patches of AKR/J mice or SAMP1/Yit mice observed from the serosal side under an intravital microscope at 50–60 min after infusion of labelled T lymphocytes. (a) In AKR/J mice, there were a few adhered lymphocytes in PCVs of Peyer's patches. (b) On the other hand, in 35 week old SAMP1/Yit mice, there were a significant number of adhered lymphocytes. (c) Anti-MAdCAM-1 antibody significantly decreased the number of adhered T lymphocytes. (d) Anti-VCAM-1 antibody also decreased the number of adhered T lymphocytes (objective lens × 10).
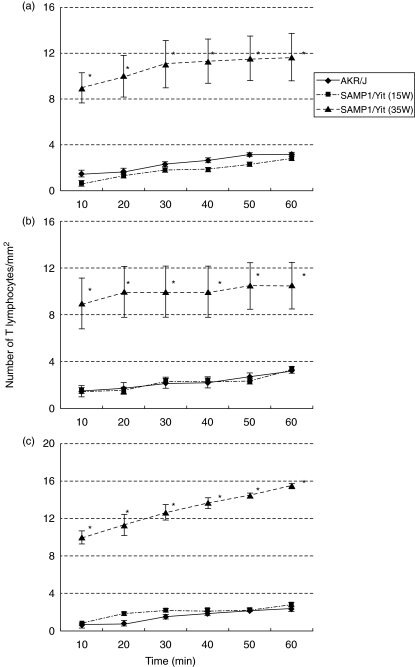
Figure 3 showes the time courses of T lymphocyte accumulation at three sites. The number of adhered T lymphocyte gradually increased up to 60 min after infusion. In control AKR/J mice and 15-week-old SAMP-1/Yit mice, a few T lymphocytes adhering to PCV of Peyer's patches were seen (AKR/J; 3·2 ± 0·2/mm2, 15 weeks-old SAMP1/Yit; 2·8 ± 0·2/mm2). The number of adhered T lymphocyte in 35 weeks-old SAMP1/Yit mice (11·7 ± 2·0/mm2) was significantly (P < 0·01) larger than that in AKR/J and 15-weeks-old SAMP-1/Yit mice. Significant increases in T lymphocyte adherence in submucosal venules and villus microvessels of the ileum were also observed in 35 weeks-old SAMP1/Yit mice (submucosal vessels; 10·5 ± 2·0/mm2, villus capillaries; 15·5 ± 0·2/mm2) compared with those in AKR/J mice and 15 weeks-old SAMP1/Yit mouse (submucosal vessels; 3·2 ± 0·2/mm2 and 3·3 ± 0·2/mm2, respectively; villus capillaries; 2·3 ± 0·2/mm2 and 2·8 ± 0·2/mm2, respectively) (P < 0·01).
Fig. 3.
Time courses of adhesion of T lymphocytes in the intestinal mucosa (a) in PCV of Peyer's patches, (b) in submucosal venules and (c) in villus microvessels. *P < 0·01 versus AKR/J mice and 15 weeks old SAMP1/Yit mice. n = 5 in each group.
The effects of antibodies against MAdCAM-1 and VCAM-1 in the ileal mucosa of SAMP1/Yit mice
As shown in Fig. 2, anti-MAdCAM-1 antibody or anti-VCAM-1 antibody significantly decreased the number of adhered T lymphocytes in PCVs of Peyer's patches of 35 week old SAMP/Yit mice (Fig. 2c,d). In submucosal venules and villus microvessels, these antibodies also inhibited T lymphocytes sticking in in vivo study.
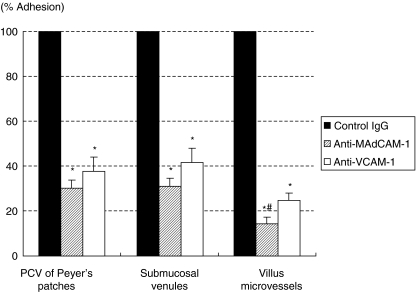
Figure 4 shows the inhibitory effects of functional blocking MAbs against adhesion molecules on the adhesion of T lymphocytes to PCVs of Peyer's patches, submucosal venules and microvessels of the villus mucosa in 35 weeks-old SAMP1/Yit mice at 60 min after T lymphocytes infusion. All results are expressed as percentages against rat-IgG-treated animals. These two MAbs significantly inhibited adhesion of T lymphocytes during the observation periods compared with control IgG treatment (P < 0·01). Anti-MAdCAM-1 MAb showed a significantly stronger inhibitory effect than the effect of anti-VCAM-1 MAb in the villus mucosa (anti-MAdCAM-1, 14·5 ± 2·5%; VCAM-1, 25·0 ± 3·2%; P < 0·05). Although it was not statistically significant in PCV of Peyer's patches and submucosal venules, anti-MAdCAM-1 MAb also markedly reduced the number of adherent T lymphocyte compared with that of anti-VCAM-1 (anti-MAdCAM-1, 30·2 ± 3·5% and 31·3 ± 3·3%; VCAM-1, 37·7 ± 6·0% and 41·7 ± 6·6%, respectively).
Fig. 4.
Effects of pretreatment with anti- MAdCAM-1 MAb or anti-VCAM-1 MAb on adherence of T lymphocytes to PCVs of Peyer's patches, submucosal venules, and microvessels of villus mucosa at 60 min after infusion of CFSE-labelled T lymphocytes. All results are expressed as percentages of those in SAMP1/Yit mice with control rat IgG. Values are means ± SE of 5 animal experiments. *P < 0·05 compared with values of SAMP/Yit mice with control rat IgG. #P < 0·05 compared with anti-VCAM-1 MAb.
Effect of periodical administration of anti-MAdCAM-1 and anti-VCAM-1 on ileitis
T lymphocyte adherence in the intestinal mucosa of SAMP1/Yit mice was significantly increased compared with that in AKR/J mice and was significantly attenuated by anti-MAdCAM-1 MAb and by anti-VCAM-1 MAb. We therefore examined whether periodical administration of anti-MAdCAM-1 MAb or anti-VCAM-1 MAb ameliorates ileitis of SAMP1/Yit mice as a prevention study.
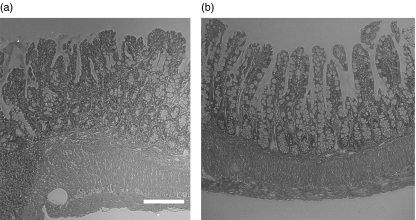
Regarding to body weight or small intestinal length, there was no difference among three groups (data not shown). Figure 5 shows representative H&E-stainings of terminal ileum of SAMP-1/Yit mice after the treatment with control IgG (Fig. 5a) and anti-MAdCAM-1 MAb (Fig. 5b). Anti-MAdCAM-1 MAb treatment showed improvement of in inflammatory cells infiltrate, but muscular hypertrophy persisted. As shown in Table 1, anti-MAdCAM-1 MAb treatment for 7 weeks significantly suppressed villus atrophy and infiltration of CD4, CD8, and β7-integrin positive cells in the ileal mucosa compared with those in mice with the control IgG. Although infiltration of cells in the ileal mucosa was significantly suppressed, submucosal thickening was not remarkably attenuated by anti-MAdCAM-1 MAb treatment. The group treated with MAb to VCAM-1 showed a trend of improvement in inflammation, but it did not reach statistical significance (Table 1). Anti-VCAM-1 MAb treatment failed to show resolution of ileitis.
Fig. 5.
Representative H&E-staining of terminal ileum of SAMP-1/Yit mice after the treatment with (a) control IgG and (b) anti-MAdCAM-1 MAb. Anti-MAdCAM-1 MAb treatment suppressed villus atrophy and infiltration of inflammatory cells in the ileal mucosa. Bar 100 µm (objective lens × 20).
Table 1.
The effect of periodical administration of anti-MAdCAM-1 and anti-VCAM-1 on the development of ileitis in SAMP1/Yit mice. Villus height, submucosal thickness, and the number of cells expressing CD4, CD8 and β7-integrin in lamina propria after 7-week treatment in each groups (The prevention study).
| Control IgG | Anti-MAdCAM-1 | Anti-VCAM-1 | |
|---|---|---|---|
| Villus height (µm) | 110 ± 18 | 187 ± 31* | 107 ± 35 |
| Submucosal thickness (µm) | 85·0 ± 12·9 | 51·3 ± 27·8 | 64·5 ± 24·0 |
| CD4 | 357 ± 51 | 170 ± 34* | 324 ± 67 |
| CD8 | 285 ± 44 | 141 ± 23* | 197 ± 84 |
| β7-integrin | 190 ± 20 | 74 ± 20* | 178 ± 86 |
The infiltrating cells were expressed as the numbers of CD4, CD8 and β7-integrin-positive cells per millimeter of muscularis mucosa. All results were expressed as means ± SE of 4 mice.
P < 0·05 versus Control IgG.
Effects of periodical administration of anti-MAdCAM-1 on established ileitis of SAMP1/Yit mice (treatment study)
We next examined whether periodical administration of anti-MAdCAM-1 MAb ameliorates ileitis of SAMP1/Yit mice age of 28 weeks. As shown in Table 2, villus height and the number of infiltrating CD4, CD8 and β7-integrin positive cells were significantly suppressed by anti-MAdCAM-1 treatment. But suppressive effects were smaller than those in the prevention study. In addition, thickened submucosal thickness was not attenuated with anti-MAdCAM-1 treatment as was demonstrated in the prevention study.
Table 2.
Effect of periodical administration of anti-MAdCAM-1 or control IgG on established ileitis of SAMP1/Yit mice age of 28 weeks. Villus height, submucosal thickness, and the number of cells expressing CD4, CD8 and β7-integrin in lamina propria after 7-week treatment in each groups (The treatment study).
| Control IgG | Anti-MAdCAM-1 | |
|---|---|---|
| Villus height (µm) | 93 ± 27 | 143 ± 29* |
| Submucosal thickness (µm) | 105 ± 26 | 76 ± 16 |
| CD4 | 287 ± 44 | 190 ± 34* |
| CD8 | 246 ± 39 | 168 ± 28* |
| β7-integrin | 168 ± 38 | 81 ± 16* |
The infiltrating cells were expressed as the numbers of CD4, CD8, and β7-integrin-positive cells per millimeter of muscularis mucosa. All results were expressed as means ± SE of 4 mice.
P < 0·05 versus Control IgG.
Discussion
In this present study, we demonstrated that there was a strong expression of MAdCAM-1 and VCAM-1 in SAMP1/Yit mice with spontaneous ileitis and that bolus administration of antibodies against MAdCAM-1 or VCAM-1 significantly attenuated the increased T lymphocyte adherence in the microvessels of the ileal mucosa. On the other hand we also demonstrated that the prophylactic effect of antibody treatment on the development of ileitis of SAMP1/Yit mice was only seen by anti-MAdCAM-1 MAb, but not by anti-VCAM-1 MAb. The treatment study also demonstrated that anti-MAdCAM-1 MAb has a suppressive effect on established ileitis.
Increased expression of MAdCAM-1 has been recognized in other animal models, such as interleukin (IL)-10-deficient mice [26], SCID mice with CD45RBhigh CD4+ T cells [31], dextran sulphate sodium (DSS)-induced colitis [32], trinitrobenzene sulphonic acid colitis [33] and granulomatous colitis induced by peptidoglycan-polysaccharide (PG-PS) [34]. These findings together with our present data on microcirculatory observation in the ileal mucosa suggest that MAdCAM-1 is largely responsible for recruiting T lymphocyte into the inflamed mucosa of SAMP1/Yit mice, thereby promoting development of ileitis. MAdCAM-1 is induced on a murine endothelial cell line, bEND.3, by tumour necrosis factor (TNF)-α and IL-1 mediated nuclear factor-kappa B protein in vitro. TNF-α and IL-1 are thought to be produced chiefly by activated macrophages [35]. Actually, it has been reported that i.p.-administered anti-TNF-α antibody ameliorated SAMP1/Yit ileitis [17]. Based on these observations, we speculate that the increased expression of MAdCAM-1 is induced by enhanced TNF-α production from activated macrophage in the inflamed mucosa of SAMP1/Yit mice.
The prophylactic effects of anti-MAdCAM-1 antibody on experimental colitis have also been reported in several animal models [32,34]. In this study, anti-MAdCAM-1 MAb treatment showed a significant improvement of inflammatory cell infiltration in the ileal mucosa of SAMP1/Yit mice, however, it did not significantly attenuate the submucosal thickening. The exact reason why muscular hypertrophy persisted after MAdCAM-1 treatment is not known. But we recently showed that in mice colitis induced by DSS anti-MAdCAM-1 treatment was effective when given 7 days after the start of DSS treatment (at a late time point). We also demonstrated that in the PG-PS colitis model significant infiltration of macrophages was not attenuated by anti-MAdCAM-1 treatment, suggesting the importance of MAdCAM-1-independent mechanisms in the histologic changes. These results suggest the possibility that MAdCAM-1 plays a key role mainly in the aggravated phase of ileal inflammation in SAMP1/Yit mice by promoting T lymphocyte migration, but not in the initiation phase of ileitis where macrophages or other types of cells are largely involved. Data showing the suppressive effects of anti-MAdCAM-1 on established ileitis also support that MAdCAM-1 play a role in the aggravated phase of ileal inflammation. We also speculate that cells including smooth musclular cells or fibroblasts could play important roles in the chronic fibrotic changes of submucosal inflammation in the affected ileum of SAMP1/Yit mice by a MAdCAM-1 independent pathway.
VCAM-1 and ICAM-1 are other adhesion molecules that may play a role in site of inflammation. In clinical studies on inflammatory bowel diseases, the levels of ICAM-1 and VCAM-1 were increased in the intestinal mucosa in active UC and CD [36,37]. It have been also reported that blockade of either ICAM-1 or VCAM-1 can reduce the leucocyte adherence and disease activity index in inflamed colon in some models of experimental colitis [38,39]. In the case of ICAM-1, however, we have already reported that up-regulated ICAM-1 is not involved in T lymphocyte-endothelial adhesion in the inflamed villus mucosa of intestine [29]. In the case of VCAM-1, our data showed that bolus administration of anti-VCAM-1 MAb significantly attenuated T lymphocyte adherence in microvessels of the ileal mucosa in SAMP1/Yit mice. However, it should be noted that periodical anti-VCAM-1 MAb treatment had no significant improvement of inflammation in SAMP1/Yit ileitis. Burns et al. [18] demonstrated in the SAMP1/Yit adoptive transfer model that blockade of either VCAM-1 or ICAM-1 had no significant beneficial effects on chronic inflammation. Their results are in accordance with the present results.
The reason why anti-VCAM-1 MAb did not reduce the inflammation in the ileum of SAMP1/Yit mice despite of its ability to inhibit T lymphocyte recruitment is not clearly known. But there are several possibilities. One possible reason is that anti-MAdCAM-1 MAb selectively blocked the β7-integrin positive T lymphocytes with gut tropism, but anti-VCAM-1 MAb did not have this selectivity. MAdCAM-1 is known to be a specific ligand for α4β7-integrin, but not for α4β1-integrin [23], while VCAM-1 can recognize α4β1-integrin, but it can also recognize α4β7-integrin under flow conditions [40]. Because we used mixed lymphocytes from spleen and mesenteric lymph nodes, which express both α4β1 and α4β7-integrins (data not shown), we can speculate that anti-MAdCAM-1 MAb treatment could inhibit the β7-integrin positive T lymphocyte adherence to the intestinal microvessels more dominantly than anti-VCAM-1 MAb, even though both antibodies appeared to inhibit significantly the total T lymphocyte sticking in in vivo study. A remarkable inhibition of β7-integrin positive cell accumulation in the ileal mucosa after anti-MAdCAM-1 MAb treatment for 7 weeks may also support this possibility. Another possibility is that anti-VCAM-1 MAb treatment has less sufficient effect to suppress repeated migration and infiltration of immune cells thereby inflammatory cytokines. In terms of this point it should be noted that bolus administration of anti-VCAM-1 MAb showed a weaker inhibitory effect on the inhibition of T cell adhesion in villus microvessels compared with anti-MAdCAM-1 MAb. In relation to this issue, T cell aberrancy in SAMP/Yit mice must also be considered. Because we injected T cells isolated from normal mice (AKR/J), there is a possibility that migration of T cells from SAMP/Yit mice may not be sufficiently blocked by anti-VCAM-1 antibody. In our preliminary studies on homing receptor of T cells by FACScan, the expression of α4-integrin was significantly greater in SAMP/Yit mice than AKR/J mice, although α4β7-integrin expression was not different between them (data not shown).
Because it is demonstrated that MAdCAM-1-positive vessels was significantly greater in the lamina propria of intestine of SAMP1/Yit mice than AKR/J mice, and that VCAM-1 was mainly increased in the basal crypt and submucosa, anti-MAdCAM-1 may explain the stronger amelioration of villus destruction and atrophy in the SAMP1/Yit mice. The results of this study on experimental ileitis in SAMP1/Yit mice suggested that MAdCAM-1, but not VCAM-1 may be an appropriate target for the therapeutic modulation of chronic ileitis.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Marteau P. Inflammatory bowel disease. Endoscopy. 2000;32:131–7. doi: 10.1055/s-2000-142. [DOI] [PubMed] [Google Scholar]
- 3.Satsangi J, Welsh KI, Bunce M, et al. Contribution of genes of the major histocompatibility complex to susceptibility and disease phenotype in inflammatory bowel disease. Lancet. 1996;347:1212–7. doi: 10.1016/s0140-6736(96)90734-5. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 5.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 6.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 7.Targan SR, Hanauer SB, Van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 8.Baert FJ, D'Haens GR, Peeters M, et al. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999;116:22–8. doi: 10.1016/s0016-5085(99)70224-6. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, Hosokawa M, Takeshita S, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–94. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizarro TT, Arseneau KO, Cominelli F. Lessons from genetically engineered animal models XI. Novel mouse models to study pathogenic mechanisms of Crohn's disease. Am J Physiol. 2000;278:G665–9. doi: 10.1152/ajpgi.2000.278.5.G665. [DOI] [PubMed] [Google Scholar]
- 12.Strober W, Nakamura K, Kitani A. The SAMP1/Yit mouse: another step closer to modeling human inflammatory bowel disease. J Clin Invest. 2001;107:667–70. doi: 10.1172/JCI12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera-Nieves J, Bamias G, Vidrich A, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–82. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 14.Kozaiwa K, Sugawara K, Smith MF, Jr, et al. Identification of a quantitative trait locus for ileitis in a spontaneous mouse model of Crohn's disease: SAMP1/Yitfc. Gastroenterology. 2003;125:477–90. doi: 10.1016/s0016-5085(03)00876-x. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S, Watanabe N, Imaoka A, et al. Preventive effects of Bifidobacterium- and Lactobacillus-fermented milk on the development of inflammatory bowel disease in senescence-accelerated mouse P1/Yit strain mice. Digestion. 2001;64:92–9. doi: 10.1159/000048846. [DOI] [PubMed] [Google Scholar]
- 16.Kosiewicz MM, Nast CC, Krishnan A, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marini M, Bamias G, Rivera-Nieves J, et al. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci USA. 2003;100:8366–71. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns RC, Rivera-Nieves J, Moskaluk CA, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 19.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 20.Butcher EC. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell. 1991;67:10033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 21.Springer T. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 22.Ishii N, Tsuzuki Y, Matsuzaki K, et al. Endotoxin stimulates monocyte–endothelial cell interactions in mouse intestinal Peyer's patches and villus mucosa. Clin Exp Immunol. 2004;135:226–32. doi: 10.1111/j.1365-2249.2003.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 24.Fong S, Jones S, Renz ME, et al. Mucosal addressin cell adhesion molecule-1 (MAdCAM-1). Its binding motif for alpha 4 beta 7 and role in experimental colitis. Immunol Res. 1997;16:299–311. doi: 10.1007/BF02786396. [DOI] [PubMed] [Google Scholar]
- 25.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 26.Connor EM, Eppihimer MJ, Morise Z, et al. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–55. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 27.Miura S, Tsuzuki Y, Kurose I, et al. Endotoxn stimulates lymphocyte–endothelial interactions in rat intestinal Peyer's patches and villus mucosa. Am J Physiol. 1996;271:G282–G292. doi: 10.1152/ajpgi.1996.271.2.G282. [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu T, Specian RD, Wolf RE, et al. MAdCAM mediates lymphocyte-endothelial cell adhesion in a murine model of chronic colitis. Am J Physiol. 2001;281:G1309–15. doi: 10.1152/ajpgi.2001.281.5.G1309. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe C, Miura S, Hokari R, et al. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am J Physiol. 2002;283:G1379–87. doi: 10.1152/ajpgi.00026.2002. [DOI] [PubMed] [Google Scholar]
- 30.Fujimori H, Miura S, Koseki S, et al. Intravital observation of adhesion of lamina propria lymphocytes to microvessels of small intestine in mice. Gastroenterology. 2002;122:734–44. doi: 10.1053/gast.2002.31899. [DOI] [PubMed] [Google Scholar]
- 31.Picarella D, Hurlburt P, Rottman J, et al. Monoclonal antibodies specific for β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–106. [PubMed] [Google Scholar]
- 32.Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183–9. [PubMed] [Google Scholar]
- 33.Viney JL, Jones S, Chiu HH, et al. Mucosal addressin cell adhesion molecule-1; A structural nad functional analysis demarcates the integrin binding motif. J Immunol. 1996;157:2488–97. [PubMed] [Google Scholar]
- 34.Hokari R, Kato S, Matsuzaki K, et al. Involvement of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the pathogenesis of granulomatous colitis in rats. Clin Exp Immunol. 2001;126:259–65. doi: 10.1046/j.1365-2249.2001.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartor RB. Cytokines in intestinal inflammation. Pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura S, Ohtani H, Watanabe Y, et al. In situ expression of the cell adhesion molecule in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Laboratory Invest. 1993;69:77–85. [PubMed] [Google Scholar]
- 37.Jones SC, Banks RE, Haider A, et al. Adhesion molecules in inflammatory bowel disease. Gut. 1995;36:724–30. doi: 10.1136/gut.36.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sans M, Panes J, Ardite E, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116:874–83. doi: 10.1016/s0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 39.Hamamoto N, Maemura K, Hirata I, et al. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1) Clin Exp Immunol. 1999;117:462–8. doi: 10.1046/j.1365-2249.1999.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sriramarao P, DiScipio RG, Cobb RR, et al. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of shear flow. Blood. 2000;95:592–601. [PubMed] [Google Scholar]