Abstract
We have shown previously that in listeric encephalitis of cattle and rats, nitrotyrosine was produced in microabscesses, implying that both superoxide anion (O2–) and nitric oxide (NO) are present and react with each other. Evidence of local synthesis of NO by macrophages was provided, but the source of O2– remained unknown. Here we have examined whether phagocytes exposed to viable and heat-killed Listeria monocytogenes (LMΔ) produce O2– and, if so, whether this results from direct interaction of phagocytes with the bacterial surface of L. monocytogenes or whether prior opsonization is required. Using lucigenin-enhanced chemiluminescence (LCL) for the measurement of O2–, we show that LMΔ induces an oxidative burst in human neutrophils, monocytes and monocyte-derived macrophages (Mφ). Viability is not required, and opsonization by antibodies and/or complement does not enhance the LCL signal. As Toll-like receptors (TLR) were shown recently to mediate an oxidative burst, TLR agonists representative for pathogen-associated molecular patterns (PAMPs) were tested for their ability to elicit an oxidative burst. These included lipoteichoic acid (LTA), bacterial peptidoglycan (PGN), recombinant flagellin, CpG-containing DNA and double-stranded RNA. Only PGN and flagellin consistently elicited an LCL signal resembling that induced by LMΔ with regard to the kinetics and cell spectrum stimulated. However, flagellin was unlikely to be responsible for the LMΔ-mediated burst, as a flagellin-deficient mutant showed no decrease in LCL. We therefore assume that in LMΔ, core PGN acts as a PAMP and directly induces an oxidative burst in all phagocyte populations. We conclude that in cerebral lesions superoxide anion is generated locally by phagocytes recognizing bacterial PGN.
Keywords: chemiluminescence, human phagocytes, Listeria monocytogenes, oxidative burst, peptidoglycan, superoxide anion
Introduction
Listeria monocytogenes (LM) is a facultative intracellular Gram-positive bacterium causing serious infections in ruminants fed with contaminated silage food. The food products of these animals such as meat and cheese may infect humans, particularly elderly or immunologically compromised subjects and children [1,2]. In both ruminants and humans the infection may be lethal. LM has been used extensively as a prototypic organism for studying host defence mechanisms in rodents [3]. It was reported that innate host defence depends on a broad panel of cells (e.g [4,5]), whereas acquired immunity rests on both CD8 cells and an interplay between specifically sensitized T cells and Mφ [6,7]. However, the ultimate effector molecules leading to listerial growth control in tissues are unclear, and the role of both reactive oxygen and nitrogen intermediates is controversial [8–11]. Mice deficient for both phagocyte oxidase (phox) and inducible nitric oxide synthase (iNOS) are more susceptible to an infection with LM than those deficient for one of these constituents only [12]. We have demonstrated previously that in both naturally infected cattle and in intracisternally infected rats, nitrotyrosine is formed locally in sites of infection, and the kinetics of its appearance and disappearance overlaps with that of inducible nitric oxide synthase (iNOS) [13,14]. This implies that superoxide is present in lesions.
In this study, we address the mechanism whereby LM induces an oxidative burst, as determined by lucigenin-enhanced chemiluminescence (LCL). It is not known whether LM triggers receptors of phagocytes via bacteria-bound opsonins or whether it is able to trigger the generation of ROI directly and, if so, which phagocyte population(s) react(s) to LM. Furthermore, the requirement of viability has not been examined. We found that both viable and heat-killed LM (LMΔ) showed an oxidative burst of similar magnitude, and no opsonization by antibody or complement was required. This prompted the search for pathogen-associated molecular patterns (PAMP) involved in triggering a LCL signal. Using various highly purified Toll-like receptor (TLR) agonists, evidence is provided suggesting that bacterial PGN is the mediator of an LCL response in all phagocyte populations tested (neutrophils, monocytes and Mφ).
Materials and methods
Preparation of Listeria monocytogenes
Bacteria from a frozen stock of LM, strain EGD [7], were cultured overnight in Tryptic soy broth (Difco, Detroit, MI, USA) at 37°C and grown to log-phase for an additional 3 h at 37°C. LM was heat-inactivated (1 h at, 65°C; LMΔ). LMΔ was either left unopsonized or opsonized with fresh-frozen human serum for 1 h at 37°C (LMS) thereby allowing the fixation of complement, or treated with polyclonal rabbit IgG specific for L. monocytogenes (serotypes 1 and 4, Difco) for 1 h at 37°C (LM + AB). Still other bacteria were treated first with antibodies, then with fresh serum (LM + ABS). Appropriate binding of antibodies and/or complement was verified either by phagocytosis experiments (see below) or by immunohistochemistry [13]. Bacterial preparations were aliquoted and stored at −80°C. In some experiments a flagellin-deficient mutant (LM flag FlaA LuxAB) and its corresponding wild-type (LM 7973 wt prfa) [15] were treated accordingly.
Phagocyte isolation and culture
Heparinized blood was collected from healthy volunteers. To remove erythrocytes blood was placed over a layer of 1·41% (w/v) methocell (methyl cellulose A15 premium; Prochem AG, Zurich, Switzerland) in sodium metrizoate (11·26% v/v; Sigma, St Louis, MO, USA) and allowed to sediment at 1 g for 45 min. Leucocyte-enriched supernatants were removed and peripheral blood leucocytes were separated by centrifugation over Lymphoprep (Biochrom KG, Berlin, Germany) into periperal blood mononuclear cells (PBMC; interphase) and granulocytes (sediment). After washing with phosphate buffered saline (PBS), fractions were resuspended at 1 × 106 cells/ml in Hanks’ balanced salt solution (HBSS) with 1·25 mm Ca2+ and 0·81 mm Mg2+, pH 7·2–7·3 (HBSS2+) and tested immediately for LCL. Neutrophils (PMN) within the granulocyte fraction (sediment) had a purity of >98%. PBMC contained, on average, 25% monocytes, the remainder being lymphocytes. Only the monocytes, but not the lymphocytes, contributed to LCL, as determined by magnetobead separation experiments (Jungi, unpublished).
For the preparation of mature Mφ blood donations from healthy volunteers were used, and PBMC were isolated essentially as described above, followed by adherence purification of monocytes as reported previously [16]. Adherent cells were cultured overnight, followed by dislodgement and culturing in Teflon bags as described previously [16]. Within 1 week, monocytes differentiated into mature resting Mφ [16,17].
Measurement of an oxidative burst
In pilot experiments, several chemiluminescence-based and flow cytometry-based methods were compared and essentially performed as described [18]. To prove that the signal measured represented superoxide anion production, LCL was blocked by superoxide dismutase (SOD, 1000 U/ml; Sigma). This prompted us to examine the effect of LM on ROI generation by LCL, as detailed below.
Purified Mφ, PMN or PBMC were transferred to 11 × 47 mm polystyrene CL tubes (0·25 ml, 1 × 106/ml). Cells were dark-adapted at 37°C with 50 µm of lucigenin (Sigma) for 45 min. They were then triggered with the positive control (5 × 10−7m phorbol 12-myristate 13-acetate), mock-triggered (HBSS2+) or triggered with various stimuli. These were viable and heat-killed LM, opsonized LM (LMS, LM + AB, LM + ABS; final concentration 20 µg/ml) and pathogen-associated molecular patterns. They included 1 µg/ml flagellin kindly provided by Dr Salzman [19], 10 µg/ml peptidoglycan (PGN; Fluka, Buchs, Switzerland), 4 µg/ml lipoteichoic acid (LTA) from both L. monocytogenes and Staphylococcus aureus, prepared as described [20] and kindly provided by Dr T. Hartung, 100 µg/ml poly(I:C) (Sigma), 2 µm CpG-ODN #2006 and the respective control motif (Biomol, Berlin, Germany). All stimuli of LCL were tested previously for endotoxin contamination by running a Limulus amoebocyte lysate (LAL) assay in the quality control laboratory of the Bern University Hospital. This assay had a detection threshold of 0·01 endotoxin units per ml, corresponding to 1 pg/ml endotoxin from Escherichia coli. Samples that had a LAL activity of higher than 0·1 EU/ml were excluded. LCL was measured in a LB 950 luminometer (Berthold, Wildbad, Germany). LCL data are expressed either as temporal traces (mean cpm of duplicates or triplicates) or as stimulation indices (signal-to-background ratio of the mean total counts over a period of 60–90 min). Stimulation indices were tested for significance using non-parametric one-way anova (Kruskall–Wallis) followed by Dunn's multiple comparison test.
Measurement of opsonization
Opsonization was tested by offering LMΔ, LMS, LM + AB or LM + ABS to Mφ monolayers (10 bacteria per Mφ, on average) for 30 min. Cells were then lysed using H20. This was followed by quantitative bacteriology, using Tryptic soy agar (Difco) plates. Data were analysed by one-way anova with Bonferroni's multiple comparison test as obtained from the graphpad prism 3·0 package.
Results
Heat-killed LM trigger the release of superoxide anion in human phagocytes
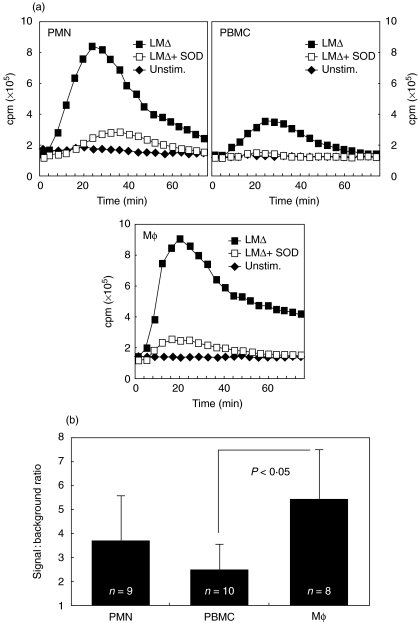
When stimulated with LMΔ all phagocyte populations tested reacted with an oxidative burst, as evidenced by a LCL signal. After the addition of LMΔ, there was an increase of LCL for 20–30 min, and LCL then dropped to baseline levels. The order of the maximal light emission was Mφ = PMN > PBMC; the order of the LMΔ-dependent increase was Mφ > PMN > PBMC (Fig. 1). LCL signals were significantly higher for LMΔ-stimulated Mφ than for PBMC (Fig. 1), but considering the percentage of monocytes within PBMC their signal was of a strength similar to that of Mφ. Regardless of the cell type tested, superoxide dismutase (SOD) abrogated LMΔ-induced CL, confirming that LCL measured superoxide anion production (Fig. 1).
Fig. 1.
LCL of PMN, PBMC and Mφ in response to LMΔ. Addition of superoxide dismutase to LMΔ-stimulated cells (LMΔ + SOD) abrogates the CL signal. Mean temporal traces of two from representative experiments (a) and average signal-to-background ratios of LMΔ-stimulated versus unstimulated cells integrated over the whole experiment (b) are shown. P-values indicate levels of statistical significance of difference.LCL of PMN, PBMC and Mφ by viable and heat-killed LM. Mean temporal traces of two from a representative experiment (a) and mean signal-to-background ratios of viable LM-stimulated versus LMΔ-stimulated cells integrated over the whole experiment (b) are shown for one representative experiment.
The effect of viability on superoxide anion production by LM
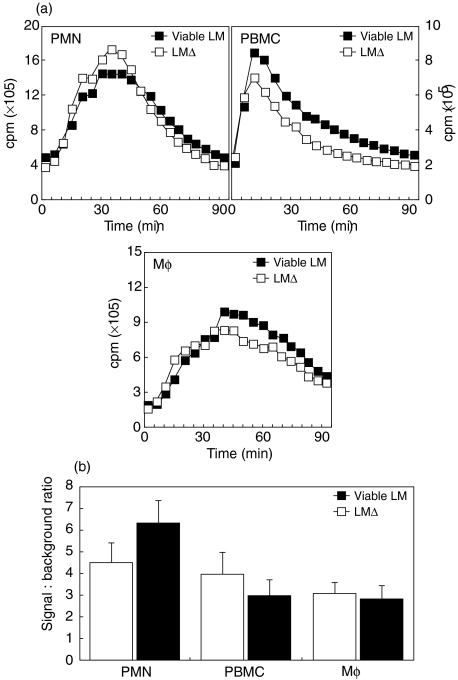
The requirement for viability of LM was tested by comparing viable and heat-inactivated LM for their LCL response. Both bacteria elicited a similar LCL signal (Fig. 2), suggesting that bacterial surfaces themselves, rather than secreted metabolic products, triggered an oxidative burst.
Fig. 2.
LCL of PMN, PBMC and Mφ by viable and heat-killed LM. Mean temporal traces of 2 from a representative experiment (A) and mean signal-to-background ratios of viable LM-stimulated versus LMΔ-stimulated cells integrated over the whole experiment (B) are shown for one representative experiment.
The effect of opsonization on superoxide anion production by LM
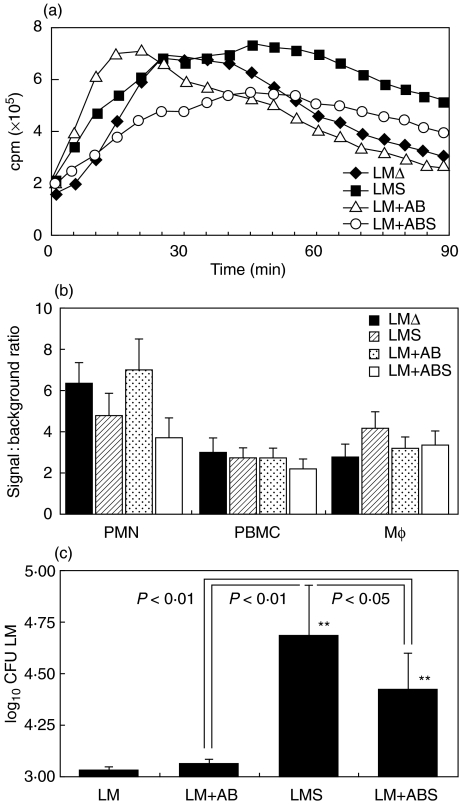
To determine whether opsonization of bacteria increased the CL signal, cells were stimulated with either LMΔ or LMΔ opsonized with antibodies or complement, or both combined. Opsonization did not enhance the LCL signal, regardless of whether antibodies or complement or both were used (Fig. 3a,b), although fresh-frozen serum as a complement source strongly augmented phagocytosis of LM by Mφ (Fig. 3c).
Fig. 3.
The effect of opsonization of bacteria with IgG or fresh-frozen serum on LCL and phagocytosis. (a) Mean temporal traces of two. (b) Signal-to-background ratio of the same LCL experiments. (c) Phagocytosis (average of three experiments; error bars indicate s.d.). Asterisks in (c) denote statistically significant differences when compared with nonopsonized bacteria (*P < 0·05, **P < 0·01).
The effect of bacterial components on superoxide anion production by LM
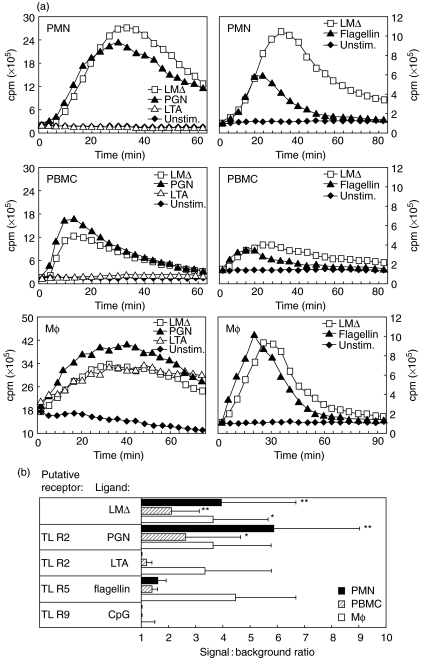
The previous results suggested that Listeria itself rather than a host constituent triggers an oxidative burst in phagocytes. To address candidate molecules involved, several Toll-like receptor (TLR) agonists were assessed for their ability to elicit an oxidative burst, because it has been shown recently that members of the TLR family promote an oxidative burst [21,22]. Nucleic acid-derived constituents such as CpG motifs and double-stranded RNA were unable to elicit a burst (Fig. 4, and data not shown). LTA induced an oxidative burst in PBMC and Mφ, but not in PMN (Fig. 4). Both PGN and flagellin induced a burst of similar kinetics, as did LMΔ. To exclude the role of flagellin, a flagellin-free mutant of LM was compared with its wild-type strain. Both elicited a LCL signal of similar magnitude and kinetics (data not shown).
Fig. 4.
LCL in response to LMΔ, PGN, LTA, flagellin and CpG by PMN, PBMC and Mφ. (a) Mean temporal CL traces of a representative experiment are shown. (b) Average signal-to-background ratios (n = 3) for each cell type of LMΔ-stimulated versus unstimulated cells integrated over the whole experiment are shown. Asterisks denote statistically significant differences (*P < 0·05, **P < 0·01).
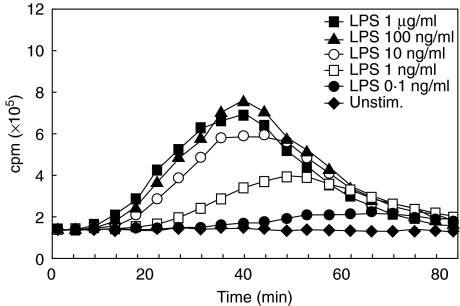
The TLR4 ligand lipopolysaccharide (LPS) is a major cell wall component of Gram-negative bacteria and a frequent contaminant of biochemical preparations. To determine whether the LCL responses observed can be explained by LPS contamination, we examined the ability of LPS to induce an oxidative burst in Mφ. A series of LPS concentrations ranging from 1 µg/ml to 0·1 ng/ml was tested in lucigenin-enhanced CL. LPS dose-dependently induced a CL signal and the amplitude of the signal was lower and appeared later during the CL with decreasing concentrations of LPS (Fig. 5). Given the very low level of LPS contamination measured by LAL assay, LPS as a cause of the observed CL responses could be ruled out. This leaves PGN of LM as the most likely candidate eliciting an oxidative burst in all phagocyte populations tested.
Fig. 5.
Dose-dependent CL in response to the TLR4 ligand LPS. Representative temporal CL traces obtained with Mφ from an individual donor (averages of duplicate measurements) are shown. Symbols of individual curves are given in the figure.
Discussion
In the present work, the ability of LM, a facultative intracellular bacterium lethal to humans and domestic animals [1,2], to trigger an oxidative burst was investigated. In vivo observations suggested that in the brain, one of the preferred sites of infection by these organisms in the naturally infected host, LM not only induces the synthesis of reactive nitrogen, but also of reactive oxygen intermediates [13,14]. It remained open whether LM induces an oxidative burst directly, or whether the oxidative burst is induced by opsonization with complement and/or antibodies, or by metabolic products. Similarly, it was not known whether the local generation of reactive oxygen is due to phagocyte stimulation and, if so, which phagocyte population is responsible. Here we show that the bacteria trigger an oxidative burst in all phagocyte populations tested, neutrophils, monocytes and Mφ. Triggering did not depend on a metabolic product as heat-killed, washed bacteria (LMΔ) elicited an undiminished response. Similarly, the LCL signal was not increased by opsonization, although opsonization with fresh-frozen serum enhanced bacterial uptake when compared with LMΔ. This is distinct from other bacteria, e.g. Pseudomonas, for which it was shown that triggering of an oxidative burst was dependent on antibodies [23]. For E. coli K1, the alternative complement pathway was reported to trigger an oxidative burst [24], and both antibodies and the classical complement pathway have an important role [25]. For LM, triggering of an oxidative burst could be clearly dissociated from phagocytosis, suggesting that the respective signalling cascades leading to these responses are different.
A comparison of the amount of PMA-induced O2– generated revealed a difference between Mφ and neutrophils. As expected, the latter were the more efficient producers of of O2– than the former. Interestingly, LMΔ elicited O2– of a similar magnitude in either cell type. This is probably related to the fact that the magnitude of the response depends both on the effector machinery and the recognition apparatus of a given cell type. This could imply that Mφ are better equipped to recognize LMΔ than neutrophils.
Phox, the enzyme catalysing the production or reactive oxygen intermediates in phagocytes, is known to be activated by opsonized zymosan [26], cross-linking of Fc receptors and other cell surface molecules [27–29], complement receptors, lipid mediators and chemokines [30], activation of protein-kinase C and by mobilizing calcium flux [30]. Although some bacteria elicit a burst without prior opsonization, the molecules involved have not been delineated. This is due in part to the poor solubility of bacterial cell wall constituents. We and others have shown that members of the TLR family mediate an oxidative burst [21,22]. Given the observation that LM itself rather than LM-bound antibodies and/or complement mediates a burst, we asked which constituent(s) of LM promote(s) the generation of superoxide anion. A list of candidates comprised pathogen-associated molecular patterns. Indeed, at least ligands of TLR2, TLR4 and TLR5 among these have been shown to be able to elicit an oxidative burst in all phagocyte populations tested. This confirms data published previously [21,22,31] and extends it to TLR5. It therefore appears that one of these receptors is involved in the triggering of an oxidative burst by bacteria. However, recent evidence suggests that receptors other than TLR such as dectin-1 are also involved in the triggering of an oxidative burst mediated by zymosan [32].
The signalling cascades following engagement of one of the TLR members extensively overlap [33]. It is therefore surprising that triggering of some but not all TLR elicit an oxidative burst. In this study, we found that agonists of TLR3 and TLR9 failed to trigger an oxidative burst in all phagocyte populations tested. This includes poly(I:C), which is an agonist of TLR3 [34], and CpG ODN, which is known to be an agonist of TLR9 [35]. Both preparations found to be devoid of activity in this study were active in other respects, and were found to trigger TNF and IL-12 production by human monocyte-derived dendritic cells [36]. It therefore appears that nucleic acid-based TLR agonists, as they are likely to be expressed by viral pathogens, do not elicit an oxidative burst in phagocytes. However, in our hands both human primary phagocytes and monocytoid cell lines fail to respond to these agonists by both an oxidative burst and by TNF production. Some studies showed that both TLR3 and TLR9 are localized intracellularly, at least in phagocytes [37,38]. The subcellular localization may be coupled to the effector response spectrum of these receptors.
The molecular mechanisms of how heat-killed LM elicits an oxidative burst remain to be determined. However, in this study the bacterium-derived TLR agonists LTA and flagellin were excluded experimentally from inducing an oxidative burst in this study. Thus, LTA was unlikely to be responsible for the burst elicited by LMΔ, although it is a TLR2 agonist [39], and TLR2 engagement was shown to elicit an oxidative burst [21]. Highly purified LTA of several bacterial species, including LM, induced a weak burst in monocytes only, and failed to trigger PMN, whereas LMΔ was a strong trigger of both monocytes and neutrophils. This may be due to low levels of expression of CD14 by neutrophils, which is an important co-receptor for LTA [40–42]. Finally, flagellin is unlikely to be the mediator of a burst elicited by LMΔ, although it is shown here that this TLR5 agonist induces an oxidative burst in all phagocyte populations tested with similar kinetics, as seen by LMΔ triggering. Evidence arguing against flagellin is twofold. First, although LM is a peritrichous organism when grown at 22°C, the bacterium grown at 37°C has few flagellae only [15] and therefore expresses low amounts of flagellin on its surface. Secondly, a flagellin-deficient mutant elicited a similar LCL signal to wild-type bacteria. This leaves PGN as a candidate for eliciting a burst in phagocytes. Indeed, both LM and PGN were reported to be a TLR2 agonist [39,43], and triggering of an oxidative burst by highly purified PGN in a TLR2-dependent manner was shown for this compound [21]. Alternatively, an unknown bacterial constituent serving as PAMP may be the trigger of an oxidative burst in all phagocyte populations tested here. When tools become available, further studies will need to demonstrate more directly that LMΔ elicits an oxidative burst by interaction of PGN with phagocytes.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation (nos 3200–52247·97, 3200–54041·98 and 3200–065036). The expert technical assistance of Mrs Eszter Benedek and the critical reading of the manuscript by Drs Ernst Peterhans and Dirk Werling, Institute of Veterinary Virology, University of Bern, are gratefully appreciated. We are grateful for the generous gifts of recombinant flagellin from Dr A. L. Salzman (Salzman Inotek Corporation, Beverly, MA, USA), of LTA from S. aureus and LM from Dr Thomas Hartung (Biochemical Pharmacology, University of Constance, Germany) and of flagellin-deficient LM by Drs C. Dodd and K. Rees, Division of Food Sciences, School of Biological Sciences, University of Nottingham, Loughborough, UK.
Note added in proof
A recent paper [44] shows that PGN from various bacterial species including L. monocytogenes indues NFκB activation and cytokine generation in a TLR2-independent manner. Although we cannot exclude the possibility that a minor contaminant of the commercial PGN preparation used triggers an oxidative burst, a TLR-2 specific monoclonal antibody completely blocks a TNF signal generated by this preparation in THP-1 cells (M. Brcic and T. W. Jungi, unpublished).
References
- 1.Schlech WFI. Foodborne listeriosis. Clin Infect Dis. 2000;31:770–5. doi: 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- 2.Rebhun WC. Listeriosis. Vet Clin North Am Food Anim Pract. 1987;3:75–83. doi: 10.1016/s0749-0720(15)31180-4. [DOI] [PubMed] [Google Scholar]
- 3.Parham P, Unanue ER, editors. Immunol Rev. Vol. 158. 1997. Immunity to Listeria monocytogenes: a model intracellular pathogen; pp. 11–70. [Google Scholar]
- 4.Unanue ER. Macrophages, NK cells and neutrophils in the cytokine loop of Listeria resistance. Res Immunol. 1996;147:499–505. doi: 10.1016/s0923-2494(97)85214-5. [DOI] [PubMed] [Google Scholar]
- 5.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–68. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harty JT, Bevan MJ. CD8 T-cell recognition of macrophages and hepatocytes results in immunity to Listeria monocytogenes. Infect Immun. 1996;64:3632–40. doi: 10.1128/iai.64.9.3632-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 8.Gregory SH, Wing EJ, Hoffman RA, Simmons RL. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993;150:2901–9. [PubMed] [Google Scholar]
- 9.Beckerman KP, Rogers HW, Corbett JA, Schreiber RD, McDaniel ML, Unanue ER. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–95. [PubMed] [Google Scholar]
- 10.Bermudez LE. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–81. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr T, Schoedon G, Odermatt B, et al. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;185:921–31. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiloh MU, MacMicking JD, Nicholson S, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 13.Remer KA, Jungi TW, Fatzer R, Täuber M, Leib SL. Nitric oxide is protective in listeric meningoencephalitis of rats. Infect Immun. 2001;69:486–93. doi: 10.1128/IAI.69.6.4086-4093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfister H, Remer KA, Brcic M, et al. Inducible nitric oxide synthase and nitrotyrosine in listeric encephalitis: a cross-species study in ruminants. Vet Pathol. 2002;39:190–9. doi: 10.1354/vp.39-2-190. [DOI] [PubMed] [Google Scholar]
- 15.Vatanyoopaisan S, Nazli A, Dodd CE, Rees CE, Waites VM. Effect of flagella on initial attachment of Listeria monocytogenes to stainless steel. Appl Environ Microbiol. 2000;66:860–3. doi: 10.1128/aem.66.2.860-863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhnert P, Schalch L, Jungi TW. Cytokine induction in human mononuclear cells stimulated by IgG-coated culture surfaces and by IgG for infusion. Clin Immunol Immunopathol. 1990;57:218–32. doi: 10.1016/0090-1229(90)90036-p. [DOI] [PubMed] [Google Scholar]
- 17.Jungi TW, Thony M, Brcic M, Adler B, Pauli U, Peterhans E. Induction of nitric oxide synthase in bovine mononuclear phagocytes is differentiation stage-dependent. Immunobiology. 1996;195:385–400. doi: 10.1016/S0171-2985(96)80054-4. [DOI] [PubMed] [Google Scholar]
- 18.Rothe G, Oser A, Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988;75:354–5. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- 19.Salzman AL, Eaves-Pyles T, Linn SC, Denenberg AG, Szabo C. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology. 1998;114:93–102. doi: 10.1016/s0016-5085(98)70637-7. [DOI] [PubMed] [Google Scholar]
- 20.Morath S, Geyer A, Hartung T. Structure–function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–8. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 22.Remer KA, Brcic M, Jungi TW. Toll-like receptor-4 mediates an LPS-induced oxidative burst. Immunol Lett. 2002;85:75–80. doi: 10.1016/s0165-2478(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 23.Allen RC, Lieberman MN. Kinetic analysis of microbe opsonification based on stimulated polymorphonuclear leucocyte oxygenation activity. Infect Immun. 1984;45:475–82. doi: 10.1128/iai.45.2.475-482.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens P, Young LS, Adamu S. Opsonization of various capsular (K) E. coli by the alterntive complement pathway. Immunology. 2004;50:497–502. [PMC free article] [PubMed] [Google Scholar]
- 25.Jungi TW, Schmid A, Morell A, Spaeth PJ, Peterhans E. Quantitative assessment of human neutrophil chemiluminescence induced by opsonized Escherichia coli K-12. Zbl Bakt Hyg A. 1989;270:406–17. doi: 10.1016/s0176-6724(89)80010-0. [DOI] [PubMed] [Google Scholar]
- 26.Channon JY, Leslie CC, Johnston RB., Jr Zymosan-stimulated production of phosphatidic acid by macrophages: relationship to release of superoxide anion and inhibition by agents that increase intracellular cyclic AMP. J Leukoc Biol. 1987;41:450–3. doi: 10.1002/jlb.41.5.450. [DOI] [PubMed] [Google Scholar]
- 27.MacIntyre EA, Roberts PJ, Jones M, van der Schoot CE, Tidman N, Linch DC. Activation of human monocytes occurs on cross-linking monocytic antigens to an Fc receptor. J Immunol. 1989;142:2377–83. [PubMed] [Google Scholar]
- 28.Trezzini C, Jungi TW, Spycher MO, Maly FE, Rao P. Human monocyte CD36 and CD16 are signaling molecules. Evidence from studies using antibody-induced chemiluminescence as a tool to probe signal transduction. Immunology. 1990;71:29–37. [PMC free article] [PubMed] [Google Scholar]
- 29.Jungi TW, Rüegg SJ, Morell A. Interferon gamma-treated human macrophages display enhanced cytolysis and generation of reactive oxygen metabolites but reduced ingestion upon Fc receptor triggering. Hum Immunol. 1989;24:77–93. doi: 10.1016/0198-8859(89)90049-9. [DOI] [PubMed] [Google Scholar]
- 30.Wymann MP, von Tscharner V, Deranleau DA, Baggiolini M. The onset of the respiratory burst in human neutrophils. Real-time studies of H2O2 formation reveal a rapid agonist-induced transduction process. J Biol Chem. 1987;262:12048–53. [PubMed] [Google Scholar]
- 31.Landmann R, Scherer F, Schumann R, Link S, Sansano S, Zimmerli W. LPS directly induces oxygen radical production in human monocytes via LPS binding protein and CD14. J Leukoc Biol. 1995;57:440–9. doi: 10.1002/jlb.57.3.440. [DOI] [PubMed] [Google Scholar]
- 32.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beutler B, Du Hoebe KX, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2004;74:479–85. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 35.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 36.Werling D, Hope JC, Howard CJ, Jungi TW. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunology. 2003;111:41–52. doi: 10.1111/j.1365-2567.2003.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto M, Funami K, Tanaba M, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–62. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 38.Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173:1179–83. doi: 10.4049/jimmunol.173.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 40.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375–9. doi: 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 41.Ellingsen E, Morath S, Flo T, et al. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med Sci Monit. 2002;8:BR149–56. [PubMed] [Google Scholar]
- 42.Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–9. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- 43.Flo TH, Halaas O, Lien E, et al. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–9. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 44.Travassos LH, Girardin SE, Philpox DJ, et al. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004;5:1000–6. doi: 10.1038/sj.embor.7400248. [DOI] [PMC free article] [PubMed] [Google Scholar]