Abstract
Acetylsalicylic acid (aspirin) is one of the most widely used drugs worldwide, due mainly to its broad therapeutic spectrum with anti-inflammatory, antipyretic, antithrombotic and analgesic effects. However, the exact mechanisms by which aspirin influences inflammation, pain and immune system activation are only partly understood. Within activation of the cellular immune system, Th1-type cytokine interferon (IFN)-γ induces enzyme indoleamine-2,3-dioxygenase (IDO) which converts tryptophan to kynurenine. In parallel, IFN-γ induces enzyme GTP-cyclohydrolase I, which gives rise to neopterin production by activated human macrophages. Similarly, tryptophan degradation and neopterin formation increase during several disease states involving Th1-type immune activation. Using stimulated human peripheral blood mononuclear cells (PBMC), the effect of aspirin on tryptophan degradation and neopterin production was investigated. Stimulation of PBMC with mitogens concanavalin A, phytohaemagglutinin and pokeweed mitogen induced significant tryptophan catabolism as was reflected by a decline in tryptophan levels and a parallel increase in kynurenine concentrations compared with unstimulated cells. In parallel, neopterin production was enhanced. Treatment of stimulated PBMC with increasing doses of 1–5 mM aspirin significantly decreased stimulation-induced tryptophan degradation and neopterin production as well. All the effects of aspirin were dose-dependent. The parallel influence of aspirin on both biochemical pathways implies that there was no direct inhibitory effect of aspirin on IDO; rather, it inhibits production of IFN-γ in mitogen-treated PBMC. The influence of aspirin on biochemical pathways induced by IFN-γ may represent an important part of its broad pharmacological effect.
Keywords: aspirin; indoleamine (2,3)-dioxygenase (IDO); neopterin; tryptophan
Introduction
Acetylsalicylic acid (aspirin) is one of the most widely used drugs worldwide, due mainly to its broad therapeutic spectrum with anti-inflammatory, antipyretic, antithrombotic and analgesic effects [1]. However, the exact mechanisms by which aspirin influences inflammation, pain and immune system activation are only partly understood. It is well known that by acetylation of the enzymes cyclooxygenase I and II the formation of prostaglandins is irreversibly blocked, but there are also other mechanisms of action of aspirin and salicylates [2]. In low millimolar ranges, aspirin and its derivatives interfere with intracellular signalling pathways, e.g. NF-κB [3,4], also leading among other effects to the modulation of cytokine production [5]. Thus, the modulation of cytokine balance could contribute to the efficacy of aspirin.
Within cell-mediated immune response, indoleamine (2,3)-dioxygenase (IDO) is activated in human monocyte-derived macrophages upon stimulation with interferon (IFN)-γ[6], and IDO converts tryptophan to kynurenine. To estimate IDO activity, the ratio of kynurenine to tryptophan concentrations (kyn/trp) can be used [7]. During immune response IDO seems to play a central role in the suppression of intracellular bacteria and viruses, as ongoing tryptophan degradation leads to deprivation of this essential amino acid [8]. Also T cell proliferation is inhibited efficiently by IDO [9]. In normal murine pregnancy, IDO was identified as a key immunosuppressive mechanism, and inhibition of IDO leads to immediate rejection of an allogeneic fetus [10]. Accordingly, tryptophan degradation was found earlier to parallel the normal course of pregnancy in healthy women [11] and in the course of several clinical conditions in which an adaptive immune response is activated [6], including HIV infection [7] and rheumatoid arthritis [12]. IFN-γ in parallel to IDO induces enzyme GTP-cyclohydrolase I, which gives rise to enhanced neopterin formation in human macrophages [13]. Accordingly, increased IDO activity as indicated by increased kyn/trp usually correlates closely with neopterin concentrations in various human diseases [6,7,12] and during pregnancy [11]. In this study, the effect of aspirin on tryptophan degradation and neopterin production in stimulated human peripheral blood mononuclear cells (PBMC) was investigated.
Materials and methods
Cell culture
PBMC were isolated from whole blood obtained from healthy voluntary blood donors by density centrifugation (Lymphoprep, Nycomed Pharma AS, Oslo, Norway). Cells were maintained in RPMI-1640 (PAA-Laboratories, Linz, Austria) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Biochrom, Berlin, Germany), 2 mM l-glutamine (Serva, Heidelberg, Germany) and 50 µg/ml gentamycin (Bio-Whitaker, Walkersville, MD, USA).
For stimulation cells were seeded at a density of 1 × 106/ml and stimulated with different concentrations of mitogens concanavalin A (Con A), phytohaemagglutinin (PHA) and pokeweed mitogen (PWM; all from Sigma, Vienna, Austria) to induce tryptophan degradation. PBMC were preincubated with aspirin (0·14–0·7 mg/ml) for 2 h, and thereafter cells were stimulated with mitogens and incubated at 37°C in 5% CO2 for 72 h. Supernatants were harvested by centrifugation (400 g, 4°C, 8 min) and frozen at −20°C until measurement. Cell cycle and apoptosis were monitored by FACS-analysis. In addition, another set of three experiments was performed in which no preincubation of PBMC was carried out; instead, cells were first stimulated with mitogens and aspirin was added after 2 h. Each experiment was performed using blood from three different healthy donors, and experiments were run with two parallels each.
Measurements
To determine IDO activity, tryptophan and kynurenine concentrations were measured in supernatants using high performance liquid chromatography (HPLC) on reversed phase [14], and kyn/trp was calculated. Experiments were performed three times with duplicates. For comparison of grouped data, the Mann–Whitney-U-test was applied and P-values <0·05 were considered to indicate significant differences. Neopterin concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (Brahms Diagnostica, Berlin, Germany) according to the manufacturer's instructions with a detection limit of 2 nM.
Statistical analysis
To correct for slight interindividual differences between donors, results are presented as fold of the unstimulated control of each experiment. For comparisons of grouped data the Mann–Whitney-U-test was applied. P-values <0·05 were considered to indicate significant differences.
Results
In PBMC stimulated with mitogens Con A and PHA, enhanced degradation of tryptophan was observed. In comparison with untreated cells, supernatants of stimulated PBMC contained significantly lower tryptophan concentrations (P < 0·01 for all stimuli, Fig. 1). When stimulated with 10 µg/ml PHA, after 72 h tryptophan concentrations were even below the limit of detection of the method used (<0·2 µM). In contrast, kynurenine concentrations increased significantly in stimulated cells (P < 0·01, details not shown). Accordingly, kyn/trp was also higher in stimulated than in unstimulated PBMC (P < 0·01 for all stimuli, Fig. 2). Neopterin concentrations also increased in cells stimulated with mitogens (P < 0·01 for all stimuli: Fig. 3).
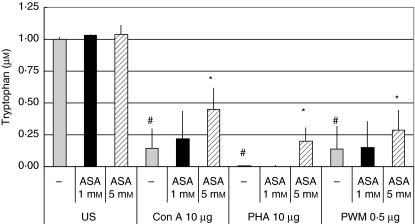
Fig. 1.
Tryptophan concentrations (µM) in unstimulated peripheral blood mononuclear cells (US) and in cells stimulated with 10 µg/ml phytohaemagglutinin (PHA), 10 µg/ml concanavalin A (Con A) and 0·5 µg/ml pokeweed mitogen (PWM) treated with increasing doses of aspirin. Tryptophan concentrations are shown as percentage of baseline in unstimulated cells; columns show ± s.e.m. of three experiments run in duplicate, total number of data included in each column = 6; *P < 0·01 compared to cells stimulated by the corresponding mitogen but without aspirin treatment; #P < 0·01 compared to untreated cells).
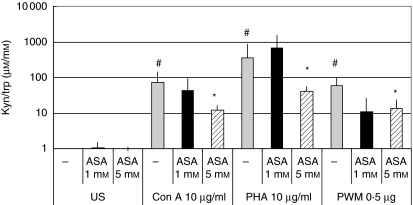
Fig. 2.
Ratio of kynurenine to tryptophan concentrations (µmol/mmol) to estimate activity of indoleamine (2,3)-dioxygenase in unstimulated peripheral blood mononuclear cells (US) and in cells stimulated with 10 µg/ml phytohaemagglutinin (PHA), 10 µg/ml concanavalin A (Con A) and 0·5 µg/ml pokeweed mitogen (PWM) treated with increasing doses of aspirin. Kyn/trp is shown as x-fold change from baseline in unstimulated cells; columns show mean values ± s.e.m. of three experiments run in duplicate, total number of data included in each column = 6; *P < 0·01 compared to cells stimulated by the corresponding mitogen but without aspirin treatment; #P < 0·01 compared to untreated cells).
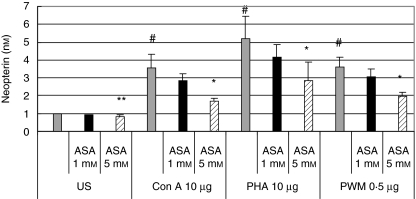
Fig. 3.
Neopterin concentrations (nM) in unstimulated peripheral blood mononuclear cells (US) and in cells stimulated with 10 µg/ml phytohaemagglutinin (PHA), 10 µg/ml concanavalin A (Con A) and 0·5 µg/ml pokeweed mitogen (PWM) treated with increasing doses of aspirin. Columns show x-fold changes from baseline in unstimulated cells; mean values + s.e.m. of three experiments run in duplicate, total number of data included in each column = 6; *P < 0·01 compared to cells stimulated with the corresponding mitogen but without aspirin treatment, #P < 0·01 compared to unstimulated cells, **P < 0·05 compared to the unstimulated control without aspirin treatment).
Pretreatment of cells with 1–5 mM aspirin only slightly increased tryptophan metabolism in resting cells: tryptophan concentrations were slightly higher compared to untreated cells (Fig. 1); kynurenine concentrations did not show any difference compared to untreated cells. Neopterin concentrations, on the other hand, were lower in cells treated with 5 mM aspirin (P < 0·05; Fig. 3). In contrast, in PBMC stimulated with mitogens both effects of the enhanced tryptophan degradation and neopterin production were influenced by aspirin in a dose-dependent manner: whereas 1 mM aspirin had only a slight effect on tryptophan concentrations and did not change kynurenine concentrations (Figs 1 and 2), pretreatment with 3 mM aspirin tended to decrease tryptophan degradation and neopterin formation, but changes observed in kynurenine and tryptophan concentrations still did not reach the level of significance. Preincubation of cells with 5 mM, however, resulted in a significant increase of tryptophan concentrations compared to stimulated cells (P < 0·01, Fig. 1) and kyn/trp and neopterin concentrations decreased significantly (P < 0·01, Figs 2 and 3). Nearly the same effects were seen in PBMC stimulated with PHA, Con A or PWM: preincubation of cells with aspirin decreased stimulation-induced tryptophan degradation and neopterin formation significantly independently from the mitogen used. If doses higher than 3 mM were used, elevated kyn/trp in supernatants of mitogen-stimulated PBMC decreased, and the decreased tryptophan concentrations increased when cells were treated with 5 mM aspirin in addition to mitogens (Figs 1 and 2).
When PBMC were not preincubated with aspirin, but aspirin was added 2 h after stimulation of cells, results observed were similar to those presented above: again tryptophan degradation and neopterin production was found in stimulated cells, and both biochemical effects could be inhibited by 5 mM aspirin (data not shown). The inhibitory effect of aspirin was less expressed than in the preincubation experiments. Tryptophan concentrations tended to be higher and kyn/trp to be lower (both P < 0·1) in cells treated with aspirin, and also neopterin formation was inhibited significantly in all experiments with 5 mM aspirin (P < 0·05).
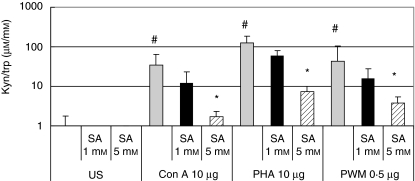
When PBMC were treated with salicylic acid, effects on tryptophan degradation and neopterin formation similar to aspirin were observed. In mitogen-stimulated cells, 5 mM salicylic decreased tryptophan degradation significantly (P < 0·01 for decline of kyn/trp, Fig. 4; P < 0·01 for increase of tryptophan); neopterin formation was lower in all experiments (P < 0·05).
Fig. 4.
Ratio of kynurenine to tryptophan concentrations (µmol/mmol) to estimate activity of indoleamine (2,3)-dioxygenase in unstimulated peripheral blood mononuclear cells (US) and in cells stimulated with 10 µg/ml phytohaemagglutinin (PHA), 10 µg/ml concanavalin A (Con A) and 0·5 µg/ml pokeweed mitogen (PWM) treated with increasing dose of salicylic acid. Kyn/trp is shown as x-fold change from baseline in unstimulated cells; columns show mean values + s.e.m. of three experiments run in duplicate, total number of data included in each column = 6; *P < 0·01 compared to cells stimulated by the corresponding mitogen but without salicylic acid treatment; #P < 0·01 compared to the unstimulated control.
Discussion
Our study demonstrates suppression of stimulation-induced tryptophan degradation and neopterin production in human PBMC by aspirin. Whereas aspirin did not influence tryptophan metabolism in resting immunocompetent cells, tryptophan degradation and neopterin production was suppressed in stimulated PBMC that were treated with aspirin in comparison with untreated stimulated cells. Cells were stimulated with high doses of mitogens, which are known to induce high-level production of IFN-γ, and all mitogens, PHA, Con A and PWM, induced tryptophan degradation and neopterin production to a similar extent. The concurrent increase of kyn/trp and neopterin production points to the view that IDO, activated by IFN-γ, is involved in changes of tryptophan metabolism in stimulated PBMC.
Whereas lower concentrations of aspirin only slightly influenced tryptophan degradation and neopterin formation, 5 mM aspirin was able to inhibit both processes in parallel. Consequently, data point to a modulating effect of aspirin on the expression of the cytokine IFN-γ, which induces tryptophan degradation via activation of IDO as well as neopterin production by triggering GTP-cyclohydrolase I [6]. In line with these findings, aspirin has been demonstrated previously to decrease the formation of interleukin-12, the cytokine, which induces the formation of IFN-γ[5].
In our investigation, salicylic acid at similar concentrations to aspirin suppressed tryptophan degradation and neopterin production in stimulated PBMC. Our data on PBMC concur with earlier studies in other cells, showing that treatment with non-steroidal anti-inflammatory drugs influences tryptophan metabolism [15,16]. Non-steroidal anti-inflammatory drugs, including aspirin, represent an important feature of basic treatment in various groups of patients suffering from chronic and inflammatory conditions [17], which go along with elevated concentrations of proinflammatory cytokines [18,19]. A modulating effect of aspirin on the production of cytokine IFN-γ has also been demonstrated recently in patients with giant cell arteritis [20]. Inhibition of cytokine cascades may be part of the beneficial effect of aspirin in such diseases in addition to the well-established effect of aspirin to block enzymes cyclo-oxygenases I and II by acetylation, thereby diminishing the formation of prostaglandins.
The well-known influence of aspirin on cyclo-oxygenases is unlikely to explain our findings. Rather, other mechanisms of aspirin and salicylates will be important, such as interference with intracellular signalling pathways, e.g. NF-κB [3,4,21], modulating cytokine production [5]. An earlier suppressive effect on tryptophan degradation and neopterin production in stimulated PBMC was found for plants and beverages containing large amounts of antioxidants [22,23]. Thus, antioxidative and radical-scavenging activity of salicylic acid and aspirin [24,25] could be important for their down-modulatory effect on IFN-γ production in stimulated PBMC.
Aspirin is also known to occasionally elicit pseudo-allergic reactions [26]. This phenomenon could also relate to the activity of aspirin to down-regulate IFN-γ. IFN-γ is a major suppressor of Th2-type cytokines which may, however, trigger allergic reactions once their suppression is abrogated [27]. However, it cannot be excluded that aspirin may also modulate release of Th2-type cytokines; this remains to be elucidated in further studies.
During pregnancy, activation of IDO trophoblasts was found to be critical for tolerance induction [10,28]. Our data, together with data from the literature, suggest that aspirin is able to counteract IFN-γ production and consequently suppression of IDO activity in several cells in which IDO is inducible by IFN-γ. Because activation of IDO was found to be crucial for successful pregnancy in mice, the inhibitory effect of aspirin on IDO activity might be important in human reproductive immunology as well; for example, it could be involved in the increased risk of miscarriage, which has been observed in a population-based cohort study [29]. Further studies are necessary to confirm this relationship and to what extent our in vitro findings can be extrapolated to the in vivo situation.
Acknowledgments
This study was supported by the Austrian Federal Ministry of Social Affairs and Generations.
References
- 1.Mehta P. Aspirin in the prophylaxis of coronary artery disease. Curr Opin Cardiol. 2002;17:552–8. doi: 10.1097/00001573-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–72. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 3.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 4.Muller DN, Heissmeyer V, Dechend R, et al. Aspirin inhibits NF-kappaB and protects from angiotensin II-induced organ damage. FASEB J. 2001;15:1822–4. doi: 10.1096/fj.00-0843fje. [DOI] [PubMed] [Google Scholar]
- 5.Mazzeo D, Panina-Bordignon P, Recalde H, Sinigaglia F, D’Ambrosio D. Decreased IL-12 production and Th1 cell development by acetyl salicylic acid-mediated inhibition of NF-Kappab. Eur J Immunol. 1998;28:3205–13. doi: 10.1002/(SICI)1521-4141(199810)28:10<3205::AID-IMMU3205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Wirleitner B, Neurauter G, Schröcksnadel K, Frick B, Fuchs D. Interferon-γ-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–91. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs D, Möller AA, Reibnegger G, Stöckle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr. 1990;3:873–6. [PubMed] [Google Scholar]
- 8.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci. 1984;81:908–12. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 11.Schroecksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996;88:47–50. doi: 10.1016/0029-7844(96)00084-1. [DOI] [PubMed] [Google Scholar]
- 12.Schroecksnadel K, Kaser S, Ledochowski M, et al. Increased degradation of tryptophan in blood of patients with rheumatoid arthritis. J Rheumatol. 2003;30:1935–9. [PubMed] [Google Scholar]
- 13.Huber C, Batchelor JR, Fuchs D, et al. Immune response-associated production of neopterin – release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–16. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–6. [PubMed] [Google Scholar]
- 15.Sayama S, Yoshida R, Oku T, Imanishi J, Kishida T, Hayaishi O. Inhibition of interferon-mediated induction of indoleamine 23-dioxygenase in mouse lung by inhibitors of prostaglandin biosynthesis. Proc Natl Acad Sci. 1981;78:7327–30. doi: 10.1073/pnas.78.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents of induction of indoleamine 23-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987;144:1147–53. doi: 10.1016/0006-291x(87)91431-8. [DOI] [PubMed] [Google Scholar]
- 17.Mandell BF. Trends in rheumatic disease: update on new diagnostic and treatment strategies. Clevel Clin J Med. 2001;68:425–32. doi: 10.3949/ccjm.68.5.425. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA. Inflammatory cytokines: interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr Opin Immunol. 1991;3:941–8. doi: 10.1016/s0952-7915(05)80018-4. [DOI] [PubMed] [Google Scholar]
- 19.Isomaki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997;29:499–507. doi: 10.3109/07853899709007474. [DOI] [PubMed] [Google Scholar]
- 20.Weyand CM, Kaiser M, Yang H, Younge B, Goronzy J. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum. 2002;46:457–66. doi: 10.1002/art.10071. [DOI] [PubMed] [Google Scholar]
- 21.Shackelford RE, Alford PB, Xue Y, Thai SF, Adams DO, Pizzo S. Aspirin inhibits tumor necrosis factor alpha gene expression in murine tissue macrophages. Mol Pharmacol. 1997;52:421–9. doi: 10.1124/mol.52.3.421. [DOI] [PubMed] [Google Scholar]
- 22.Zvetkova E, Wirleitner B, Tram NT, Schennach H, Fuchs D. Aqueous extracts of Crinum latifolium (L) and Camellia sinensis show immunomodulatory properties in human peripheral blood mononuclear cells. Intern Immunopharmacol. 2001;1:2143–50. doi: 10.1016/s1567-5769(01)00140-0. [DOI] [PubMed] [Google Scholar]
- 23.Winkler C, Wirleitner B, Schreocksnadel K, Schennach H, Mur E, Fuchs D. In vitro effects of two extracts and two purealkaloid preparations of Uncaria tomentosa on peripheral blood mononuclear cells. Plant Med. 2004;70:205–10. doi: 10.1055/s-2004-815536. [DOI] [PubMed] [Google Scholar]
- 24.Maskos Z, Rush JD, Koppenol WH. The hydroxylation of the salicylate anion by a Fenton reaction and T-radiolysis: a consideration of the respective mechanisms. Free Radic Biol Med. 1990;8:153–62. doi: 10.1016/0891-5849(90)90088-z. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn W, Muller T, Büttner T, Gerlach M. Aspirin as a free radical scavenger: consequences for therapy of cerebrovascular ischemia. Stroke. 1995;26:1959–60. [PubMed] [Google Scholar]
- 26.Szczeklik A. Adverse reactions to aspirin and nonsteroidal anti-inflammatory drugs. Ann Allergy. 1987;59:113–8. [PubMed] [Google Scholar]
- 27.Adorini L, Guery JC, Trembleau S. Manipulation of the Th1/Th2 cell balance: an approach to treat human autoimmune diseases? Autoimmunity. 1996;23:53–68. doi: 10.3109/08916939608995329. [DOI] [PubMed] [Google Scholar]
- 28.Hönig A, Rieger L, Kapp M, Sütterlin M, Dietl J, Kämmerer U. Indoleamine 23-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J Reprod Immunol. 2004;61:79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. Br Med J. 2003;327:368. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]