Abstract
Neutrophils can be primed by bacterial lipopolysaccharide (LPS) for an enhanced oxidative burst, which is a key element in the pathogenesis of Gram-negative sepsis. Some serum proteins (e.g. lipopolysaccharide-binding protein) avidly bind LPS and markedly enhance receptor binding and cellular activation while other serum factors (lipoproteins, bactericidal/permeability-increasing protein) neutralize LPS and prevent neutrophil activation. In this paper we examined the kinetics of this priming reaction in whole blood. To study the balance between neutrophil activation and LPS neutralization a sensitive chemiluminescence assay was used in a whole blood system. LPS was able to prime neutrophils for enhanced oxidative burst in whole blood with an optimum incubation time of 25 min. However, LPS was neutralized very rapidly with a t1/2 of 10 min. After 20 min a second priming factor was already generated, which was shown to be monocyte-derived tumour necrosis factor (TNF).
Keywords: chemiluminescence, endotoxin, neutralization, neutrophils, priming, whole blood
Introduction
In Gram-negative infections, lipopolysaccharide (LPS, endotoxin), a constituent of the outer membrane of Gram-negative bacteria, can initiate a cascade of inflammatory mediators that can lead to systemic inflammation [1]. Neutrophils play a key role in tissue injury during systemic inflammation [2]. The activation of neutrophils by LPS is mediated by CD14, a 55-kDa glycosylphosphatidylinositol (GPI)-linked cell surface receptor found on monocytes/macrophages and neutrophils [3]. Lipopolysaccharide binding protein (LBP), a 60 KD serum glycoprotein, binds to the toxic lipid A moiety of bacterial lipopolysaccharides and markedly facilitates the effect of LPS on leucocytes [4]. Recently, Toll like receptor-4 (TLR4) was found to mediate the intracellular signalling after LPS binding to CD14 [5].
In contrast, other serum factors and membrane receptors have been identified which bind LPS and neutralize its toxic activity. Lipoproteins, apolipoprotein A-1, apolipoprotein B, lactoferrin, bactericidal/permeability increasing protein (BPI), soluble CD14 (sCD14) and receptors on macrophages (scavenger receptors, CD11/CD18 receptors) have all been implicated in the clearance and detoxification of LPS [6–10].
In this paper we describe the dynamic interaction between LPS and neutrophils in the presence of these serum factors. A whole blood assay was used as an ex vivo model to mimic the pathophysiological conditions found in vivo. In addition, a very sensitive luminol enhanced chemiluminescence (CL) assay was used [11] to measure neutrophil priming for enhanced oxidative burst by LPS at concentrations in the picogram range, amounts usually found in patients with septic shock [12,13].
Materials and methods
LPS was extracted from Salmonella typhimurium M206 by the hot phenol–water method according to Galanos, as described previously [14]. Human recombinant tumour necrosis factor (TNF)-α was obtained from Sigma Chemicals Co., St Louis, MO, USA. Polymyxin B sulphate (Sigma Chemicals Co.) and anti-human TNF monoclonal antibody (E11, ECACC no. 92030602) [15] were added to the test samples at a final concentration of 10 µg/ml and incubated for 30 min on ice. In control experiments, LPS (1 ng/ml) and TNF (1 n m) were incubated with polymyxin B and anti-TNF for 15 min at room temperature. Anti-CD14 monoclonal antibody 60bca (ATCC, Rockville, MD, USA) was added to blood samples at a final concentration of 10 µg/ml and incubated for 30 min at room temperature. For each experiment fresh venous blood was obtained with informed consent from healthy donors. For collection of anticoagulated blood, glass tubes with a pyrogen free anticoagulant were used. Plain pyrogen free glass tubes were used to isolate serum. For complement inactivation, serum was heated for 30 min at 56°C.
Whole blood priming
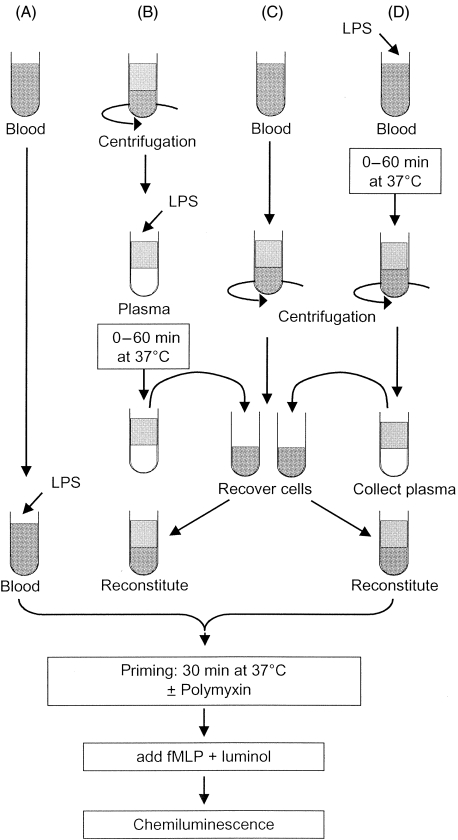
EDTA anticoagulated blood was kept on a nutator at room temperature and used within 2 h. To prime neutrophils in whole blood, 50 µl LPS (1 ng/ml) or TNF (1 n m) was added to 200 µl whole blood and incubated for 30 min at 37° on a shaking platform (protocol A in Fig. 1). To distinguish between cellular and plasma derived mediators, blood was separated into plasma and leucocytes by centrifugation for 10 min at 300 g. Packed leucocytes and erythrocytes were washed twice with pyrogen free phosphate-buffered saline (PBS) to remove remaining plasma (protocol C in Fig. 1). LPS was incubated with cell free plasma (or serum) and subsequently reconstituted with the washed leucocytes to the original blood volume to establish neutrophil priming (protocol B in Fig. 1) Additionally, to measure cell derived soluble priming factors, LPS was first incubated with whole blood at 37°C and then centrifuged for 10 min at 300 g. The leucocyte free plasma was reconstituted with fresh packed leucocytes to establish priming of naive neutrophils in their original blood volume (protocol D in Fig. 1).
Fig. 1.
Layout for the different experimental settings. All experimental conditions eventually lead to a tube containing complete whole blood with cells and plasma that are incubated for 30 min at 37°C to allow priming. Samples are diluted in luminol containing buffer and stimulated with 1 µm fMLP to measure the chemiluminescence response continuously for 10 min. The area under the curve is calculated and the LPS-induced priming is expressed relative to buffer treated samples. Different experimental settings are used to distinguish the effect of plasma and/or the cells in the ability to prime fresh cells. Therefore, whole blood is separated into cells and plasma before or after the challenge with LPS and reconstituted subsequently with fresh leucocytes with or without polymyxin to neutralize remaining LPS molecules. (A) Assay of LPS activity in whole blood, (B) assay of LPS activity after incubation with plasma and (D) assay of LPS activity after the incubation with whole blood. (C) Generation of unstimulated leucocytes in packed cells for reconstitution.
Leucocyte priming
Blood was collected in heparin anticoagulated tubes and mixed with dextran T70 to a final concentration of 0·6% to obtain leucocytes. After sedimentation for 30 min at room temperature, leucocyte-rich plasma was withdrawn, centrifuged and subjected to hypotonic lysis with pyrogen free aqua dest to lyse residual erythrocytes. After a final wash with RPMI (Gibco BRL, Gaithersburg, MD, USA) plus 0·05% human serum albumin (HSA; Central Laboratory for Blood Transfusion, Amsterdam, the Netherlands), leucocytes were suspended in RPMI/HSA at a concentration of 5 × 106 cells/ml. For priming, LPS was added together with plasma or serum at varying concentrations.
To eliminate monocytes from the leucocyte fraction, M450 magnetic beads coated with a rabbit antimouse IgG antibody (DYNAL) were loaded with purified anti-CD14 MoAb 60bca at 10 µg per 107 beads for 60 min at room temperature in PBS. Beads were washed three times with PBS containing 1% bovine serum albumin (BSA) for 30 min and stored in PBS/azide at 4°C. An aliquot of the anti-CD14 beads were pelleted with a magnet and mixed with isolated leucocytes for 10 min at room temperature on an end-over-end rotator. Beads were pelleted rapidly and the supernate of cells removed. Cell preparations were analysed for purity by cytospin preparations and by analysis with anti-CD45-FITC and anti-CD14-PE (Becton Dickinson, Franklin Lakes, NJ, USA) on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems).
Neutrophils were isolated from heparin-anticoagulated blood by a Ficoll-Histopaque gradient centrifugation as previously described [11]. Priming experiments were performed as described above.
Chemiluminescence
After the priming of neutrophils in whole blood or as isolated cells the fMLP-induced oxidative burst was measured as luminol-dependent chemiluminescence in a thermocontrolled luminometer (Autolumat LB 953, Berthold GmbH & Co., Wildbad, Germany) as described previously [16]. In brief, the samples were diluted 10-fold and 100 µl transferred to the luminometer tubes. To each sample luminol was added automatically and the reaction started by the injection of fMLP to a final concentration of 1 µm. The amount of light produced during 20 min was calculated from the area under the curve (AUC). The results from the test samples were expressed either as ‘factor increase in chemiluminescence’ by dividing the results from the test sample by the result from the control (PBS) sample, or as a percentage of priming capacity by comparing the results from the test samples with the results from the negative control (PBS) sample and the positive control sample (LPS), the value of which were set at 0% and 100%, respectively.
LPS and TNF determinations
LPS concentrations were determined by a chromogenic limulus amebocyte lysate (LAL) assay (Chromogenix, Mölndal, Sweden) according to the manufacturer's instructions. An additional standard curve with S. typhimurium M206 LPS was run in parallel with the O55 LPS provided by the manufacturer. Values were calculated according to the M206 LPS standard curve. For quantitative measurements of TNF levels, a TNF sandwich enzyme-linked immunosorbent assay (ELISA) was used as described previously [17].
Statistical analysis
Values are expressed as mean ± s.e.m. The data were analysed with Student's t-test using spssversion 11·0 (SPSS Inc., Chicago, IL, USA). A P-value of <0·05 was considered significant.
Results
Neutrophil priming by LPS in whole blood ex-vivo
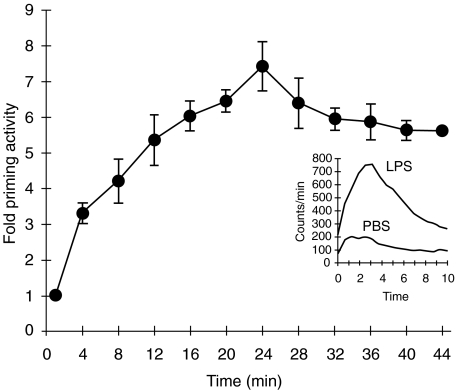
Incubation of whole blood with solubilized LPS extracted from S. typhimurium M206 for 30 min at 37°C resulted in a dose-dependent enhancement of the fMLP-triggered chemiluminescence response of neutrophils with an optimal response after 30 min incubation (Fig. 2). Because no cell separation is required and only a small amount of blood is required, this method is an easy and sensitive assay for neutrophil priming by LPS. In addition, possible priming due to cell isolation procedures is avoided. Consequently, LPS concentrations as low as 10 pg/ml already generate a significantly enhanced CL response. Both heparin and EDTA anticoagulated blood can be used and provide comparable results. It should be noted that some commercially available blood-collecting tubes with heparin are contaminated with a pyrogen.
Fig. 2.
LPS time-dependently primes the neutrophil oxidative burst in whole blood. EDTA anticoagulated blood was incubated with 1 ng/ml LPS for different time periods. Blood was diluted and the oxidative burst was measured as luminol-enhanced chemiluminescence after stimulation with fMLP (1 µm). Samples were measured continuously for 10 min and the amount of chemiluminescence was quantified as area under the curve (AUC). Data are expressed as factor increase in chemiluminescence relative to the AUC at t = 0 for blood without LPS preincubation (mean ± s.e.m., n = 3). The inset shows a representative example of the chemiluminescence of blood preincubated with PBS or LPS for 30 min.
Inhibition by polymyxin B, anti-CD14 and anti-TNF MoAb
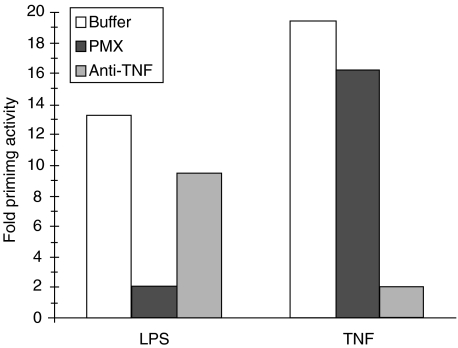
Preincubation of LPS with polymyxin B completely abolished the LPS-mediated priming up to concentrations of 1 ng/ml. A LPS concentration of 10 ng/ml is still 80% inhibited by 10 µg/ml polymyxin B (Fig. 3). Additional experiments have shown that polymyxin B, in the concentration that we used, had no effect on the oxidative burst of neutrophils (data not shown). The LPS-mediated priming is CD14-dependent, because a specific anti-CD14 MoAb (60bca) completely blocks the priming with LPS (data not shown). Priming with TNF is not affected by MoAb 60bca, indicating that the MoAb does not interfere with neutrophil function in general but is specific for LPS-mediated priming under these conditions in a whole blood system. Furthermore, an anti-TNF MoAb (E11) almost completely blocks TNF-mediated neutrophil priming, while it hardly interferes with LPS-mediated neutrophil priming (Fig. 3). It should be noted that the MoAbs were purified and stored without azide as preservative. Azide effectively inhibits myeloperoxidase (MPO), a haem containing protein necessary for luminol-enhanced CL response in neutrophils.
Fig. 3.
Polymyxin B and anti-TNF monoclonal antibody specifically inhibit LPS- and TNF-induced priming of neutrophils in whole blood. EDTA anticoagulated blood was incubated with LPS (1 ng/ml) or recombinant TNF (10 n m) after preincubation with polymyxin B (10 µg/ml), anti-TNF monoclonal antibody (10 µg/ml) or PBS for 15 min. After 30 min incubation, the fMLP-induced chemiluminescence response was measured. Data are expressed as fold priming activity compared to buffer treated cells.
LPS stability in whole blood
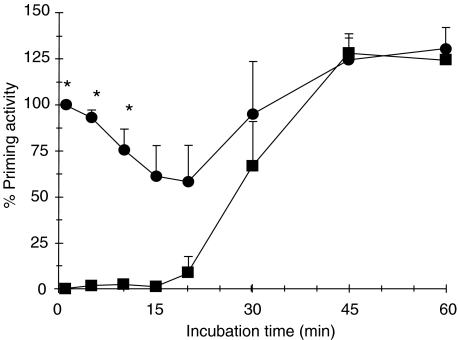
Plasma samples from LPS-stimulated blood are still able to prime fresh neutrophils (Fig. 4). However, a non-LPS soluble component is generated after 20 min, which efficiently primes fresh neutrophils. Addition of polymyxin B to the plasma sample taken at time zero efficiency neutralizes LPS. The time zero sample reflects the standard priming procedure as shown in Fig. 2. The fact that the priming capacity of the plasma samples generated after a longer than 20-min incubation period of blood with LPS was not influenced by polymyxin B could indicate that (i) LPS is no longer available for direct neutrophil priming and the newly generated factor(s) has taken over completely, or (ii) LPS is bound to a factor rendering it unavailable for polymyxin B neutralization.
Fig. 4.
Loss of LPS priming capacity but concomitant generation of priming activity in blood incubated with LPS over time. Whole blood was incubated with 1 ng/ml LPS for different time periods. Thereafter the cell free supernate was recovered and tested for priming activity by reconstitution of the plasma samples with unstimulated leucocyte pellet from fresh whole blood (•). Half the supernate sample was treated with 10 µg/ml polymyxin B to neutralize LPS before reconstitution with leucocyte pellet (▪). After 30 min incubation (optimal priming period), the fMLP-induced chemiluminescence response was measured. Data are shown as percentage priming activity, relative to the chemiluminescence of fresh whole blood primed with LPS and after subtraction of the value for unstimulated fresh whole blood (mean ± SEM). *Denotes significant difference (P < 0·05).
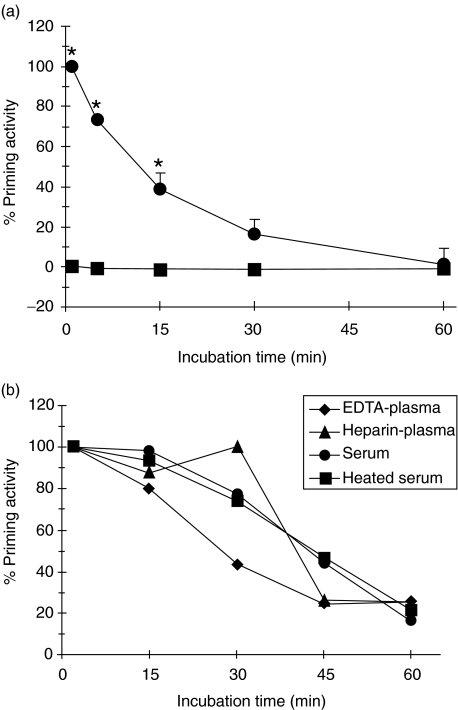
LPS stability in plasma
Preincubation of LPS with plasma results in a time-dependent decline in neutrophil priming capacity (Fig. 5a). Both heparin and EDTA plasma as well as serum and complement-inactivated serum caused this LPS neutralization with a comparable time-course, indicating that complement was not involved (Fig. 5b). This apparent neutralization of LPS bioactivity in plasma or serum over time was demonstrated further by the parallel inability of these samples to stimulate TNF production in monocytes. The LPS molecule is still present, as shown by the ability to activate LAL after heating the samples for 5 min at 75°C according to the manufacturer's instructions (data not shown). The priming inability of the plasma-treated LPS samples is not due to the inactivation of LBP, because addition of fresh LPS just prior to leucocyte priming results in a full response equivalent to the time zero sample (data not shown).
Fig. 5.
Cell-free plasma rapidly neutralizes LPS priming capacity. (a) LPS (1 ng/ml) was added to EDTA plasma and incubated for different time periods at 37°C. The samples were reconstituted with pelleted leucocytes obtained from fresh whole blood (•) and half of each sample was mixed with 10 µg/ml polymyxin B to bind LPS (▪). After a fixed 30 min incubation at 37°C to allow priming, the fMLP-induced chemiluminescence response was measured. Data are shown as percentage priming activity (mean ± s.e.m., n = 3). (b) A representative experiment shows the comparable LPS neutralization of EDTA plasma, Heparin plasma, serum and heated serum (30 min at 56°C) from a single donor after reconstitution with autologous leucocytes obtained from EDTA blood. Results are normalized to the initial LPS priming response (t = 0) for each plasma source. *Denotes significant difference (P < 0·05).
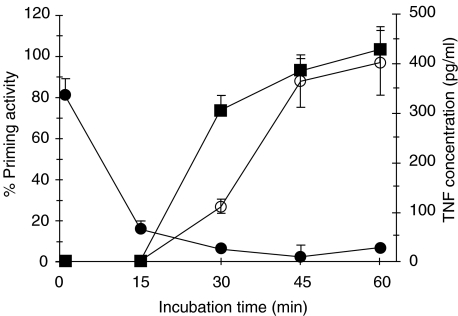
TNF as secondary mediator for neutrophil priming
TNF was considered as a possible candidate for priming factor. It is well known that LPS stimulation of monocytes initiates the production and release of TNF, which efficiently primes the neutrophil oxidative burst. The addition of a anti-TNF MoAb (E11) completely blocked the priming capacity of the plasma samples over time, and shows the inverse pattern of the polymyxin B-containing samples (Fig. 6).
Fig. 6.
Prolonged neutrophil priming ability of LPS-challenged blood is due to the rapid generation of TNF. Whole blood was challenged with 1 ng/ml LPS for increasing periods of time and subsequently centrifuged to recover the cell-free plasma. The plasma was reconstituted with pelleted leucocytes obtained from fresh blood and divided into two separate tubes: one was mixed with 10 µg/ml polymyxin B (PMX) (▪), and the other with 20 µg/ml anti-TNF monoclonal antibody (•). All reconstituted samples were allowed to prime neutrophils for the fMLP-induced chemiluminescence response. Fresh whole blood served as the basal values and data were expressed as priming activity (mean ± s.e.m., n = 3–4). TNF concentrations were determined in the plasma recovered from whole blood challenged with LPS during different time periods. Levels were measured using a specific ELISA, and are expressed as pg/ml (○) (mean ± s.e.m., n = 3).
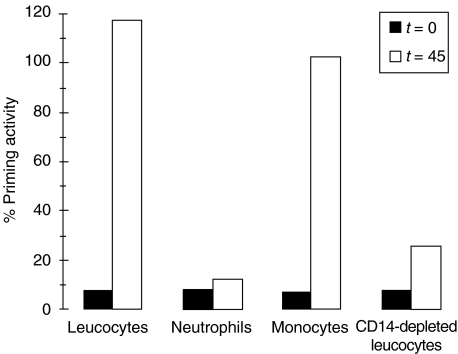
Monocytes as the source of TNF
Monocytes were the most probable source of the newly generated TNF, although most studies reported longer incubation periods to generate detectable TNF quantities. Elimination of monocytes from neutrophils by anti-CD14-coated beads or Ficoll gradient centrifugation abolishes the ability of LPS to induce TNF and generate LPS-independent priming (Fig. 7). To verify that TNF is released within the relatively short incubation period, cell-free plasma samples after LPS challenge of whole blood were analysed in a TNF-specific ELISA. As shown in Fig. 6, the amount of TNF released over time fits exactly the induction of non-polymyxin B inhibitable priming capacity.
Fig. 7.
Monocytes are the source for TNF in LPS-stimulated whole blood. To obtain different cell populations, total leucocyte fractions were obtained with dextran sedimentation of whole blood, while neutrophils and mononuclear cells were isolated from blood by Ficoll–Histopaque density gradient centrifugation. Monocytes were specifically depleted from the leucocyte fraction with anti-CD14 monoclonal antibody coated magnetic beads. The different cell fractions were challenged with 1 ng/ml LPS plus 1% NHS for 0 (black bars) and 45 min (open bars), centrifuged and the cell-free supernates reconstituted with fresh leucocytes in the presence of polymyxin B (to bind LPS) to determine the priming activity. Data from one typical experiment of three are given.
Discussion
LPS is an important triggering factor for the systemic inflammatory response in vivo[1]. LBP, an acute-phase protein in which plasma levels rise during inflammation, is an essential factor for recognition and response of CD14 bearing cells to LPS. LPS–LBP complexes prime the oxidative burst of neutrophils much more rapidly than LPS alone [18]. This implies that neutrophil activation can be achieved ex vivo, with LPS concentrations in the picogram-per-millilitre range, amounts usually found in vivo in patients with sepsis [12]. The fact that serum factors are important in mediating LPS-induced cellular activation makes whole blood a valuable system to measure neutrophil activation. In addition, this technique prevents the priming of neutrophils during the isolation procedure and provides a physiological environment where cellular interactions are preserved. It should be noted, however, that this system lacks the interaction with endothelial cells and the increase in serum acute phase proteins, factors essential for the inflammatory response and neutrophil activation in vivo.
Our results indicate that, in a whole blood system, neutrophils can be primed for enhanced oxidative burst with LPS concentrations in the picogram-per-millilitre range, with an optimal response after 30 min incubation. Interestingly, this system may be used to detect small amounts of LPS or other priming substances in patients at risk for developing a systemic inflammatory response syndrome, and may prove to be a valuable tool in patient management [19].
As shown earlier for TNF-α[3] and eicosanoid [20] production, neutrophil priming for enhanced oxidative burst in whole blood was also CD14-dependent. In addition, polymyxin B was able to completely abolish neutrophil priming. This implies the presence of both the toxic lipid A part of the LPS molecule and its subsequent binding to the CD14 receptor, for adequate neutrophil activation in whole blood. It must be noted that the presence of plasma in whole blood is a precondition for neutrophil priming at low LPS concentrations.
In contrast to the enhancement of LPS-induced neutrophil priming by plasma, it was already noted in 1957 that the febrile response of rabbits to LPS could be reduced by preincubating LPS with serum [21]. Subsequent studies, however, reported a 3–24-h incubation period with 20–25% serum for effective neutralization [22,23]. This study shows that in whole blood, serum or plasma LPS is neutralized within 60 min, with a t1/2 as short as 10–30 min. The difference in the neutralization kinetics between the different anticoagulants has been described previously, and has been ascribed to the effect of divalent cations (Mg2+, Ca2+) on the micellar confirmation of LPS [22–27]. Chelation of divalent cations by EDTA releases LPS from an aggregated micellar confirmation to a monomeric confirmation that may render it more susceptible for neutralization. The detection of LPS in the LAL assay after 60 min may be ascribed to the heating process of the samples before measurement, which may release LPS from its neutralizing serum factors. This implies that LPS is not degraded in whole blood or serum but rendered biologically unavailable for receptor binding and cellular activation. This may account for the variable correlation found between endotoxaemia measured by LAL assay and clinical outcome in patients with sepsis [13,28]. The rapid neutralization of LPS in whole blood, however, does not prevent neutrophils being primed, which requires only 25–30 min.
Interestingly, TNF-α was being released by monocytes in whole blood after only 20 min of LPS incubation. The kinetics of TNF production are in contrast with in vivo data of TNF-α levels in healthy volunteers after endotoxin administration, where TNF-α levels rose significantly only after 90 min, as measured by TNF ELISA [29], and 60 min as measured by radioimmunoassay (RIA) [30]. In an earlier in vitro study with LPS-stimulated whole blood, TNF-α m-RNA was detected after only 30 min and TNF-αas late as 3 h [31]. An explanation for this finding may be the fact that in our assay TNF functions as a priming agent and thus can be detected at very low levels. Our TNF–ELISA results confirm this assumption. Alternatively, TNF can be expressed by monocytes as a transmembrane protein and proteolytically cleaved upon cellular activation [32]. This mechanism would explain the rapid release of preformed TNF.
Neutrophil priming for enhanced oxidative burst by LPS, TNF as well as a number of other cytokines, eicosanoids and bacterial products has been described in the literature [33]. In these studies, however, isolated neutrophils and single priming agents were used. The whole blood system used in this study examines neutrophil activation in a physiological environment and shows the successive interplay between exogenous (LPS) and endogenous (TNF) priming substances. This ex-vivo model of endotoxaemia may therefore be the closest reflection of the innate immune response in vivo.
In conclusion, this study describes the kinetics of LPS-induced neutrophil priming in whole blood ex vivo, a system that may reflect events in patients with endotoxaemia. During the first phase LPS primes neutrophils and stimulates monocytes to produce TNF-α. This process requires plasma, while at the same time LPS is neutralized by plasma. During the second phase LPS has lost its bioactivity, and monocyte-derived TNF-α now functions as a second priming agent for neutrophils. Thus, although LPS is neutralized rapidly in whole blood, this does not prevent neutrophils being primed by LPS and in a second phase by monocyte derived TNF-α. Recent studies have shown that lipoproteins play an important role in the LPS-neutralizing capacity of human serum [34]. Further studies are needed to unravel the mechanism of LPS neutralization by human serum that in the future may enable us to prevent the devastating sequelae of endotoxaemia and sepsis.
Acknowledgments
The authors wish to thank Mrs R. Busser-de Rooij and Mrs H. Morren-Klinkvis for their excellent secretarial assistance.
References
- 1.Bone RC. Gram-negative sepsis. Background, clinical features, and intervention. Chest. 1991;100:802–8. doi: 10.1378/chest.100.3.802. [DOI] [PubMed] [Google Scholar]
- 2.Fujishima S, Aikawa N. Neutrophil-mediated tissue injury and its modulation. Intens Care Med. 1995;21:277–85. doi: 10.1007/BF01701489. [DOI] [PubMed] [Google Scholar]
- 3.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein [see Comments] Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 4.Schumann RR, Leong SR, Flaggs GW, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–31. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Du Hoebe KX, Ulevitch RJ. How we detect microbes and respond to them. the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479–85. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 6.Appelmelk BJ, An YQ, Geerts M, et al. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–32. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emancipator K, Csako G, Elin RJ. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsbach P, Weiss J. Bactericidal/permeability increasing protein and host defense against Gram-negative bacteria and endotoxin. Curr Opin Immunol. 1993;5:103–7. doi: 10.1016/0952-7915(93)90088-a. [DOI] [PubMed] [Google Scholar]
- 9.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–4. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 10.Tobias PS, Mathison JC, Ulevitch RJ. A family of lipopolysaccharide binding proteins involved in responses to Gram-negative sepsis. J Biol Chem. 1988;263:13479–81. [PubMed] [Google Scholar]
- 11.Troelstra A, Giepmans BN, van Kessel KP, Lichenstein HS, Verhoef J, van Strijp JA. Dual effects of soluble CD14 on LPS priming of neutrophils. J Leukoc Biol. 1997;61:173–8. doi: 10.1002/jlb.61.2.173. [DOI] [PubMed] [Google Scholar]
- 12.Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. Endotoxemia in human septic shock. Chest. 1991;99:169–75. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 13.van Deventer SJ, Buller HR, ten Cate JW, Sturk A, Pauw W. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet. 1988;1:605–9. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]
- 14.Conrad RS, Galanos C. Fatty acid alterations and polymyxin B binding by lipopolysaccharides from Pseudomonas aeruginosa adapted to polymyxin B resistance. Antimicrob Agents Chemother. 1989;33:1724–8. doi: 10.1128/aac.33.10.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meager A, Parti S, Leung H, Peil E, Mahon B. Preparation and characterization of monoclonal antibodies directed against antigenic determinants of recombinant human tumour necrosis factor (rTNF) Hybridoma. 1987;6:305–11. doi: 10.1089/hyb.1987.6.305. [DOI] [PubMed] [Google Scholar]
- 16.de Haas CJC, Poppelier MJJG, van Kessel KPM, Van Strijp JAG. Serum amyloid P component prevents high-density lipoprotein-mediated neutralization of lipopolysaccharide. Infect Immun. 2000;68:4954–60. doi: 10.1128/iai.68.9.4954-4960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattsson E, Van Dijk H, Van Kessel K, Verhoef J, Fleer A, Rollof J. Intracellular pathways involved in tumor necrosis factor-alpha release by human monocytes on stimulation with lipopolysaccharide or staphylococcal peptidoglycan are partly similar. J Infect Dis. 1996;173:212–18. doi: 10.1093/infdis/173.1.212. [DOI] [PubMed] [Google Scholar]
- 18.Hailman E, Lichenstein HS, Wurfel MM, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–77. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall JC, Walker PM, Foster DM, et al. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care. 2002;6:342–8. doi: 10.1186/cc1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surette ME, Palmantier R, Gosselin J, Borgeat P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med. 1993;178:1347–55. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rall DP, Gaskins JR, Kelley M. Reduction of febrile response to bacterial polysaccharide following incubation with serum. Am J Physiol. 1957;188:559–62. doi: 10.1152/ajplegacy.1957.188.3.559. [DOI] [PubMed] [Google Scholar]
- 22.Flegel WA, Wolpl A, Mannel DN, Northoff H. Inhibition of endotoxin-induced activation of human monocytes by human lipoproteins. Infect Immun. 1989;57:2237–45. doi: 10.1128/iai.57.7.2237-2245.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren HS, Novitsky TJ, Ketchum PA, Roslansky PF, Kania S, Siber GR. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985;22:590–5. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggesbo JB, Lyberg T, Aspelin T, Hjermann I, Kierulf P. Different binding of 125I-LPS to plasma proteins from persons with high or low HDL. Scand J Clin Lab Invest. 1996;56:533–43. doi: 10.3109/00365519609088809. [DOI] [PubMed] [Google Scholar]
- 25.Eggesbo JB, Hjermann I, Hostmark AT, Kierulf P. LPS induced release of IL-1 beta, IL-6, IL-8 and TNF-alpha in EDTA or heparin anticoagulated whole blood from persons with high or low levels of serum HDL. Cytokine. 1996;8:152–60. doi: 10.1006/cyto.1996.0022. [DOI] [PubMed] [Google Scholar]
- 26.Schlichting E, Aspelin T, Lyberg T. Interactions of endotoxin with human blood cells and serum proteins. Scand J Clin Lab Invest. 1996;56:167–76. doi: 10.3109/00365519609088604. [DOI] [PubMed] [Google Scholar]
- 27.Ulevitch RJ, Johnston AR. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978;62:1313–24. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casey LC. Immunologic response to infection and its role in septic shock. Crit Care Clin. 2000;16:193–213. doi: 10.1016/s0749-0704(05)70107-x. [DOI] [PubMed] [Google Scholar]
- 29.Michie HR, Manogue KR, Spriggs DR, et al. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–6. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 30.van Deventer SJ, Buller HR, ten Cate JW, Aarden LA, Hack CE, Sturk A. Experimental endotoxemia in humans. analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–6. [PubMed] [Google Scholar]
- 31.Allen JN, Herzyk DJ, Allen ED, Wewers MD. Human whole blood interleukin-1-beta production: kinetics, cell source, and comparison with TNF-alpha. J Lab Clin Med. 1992;119:538–46. [PubMed] [Google Scholar]
- 32.Black RA, et al. A metalloproteinase desintegrin that releases tumor-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 33.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms [Editorial] Clin Sci Colch. 1998;94:461–71. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 34.Read TE, Harris HW, Grunfeld C, Feingold KR, Kane JP, Rapp JH. The protective effect of serum lipoproteins against bacterial lipopolysaccharide. Eur Heart J. 1993;14(Suppl. K):125–9. [PubMed] [Google Scholar]