Abstract
Activated T cells that express activation antigens are termed nonprofessional antigen-presenting cells (T-APCs). This study evaluates the ability of lamina propria lymphocytes (LPLs) in inflammatory bowel disease (IBD) to become T-APCs. LPLs were stained by two-colour immunofluorescence to determine the expression of activation antigens on T cells. Those from actively inflamed IBD mucosa expressed greater amounts of MHC class II (DR) and CD86 than did LPL T cells from disease controls or normal individuals. After culture in IL-2 with or without IL-10, the ability of the T-APCs from IBD colon to stimulate allogeneic peripheral blood T cell proliferation was measured. The T-APCs from IBD stimulated an allogeneic mixed lymphocyte reaction, particularly through their expression of DR and CD86, as demonstrated by antibody blocking. Normal LPLs acquired these properties only if repeatedly stimulated with allogeneic peripheral blood lymphocytes (PBLs) used as cell lines in the presence of IL-2. Addition of IL-10 reduced expression of activation antigens and the stimulatory ability of LPLs from either IBD patients or from these cell lines. In summary, LPLs from active IBD, but not from disease controls, express activation antigens that stimulate naïve T cells, a process that is reduced by IL-10. This may contribute to perpetuation of the inflammation.
Keywords: inflammatory bowel disease, IL-10, CD80, CD86, MHC class II
Introduction
While Crohn's disease (CD) and ulcerative colitis (UC) differ in their predominance of Th1 and Th2 cells and probably in their initiating events, they are both characterized by chronic inflammation [1]. The hypothesis in this study is that this inflammation is partly mediated by up-regulatory circuits, namely activated T cells serving as ‘nonprofessional’ antigen-presenting cells (T-APCs) that stimulate other T cells.
Various activation antigens have been found on T cells in the mucosa of patients with IBD. The major lymphocytes in the colon are lamina propria lymphocytes (LPLs), comprised predominantly of T cells. In IBD, the LPLs express acute activation antigens, such as receptors for IL-2, transferrin, and MHC class II molecules [2,3], as well as chronic activation antigens, such as the very late activation (VLA) antigens and CD45RO. The unstudied, and perhaps most important, are antigens found on ‘professional’ APCs, namely, CD80 and CD86 which trigger proliferation and cytokine production by naïve T cells.
Intestinal epithelial cells (ECs) in IBD but not in normal subjects express low levels of MHC class II on their basolateral surfaces [3,4] at a density that is one order of magnitude less than that on conventional APCs. Not surprisingly, they serve as incomplete APCs, lacking the ability to support T cell proliferation toward superantigen or CD3 stimulation [5]. Yet, they present high concentrations of antigen to CD4+ T cells through an interferon (IFN)γ-independent pathway [6]. With up-regulation of MHC class II by IFN-γ treatment, ECs present antigen in a manner similar to conventional APCs.
Activated T cells, studied from nonintestinal sources, can serve as nonprofessional APCs, presenting autoantigen, HIV-gp120, tetanus toxoid, or hepatitis B envelope antigen to naïve T cells [7–9]. They can be powerful stimulators of allogeneic T cells, due to a combined effect of MHC class II and either CD80 or CD86 (also called B7-1 and B7-2) [10,11]. They can present superantigen and costimulate CD3 triggering in the absence of conventional APCs [12,13]. Such T-APCs can also induce IFNγ production and the development of cytotoxic CD4+ T cells [13,14]. Unlike conventional APCs, T-APCs activate T cells with diverse TCR repertoires and without HLA restriction [15].
T-APCs have also been shown to lack stimulatory activity and, in fact, can promote anergy and tolerance [16–18]. The presence of IL-2 during priming with T-APCs can block tolerance and elicit stimulation. Certainly, the presence of CTLA4 on the surface of the naïve responder T cell will lead to inhibition since this ligand has a higher affinity for the B7 molecules than does CD28 [19]. However, most studies showing tolerance use nonhuman cells or cell lines. These findings may not be applicable to humans for several reasons. First, there are species differences, that is, CD86 is absent on resting human T cells and appears with activation, while for murine T cells, maximum expression is seen constitutively. Secondly, the density of B7 expression is generally much higher on transfected cells than on naturally activated T cells.
T-APCs may contribute to the pathogenesis of certain diseases. For example, expression of B7 and MHC class II on synovial T cells in rheumatoid arthritis can stimulate naïve T cells [20]. In graft-versus-host disease, CD80 expression on donor T cells is critical for optimal lethality [21]. It is certainly possible, then, that T-APCs also play a role in IBD.
The present study begins to define the presence and function of T-APCs in the mucosa of inflamed bowel, focusing on CD80 and CD86. the expression of activation antigens on lamina propria lymphocyte (LPL)-T cells in IBD are defined as well as their ability to stimulate allogeneic T cells. The effects of IL-10 on the expression and function of activation antigens were investigated since this cytokine down-regulates B7 and MHC class II on classical APCs and has been used as a treatment for IBD [22,23].
Methods
Patient populations
Colonic T cells were isolated from the following:
A Normal-appearing colonic mucosa from patients undergoing resection for cancer (n = 10), polyps (n = 2), or diverticulitis (n = 6) (average age 58 ± 8 years);
B Ulcerated, friable colonic mucosa from patients with IBD obtained from biopsy specimens during colonoscopy or from surgical resections (n = 46, average age 41 ± 5 years);
C Ulcerated, friable colonic mucosa from disease controls (radiation colitis (n = 2), pseudomembranous colitis (n = 7), ischemic colitis (n = 1), infectious colitis (n = 4)) obtained during colonoscopy (average age 49 ± 6 years).
For B and C, only symptomatic patients with more than 5 bowel movements daily were included in the study. There were 25 patients with UC, 7 of which were on oral immunosuppressive medications (corticosteroids, 6-mercaptopurine) and 19 on mesalamine products. Ten had left-sided disease, and 15 had pancolitis. Of the 21 patients with CD, 6 were on immunosuppressive medications and 18 on mesalamine products. Twelve had disease restricted to the colon, while 9 had ileocolonic disease. Patients with ileal disease alone were not included. Informed consent was obtained beforehand. This study was approved by the Institutional Review Board at UMDNJ.
Isolation of lymphocytes
Mucosa was treated with deoxyribonuclease for 30 min and then ethylene-diamine-tetraacetic acid for 1 h. Only with normal mucosa were the cells from this treatment discarded as they were mainly epithelial cells (ECs). For inflamed mucosa because of denuded epithelium, the cells released by this treatment were kept as they were mainly lymphocytes with little EC contamination. Next the tissue was treated with collagenase (20 U/ml, Worthington) for 3 h. Tissue was pressed with a wire mesh sieve to obtain a single-cell suspension. Lymphocytes were purified by Percoll density gradient centrifugation (Pharmacia, Piscataway, NJ, USA) as described previously [24]. These cells will be referred to as LPLs.
Peripheral blood lymphocytes (PBLs) were isolated from whole blood by Ficoll density gradient centrifugation. All T cells were isolated by nylon wool adherence followed by removal of macrophages and B cells by Petri dish adherence and depletion of CD14+ and CD20+ cells with immunomagnetic beads [24].
Immunofluorescence
Two-colour immunofluorescence was performed on fresh and cultured LPLs using mAbs against MHC class I (clone B9·12·1), DR (BL2), CD80 (MAB104), CD86 (HA5·2B7), or isotype-matched IgG control, each conjugated to phycoerythrin (PE). This was followed by mAb against CD2 or isotype-matched IgG controls, each conjugated to fluorescene isothiocyanate (FITC)(all from Immunotech, Westbrook, ME, USA). Indirect single-colour immunofluorescence was carried out to detect CD2 (C1·5), CD3 (UCHT-1), CD4 (13B8·2), CD8 (B9·11), CD14, CD20, CD28, CD83 (HB15a), CTLA4 (Immunotech), and cytokeratin 18 (Sigma) as well as the markers listed above. Data were analysed on a Coulter Epics Profile analytical flow cytometer and expressed as the percentage of positive cells and the relative fluorescence intensity (RFI) compared to IgG-FITC or IgG-PE controls. The RFI is the ratio of the mean channel number for the test samples to that for the control samples.
Lymphocyte cultures
Cell lines were initiated by culturing LPLs or PBLs (donor 1) with allogeneic irradiated (2000 rads) PBLs (donor 2). They were supplemented with IL-2 (10 ng/ml)(R & D Systems) every week and stimulator PBLs (donor 2) every two weeks. The lines were used from four to eight weeks after initiation, thawing fresh aliquots that were frozen at four weeks. These lines, described in detail previously [25], were 100% CD2+ and 96% CD3+ when analysed 7 days after addition of stimulator PBLs. They contained no CD14+, CD20+, or CD83+ cells. These lines demonstrated alloantigen-specific proliferation and cytotoxicity and doubled every four to seven days, depending upon the concentration of IL-2 added [25].
For functional assays, lymphocytes from cell lines and tissue were cultured with IL-2 in the presence or absence of IL-10 (both at 10 ng/ml, R & D Systems) for 5 days. For cell lines, this was initiated 7 days after stimulator PBLs were added. After this culture, the cells, now considered to be T-APCs, were stained by immunofluorescence to establish their expression of activation antigens. In addition, they were irradiated (2000 rads) and then re-cultured at a 1 : 1 ratio for 5 days with allogeneic PB T cells isolated by nylon wool columns and depleted of CD14+ and CD20+ cells by immunomagnetic depletion. These T cells were from donor 2 so that they would react minimally to any residual autologous feeder PBLs, also from donor 2. Proliferation was measured by 3H-thymidine incorporation after another 5 days, a time found to be optimal according to preliminary kinetics experiments. The stimulation index is the ratio of counts per minute (cpm) with T cells stimulated by allogeneic T-APCs divided by the cpm of T cells cultured in medium alone. in some experiments, PHA (1 µg/ml, Burroughs-Wellcome, Hialeah, FL, USA) was added. in others, blocking mAbs against MHC class I, DR, CD80, CD86, or isotype-matched IgG controls (5 µg/ml) were included in the cultures.
Statistical analysis
For each data set, an arithmetic mean and SD were calculated. Pairs of data sets were compared by the Student's t-test or Wilcoxon rank sum test.
Results
Expression of DR and CD86 by LPL-T cells and ECs in IBD
LPLs from patients with IBD or disease controls were obtained by biopsies or surgical resections of grossly diseased areas with ulcerations and friability. LPLs from normal mucosa were obtained from surgical resections only since the yield of lymphocytes from biopsies was too low to use. Despite separation of lymphocytes by a discontinuous Percoll gradient, the LPLs from normal mucosa contained 30 ± 8% contaminating ECs while those from the denuded epithelial mucosa in IBD and disease controls had only 9 ± 2%. Therefore, phenotyping by immunofluorescence was performed by gating on the lymphocytes to avoid variations due to EC contamination. This showed that virtually all of the LPLs were CD2+CD3+ with rare CD14+, CD20+, or CD83+ cells (Table 1). Few lymphocytes or APCs were found outside this gate. These data were derived from IBD mucosa (4 with CD, 4 with UC) and normal mucosa (4 patients) with no significant differences noted among the groups so the results were combined.
Table 1.
Phenotype of LPL preparation
| Cell marker | Percentage positive |
|---|---|
| CD2 | 98 ± 4 |
| CD3 | 96 ± 4 |
| CD4 | 75 ± 5 |
| CD8 | 19 ± 10 |
| CD28 | 53 ± 10 |
| CTLA4 | 3 ± 1 |
| CD14 | 1 ± 0 |
| CD20 | 0 |
| CD83 | 0 |
| Cytokeratin 18 | 2 ± 1 |
LPLs from 4 patients with CD, 4 with UC, and 4 normal controls, were phenotyped by immunofluorescence. The results were obtained by flow cytometry, gating on the lymphocytes. Since the data was the same for the 3 groups, the results were combined.
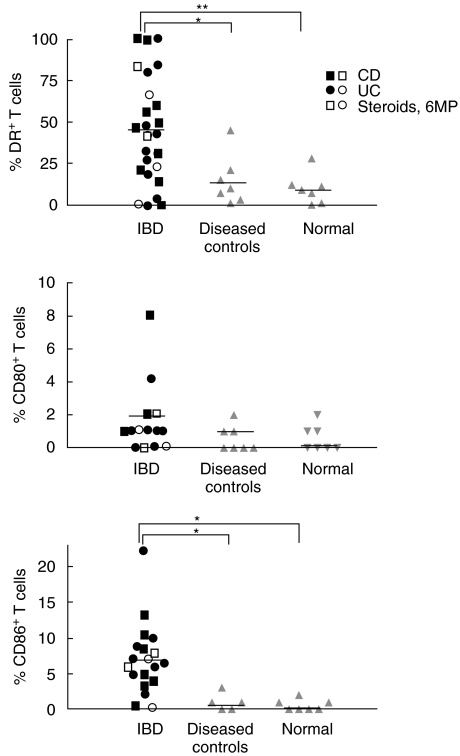
The activation antigens, DR, CD80, and CD86, were identified on freshly isolated LPLs by two-colour immunofluorescence using PE-conjugated Abs against each marker combined with CD2-FITC, again gating on the lymphocyte populations (Figs 1 and 2). The most prevalent antigen was DR found on 46 ± 6% of T cells derived from IBD mucosa (13 patients with CD and 12 with UC), compared to only 10 ± 4% from mucosa of normal colon and 12 ± 3% from disease controls (both P < 0·05). CD86 was found on 8 ± 1% of T cells from IBD but rarely on cells from normal mucosa or disease controls (both P < 0·05). CD80 was found on 2 ± 1% of cells from IBD, not significantly different from the controls. The intensity of DR expression was widely variable among the T cells (Fig. 2). CD80 and CD86, in contrast, were found at low densities, generally with an RFI below 3. The numbers of activated T cells were no different for CD compared to UC and for those treated or untreated with immunosuppressive medications (Fig. 1). Although the patients with non-IBD diseases were very ill, all requiring hospitalizations, and biopsies taken from markedly abnormal sites, fewer activation antigens were detected than in IBD. The disease control mucosa averaged only 16 ± 7% DR+ T cells (no different from normal mucosa) and rare CD80+ or CD86+ T cells.
Fig. 1.
Fresh colonic T cells were stained with CD2-FITC and PE-conjugated Abs recognizing (a) DR, (b) CD80 or (c) CD86. The percentages of double-positive cells are shown. (*P < 0·05, **P < 0·01).
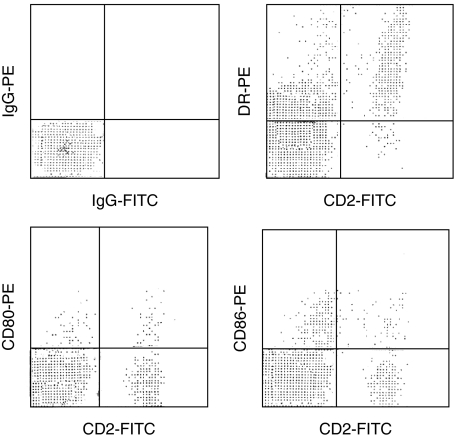
Fig. 2.
Fresh colonic T cells from a patient with active Crohn's disease were stained with CD2-FITC and PE-conjugated Abs against DR, CD80, or CD86. Two-colour analysis is shown. This is a representative study of 25 similar experiments.
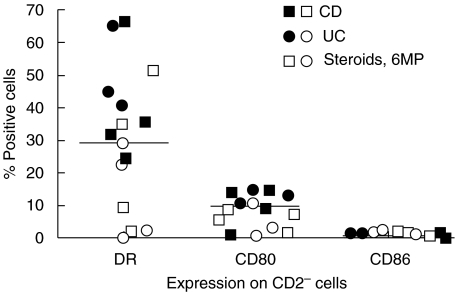
The CD2− cells, identified by ungated analyses, lacked CD45 (>98%) and expressed cytokeratin (>99%), indicating that they were mainly epithelial cells (ECs). In both UC and CD, they displayed DR (35 ± 30%) and CD86 (10 ± 5%), but rarely CD80 (0 ± 1%), in agreement with a previous report (Fig. 3 and 4). The density of DR expression was about 3-fold less than that on CD2+ T cells (RFI of 1·9 ± 0·3 versus 6 ± 1, respectively). for comparison, DR expression on peripheral blood monocytes had an average RFI of 10 ± 2. The density of CD86 expression on ECs and LPLs were similar (RFI of 2 ± 2 and 2 ± 1, respectively), both less than that found on monocytes (RFI of 5 ± 2). ECs from normal mucosa or disease controls had low-level DR-positivity (3 ± 3% in normal individuals and 5 ± 3% in disease controls), but no B7 expression.
Fig. 3.
Fresh colonic ECs were stained with CD2-FITC and PE-conjugated Abs against DR, CD80, or CD86. The percentages of CD2− cells expressing each activation antigen are shown.
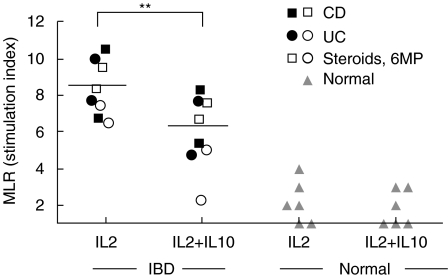
Fig. 4.
LPL T-APCs from IBD (4 UC, 4 CD) or from normal individuals were cultured for 5 days with IL-2 with or without IL-10. Their ability to stimulate allogeneic T cell proliferation in an MLR is shown. (**P < 0·01).
IFNγ, IL-2, and IL-15 do not alter expression of activation antigens on LPL T-APCs
To determine which cytokine(s) may promote T-APCs, LPLs from normal and IBD colon were cultured with IFNγ, IL-2, or IL-15 and changes in expression of MHC class I, class II (DR), CD80, and CD86 were documented. The cells were double-stained for CD2 and each marker listed so that expression on the LPL T cells (CD2+) and ECs (CD2−) could be determined. The 24- h time point was optimal as viability remained over 90%, but progressively declined thereafter in cultures lacking IL-2 or IL-15. IFNγ alone, at 100 ng/ml, augmented expression of DR and CD86 on normal ECs (Table 2). With LPL T cells, IFNγ up-regulated MHC class I expression but had no effects on DR, CD80, or CD86. The same findings were obtained using 500 ng/ml of IFNγ, while at 50 ng/ml, the increases in expression were less marked. When repeating these experiments with CD and UC LPLs, the findings were the same so the results were pooled. Again, IFNγ increased expression of DR and CD86 on the ECs, although the changes were less dramatic due to higher baseline levels compared to normal colonic ECs. IFNγ only affected MHC class I expression on LPLs, not DR or CD86. When IL-2 or IL-15 (10 ng/ml) were tested, the expression of MHC class I, DR, CD80, and CD86 was unchanged for both CD2+ and CD2− populations, in normal individuals and IBD. These studies show that IFNγ affects activation antigens on ECs but not T cells, while IL-2 and IL-15 had no effects on either one.
Table 2.
Effect of IFNγ on marker expression in normal and IBD LPLs
| HLA-DR (% positive cells) | MHC cl I (RFI) | CD86 (% positive cells) | ||||
|---|---|---|---|---|---|---|
| W/o IFNγ | W/IFNγ | W/o IFNγ | W/IFNγ | W/o IFNγ | W/IFNγ | |
| Nl T cells | 10 ± 3 | 11 ± 3 | 50 ± 10 | 170 ± 22* | 1 ± 1 | 1 ± 1 |
| Nl ECs | 3 ± 2 | 9 ± 2* | 41 ± 8 | 162 ± 10* | 1 ± 1 | 5 ± 1* |
| IBD T cells | 44 ± 9 | 47 ± 8 | 59 ± 11 | 180 ± 19* | 6 ± 2 | 6 ± 3 |
| IBD ECs | 34 ± 8 | 59 ± 9* | 51 ± 9 | 179 ± 12* | 9 ± 2 | 19 ± 3* |
LPLs were obtained from 4 normal colons, 4 with UC, and 4 with CD and cultured for 24 h with or without IFNγ (100 ng/ml) in the absence of other stimuli. The expression of the markers listed was determined by double immunofluorescence using CD2-FITC and PE-conjugated mAbs against each marker. The percentage positive cells is shown (mean ± SD) for HLA-DR and CD86 expression, while the RFI is listed for MHC class I since it is found on all cells. Since data for UC and CD did not differ, these values were pooled. The asterisks mark those values that significantly differed in the presence or absence of IFNγ.
P < 0·05
Next, mAbs neutralizing IFNγ, IL-2, IL-15, or IL-10 were added to LPLs from normal, CD, or UC colon (each n = 4). No changes occurred in expression of MHC class I, DR, CD80, or CD86 at 24 h (not shown). This suggests that the display of activation antigens on the T-APCs is not altered by endogenous availability of these cytokines.
LPL T-APCs from IBD stimulate an allogeneic MLR through DR and CD86, but have less of an effect if pretreated with IL-10
To determine whether T-APCs from IBD mucosa could stimulate allogeneic T cells better than T-APCs from normal mucosa, LPLs were first depleted of CD14+ and CD20+ cells, then cultured for 5 days with IL-2 so as to maintain viability of the CD2+ cells. This approach was taken so that the ECs, which were difficult to remove completely from fresh preparations, were allowed to disintegrate during culture, leaving pure T-APCs. After culture, the resulting population from both IBD and normal mucosa were 100% CD2+, 98% CD3+, and negative for CD14, CD20, CD68, and CD83. Expression of activation antigens was assessed by double immunofluorescence (Table 3), and found to be no different from that on fresh T-APCs (Table 2 and Fig. 1). That is, HLA-DR was the most prevalent, followed by CD86, with CD80 being rarely detected. These cells were irradiated and mixed with allogeneic PB T cells in the presence of mAbs blocking MHC class I, HLA-DR, CD80, CD86, or the combination of HLA-DR, CD80 and CD86. The incorporation of 3H-thymidine was determined on day 5 when the greatest allogeneic response occurred, according to preliminary kinetics experiments (Fig. 4). The alloantigen-induced T cell proliferation was greater using LPLs from IBD versus normal mucosa. The MLR induced by IBD LPL-T-APCs was blocked by mAbs recognizing HLA-DR and CD86, particularly when the Abs were combined, whereas it was unaffected by mAb against CD80 or MHC class I.
Table 3.
T-APCs from IBD colonic LPLs: activation antigens and stimulatory activity
| IL-2 | IL-2 + IL-10 | |
|---|---|---|
| Activation antigens (% positive cells (RFI)) | ||
| MHC class I | 100 (65 ± 8) | 100 (53 ± 9) |
| HLA-DR | 28 ± 2% (6 ± 1) | 19 ± 3%* (5 ± 1) |
| CD80 | 2 ± 1 (2 ± 1) | 2 ± 1 (2 ± 1) |
| CD86 | 6 ± 1 (2 ± 1) | 1 ± 1* (2 ± 1) |
| Stimulatory effect (Specificity of MAb added) Stimulation index | ||
| IgG | 8·9 ± 1·0 | 6·8 ± 0·9* |
| MHC class I | 8·5 ± 1·2 | 6·5 ± 1·0* |
| HLA-DR | 5·5 ± 0·8† | 4·2 ± 0·8† |
| CD80 | 8·6 ± 1·2 | 6·3 ± 0·8* |
| CD86 | 5·4 ± 0·8† | 4·4 ± 1·1† |
| DR, CD80, CD86 | 3·3 ± 0·9‡ | 2·9 ± 0·6‡ |
LPLs from 8 IBD patients (4 with UC and 4 with CD) were cultured for five days with IL-2 in the presence or absence of IL-10 (each at 10 ng/ml). The data represent a mean ± SD from all patients. The percentages of CD2+ cells expressing each antigen are shown followed by the RFI in parentheses. The asterisks identify values that significantly differed with and without IL-10(*P < 0·05). The T-APCs were irradiated, then cultured with allogeneic T cells depleted of conventional APCs. Proliferation in this MLR was measured by 3HTdr incorporation on day 5 in the presence of blocking Abs. The results are expressed as a stimulation index, that is, the counts per min (cpm) for the test divided by the cpm of T cells in medium alone. The asterisks identify values that significantly differed with and without IL-10 (*P < 0·05). The triangles identify test values that significantly differed from those obtained with irrelevant IgG († = P < 0·05; ‡ = P < 0·01).
The initial cultures of LPLs from IBD patients were also propagated with IL-2 combined with IL-10. After 5 days, expression of HLA-DR and CD86 on the T-APCs was reduced in cultures containing IL-10 (Table 3). In addition, when these T-APCs were washed extensively, irradiated, and mixed with allogeneic T cells, the subsequent proliferation was also lower than when the initial cultures were performed without IL-10. The results were the same regardless of whether UC or CD was studied. When LPLs from normal colon were cultured in the same way, their expression of activation antigens was no different from that seen in fresh cells (Fig. 1), and they were unable to significantly stimulate allogeneic T cells (Fig. 4). These findings suggest that T-APCs are present in IBD but not normal mucosa, and they have the ability to stimulate allogeneic T cells through DR and CD86 expression. Both activation antigen expression and stimulatory potential were reduced by IL-10.
T-APCs develop after repeated activation of normal LPLs and PBLs
The hypothesis is that the development of T-APCs in IBD is due to repeated stimulation rather than to an intrinsic defect in the T cells. First, the expression of activation antigens on LPLs from normal mucosa was tested using a strong single stimulus, PHA. After 1 day, double-staining revealed that most T cells expressed HLA-DR (55 ± 18%) while few expressed CD80 and CD86 (1 ± 0% and 1 ± 1%, respectively). This lack of B7 expression early after a strong stimulus correlates with that found in the acute disease controls but not in IBD. If cells were reanalysed at 7 days, DR was found on 33 ± 12% of the cells, while CD86 could now be detected in 8 ± 2% of the cells. This suggests that CD86 appears late after activation as described with naïve T cells.
Next, expression of activation antigens was tested after repeated stimulation. Normal LPLs and PBLs (donor 1) were cultured with irradiated allogeneic PBLs (donor 2) and then propagated using IL-2 with or without IL-10 on a weekly basis with stimulator irradiated PBLs (donor 2) added every other week (Table 4). The cells were used 5 days after administration of IL-2 to parallel the conditions used in the previous experiments (Table 3) and 12 days after addition of feeder PBLs (donor 2). The cells, entirely CD2+CD14−CD20−CD83−, were tested for their expression of activation Ags and their ability to stimulate allogeneic T cells from donor 2 as explained in the Methods. As before, the predominant activation Ag was HLA-DR, as determined by the number of positive cells as well as the intensity of expression (Table 4). CD86 was found on a smaller number of T cells, while CD80 was rarely displayed. Again, their stimulatory potential was reduced by mAbs blocking HLA-DR and CD86, particularly when the mAbs were combined. Addition of IL-10 reduced expression of activation antigens and the resulting allogeneic response. There were no differences in any of these functions whether the T-APCs were derived from PBLs or LPLs. This shows that normal T cells from blood or mucosa can acquire the characteristics of T-APCs seen in IBD after repeated stimulation with alloantigen but not immediately after a strong PHA stimulus. This may parallel the differences between IBD and disease controls.
Table 4.
T-APCs from normal PBLs and colonic LPLs: activation antigens and stimulatory activity
| T-APCs from PBLs | T-APCs from LPLs | |||
|---|---|---|---|---|
| IL-2 | IL-2 ± IL-10 | IL-2 | IL-2 ± IL-10 | |
| Activation antigens (% positive cells (RFI)) | ||||
| MHC class I | 100% (55 ± 8) | 100% (49 ± 8) | 100% (51 ± 9) | 100% (49 ± 10) |
| HLA- DR | 42 ± 5 (6 ± 2) | 29 ± 4* (5 ± 2) | 39 ± 4 (6 ± 2) | 21 ± 5* (5 ± 1) |
| CD80 | 3 ± 1 (3 ± 1) | 2 ± 2 (2 ± 1) | 4 ± 1 (3 ± 1) | 3 ± 2 (2 ± 1) |
| CD86 | 8 ± 2 (2 ± 2) | 3 ± 1* (2 ± 1) | 9 ± 2 (2 ± 1) | 4 ± 1* (2 ± 1) |
| Stimulatory activity (Specificity of MAb added) Stimulation index | ||||
| IgG | 7·6 ± 1·1 | 4·9 ± 1·2* | 8·1 ± 1·1 | 5·9 ± 1·8* |
| MHC class I | 7·5 ± 1·0 | 4·7 ± 1·1* | 7·9 ± 1·0 | 5·5 ± 1·4* |
| HLA-DR | 5·8 ± 0·9† | 3·6 ± 0·9† | 5·5 ± 1·1† | 3·8 ± 0·9† |
| CD80 | 7·3 ± 1·1 | 4·4 ± 1·1* | 7·1 ± 0·9 | 4·6 ± 0·9* |
| CD86 | 5·6 ± 1·1† | 3·1 ± 0·8† | 5·1 ± 1·1† | 3·3 ± 0·9† |
| DR, CD80, CD86 | 4·9 ± 1·0‡ | 2·8 ± 1·0† | 3·1 ± 0·8‡ | 1·8 ± 0·9‡ |
Normal PB T cells and LPLs (depleted of APCs) from 4 individuals were repeatedly activated with both allogeneic irradiated feeder PBLs and IL-2 in the presence or absence of IL-10 (each at 10 ng/ml). The data represent a mean ± SD from all patients. The percentages of CD2+ cells expressing each antigen are shown followed by the RFI in parentheses. The asterisks identify values that significantly differed with and without IL-10(*P < 0·05). The proliferation of allogeneic T cells (depleted of APCs) in response to irradiated T-APCs was measured by 3HTdr incorporation in the presence of blocking Abs. The results are expressed as the stimulation index. The asterisks identify values that significantly differed with and without IL-10 (* P < 0·05). The bars identify test values that significantly differed from those obtained with irrelevant IgG(† = P < 0·05; ‡ = P < 0·01).
Discussion
This study shows that:
T-APCs in LPLs from patients with active IBD express HLA-DR and to a lesser extent CD86;
such T-APCs stimulate alloproliferation through HLA-DR and CD86;
similar T-APCs can be generated from PBLs and LPLs derived from normal colonic mucosa after repeated exposures to allogeneic PBLs with IL-2 but not after a single exposure to PHA;
IL-10 reduces expression of the activation antigens and the stimulatory activity of the T-APCs.
The perpetuation of chronic inflammation, the hallmark of IBD, may be due to persistent antigens that cannot be cleared by the immune system or to defects in down-regulatory mechanisms. This study shows one mechanism that may contribute to the persistent inflammation – T-APCs that activate T cells. Actively inflamed mucosa was studied, both from IBD and disease controls. The majority of CD45+ cells were CD2+CD3+CD4+. Although dendritic cells can express CD2 and intestinal macrophages lack CD14 [26,27], there were few classical APCs as indicated by the rare numbers of CD14+ and CD83+ cells. Previous reports have shown only rare classical APCs in cell isolates from normal and UC mucosa, although they are seen in greater numbers in the mucosa of CD patients [28]. It is possible that some of these cells remain adherent to the tissue, so that they do not appear in the cell suspensions. Most of the CD45− cells were also CD2−, CD3−, CD14−, CD20−, CD83−, and positive for cytokeratin, representing primarily ECs. Separation of IELs from LPLs was not performed with the denuded and friable mucosa in IBD. However, separation of IELs and ECs from LPLs was performed in normal colon with intact epithelium. This is important since IELs from normal individuals but not from IBD patients down-regulate proliferative responses of allogeneic PBLs [29].
The expression of activation Ags in IBD occurred in active disease, regardless of whether UC or CD was studied. HLA-DR expression was found at varying densities, while CD80 and CD86 expression was usually of low-density. In diseased controls, despite the severe inflammation, the presence of activation Ags, particularly HLA-DR and CD86, was lower than in IBD.
When stimulating normal colonic T cells with PHA, the main Ag to appear early was DR rather than CD80 or CD86. CD86 could be detected after 7 days, indicating that it developed with prolonged stimulation. When LPLs were repeatedly stimulated with alloantigen and IL-2, DR and CD86 were strongly expressed. This is consistent with the notion that the B7 Ags appear on T cells late after activation or after repeated stimulation. To determine whether this is a property only of memory T cells found in LPLs, the naive T cells in PBLs were repeatedly stimulated. They expressed similar levels of activation antigens, indicating that this phenomenon was not restricted to a particular lymphocyte compartment.
T-APCs from IBD-LPLs or from alloantigen-specific cell lines stimulated T cell proliferation. This response was inhibited to an equal extent by blocking DR or CD86. This is surprising since DR was expressed on many more cells and at a higher density. However, it agrees with the findings of another study looking at T-APC function [10] as well as the demonstration of a high stimulatory potential of CD86 on ECs [4]. Since CTLA4 binds with the B7 molecules more avidly than does CD28, the lack of surface CTLA4 on the T-APCs may permit the B7 Ags to be available to CD28 on the responder T cells.
To further define the cytokine environment required for the development of these activation antigens, LPLs from normal or IBD colon were cultured for 24 h with IFNγ, IL-2, IL-15, or mAbs neutralizing each of these cytokines. Although IFNγ up-regulated MHC class I on T cells, it had no effect on expression of DR or CD86. IL-2 and IL-15 did not alter the expression of any of these markers. Inhibiting endogenous sources of these cytokines also did not affect activation antigens. The T-APCs, then, may be generated by repeated stimulation rather than by the availability of certain cytokines although other, as yet unidentified cytokine(s) may promote the development of these cells.
The findings of this study suggest that the LPL T-APCs may activate T cells in the mucosa. Several caveats, however, must be borne in mind. One is that T cells in the lamina propria are generally less responsive to CD3/TCR signalling than are T cells from peripheral blood [30]. In addition, LPL-T cells from IBD are hyporesponsive to activation by intestinal ECs when compared to control LPL-T cells [31], so their response to T-APCs may also be reduced. Furthermore, T-APCs may induce tolerance, an action blocked by IL-2 [16]. In the present study, IL-2 or IL-15 was added to the LPL-T-APCs, perhaps converting them from tolerogenic to immunogenic. This was done since their viability could not be maintained in medium alone. Also, it may reflect the environment in vivo since IBD is characterized by excess IL-15 in the mucosa [32].
IL-10 reduced expression of DR and CD86 on T-APCs in the presence of IL-2. IL-10 is synthesized by T cells (including LPLs 33), B cells, and APCs. It reduces production of IL-1 and TNFα and expression of B7 and MHC class II on APCs, thereby indirectly down-regulating T cell activation [34]. IL-10 is thought to protect against gut inflammation. IL-10-deficient mice develop inflammation resembling UC [35], while IL-10 administration can reduce inflammation in colitis in preliminary human studies [22,23]. But IL-10 can also stimulate proliferation and cytotoxicity of CD8+ T cells cultured with IL-2, an effect independent of CD3/TCR triggering and C-APCs [24,36]. This has not been shown with CD4+ T cells. In the present study, the down-regulation of T-APC development and function by IL-10 is likely due to the preponderance of CD4+ T cells. This finding favours IL-10 as a possible therapy to interrupt this cycle of T cell stimulation by T-APCs.
This study, then, identifies a process in the inflamed bowel in IBD but not normal mucosa that may perpetuate inflammation, leading to the chronicity of the disease, a process down-regulated by IL-10. The study of T-APCs may provide a good in vitro model to test the effectiveness of therapeutic options in IBD.
References
- 1.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria lymphokine secretion profiles in inflammatory bowel disease. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 2.Pallone F, Fais S, Squarcia O, Biancone L, Pozzilli P, Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28:745–53. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirata I, Austin LL, Blackwell WH, Weber JR, Dobbins WO. Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:1317–30. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- 4.Nakazawa A, Watanabe M, Kanai T, et al. Functional expression of costimulatory molecule CD86 on epithelial cells in the inflamed colonic mucosa. Gastroenterology. 1999;117:536–45. doi: 10.1016/s0016-5085(99)70446-4. [DOI] [PubMed] [Google Scholar]
- 5.Aisenberg AI, Ebert EC, Mayer L. T cell activation in human intestinal mucosa. the role of superantigens. Gastroenterology. 1993;105:1421–30. doi: 10.1016/0016-5085(93)90147-5. [DOI] [PubMed] [Google Scholar]
- 6.Hershberg RM, Framson PE, Cho DH, Lee LY, Kovats S, Beitz J, Blum JS, Nepon GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–15. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haffar OK, Smithgall MD, Bradshaw J, Brady B, Damle NK, Linsley PS. Costimulation of T-cell activation and virus production by B7 antigen on activated CD4+ T cells from human immunodeficiency virus type 1-infected donors. Proc Natl Acad Sci USA. 1993;90:11094–8. doi: 10.1073/pnas.90.23.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnaba V, Watts C, de Boer M, Lane P, Lanzavecchia A. Professional presentation of antigen by activated human T cells. Eur J Immunol. 1994;24:71–5. doi: 10.1002/eji.1830240112. [DOI] [PubMed] [Google Scholar]
- 9.LaSalle MJ, Ota K, Hafler DA. Presentation of autoantigen by human T cells. J Immunol. 1991;147:774–480. [PubMed] [Google Scholar]
- 10.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J Exp Med. 1993;177:845–50. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyss-Coray T, Mauri-Hellweg D, Baumann K, Bettens F, Grunow R, Pichler WJ. The B7 adhesion molecule is expressed on activated human T cells: functional involvement in T–T cell interactions. Eur J Immunol. 1993;23:2175–80. doi: 10.1002/eji.1830230919. [DOI] [PubMed] [Google Scholar]
- 12.Nisini R, Matricardi PM, Fattorossi A, Biselli R, D'Amelio R. Presentation of superantigen by human T cell clones. a model of T–T cell interaction. Eur J Immunol. 1992;22:2033–9. doi: 10.1002/eji.1830220812. [DOI] [PubMed] [Google Scholar]
- 13.Jeannin P, Herbault N, Delneste Y, Magistrelli G, Lecoanet-Henchoz S, Caron G, Aubry J-P, Bonnefoy J-Y. Human effector memory T cells express CD86: a functional role in naïve T cell priming. J Immunol. 1999;162:2044–8. [PubMed] [Google Scholar]
- 14.Mauri D, Wyss-Coray T, Gallati H, Pichler WJ. Antigen-presenting T cells induce the development of cytotoxic CD4+ T cells. I. Involvement of the CD80-CD28 adhesion molecules. J Immunol. 1995;155:118–27. [PubMed] [Google Scholar]
- 15.Bouchonnet F, Lecossier D, Bellocq A, Hamy I, Hance AJ. Activation of T cells by previously activated T cells. HLA-unrestricted alternative pathway that modifies their proliferative potential. J Immunol. 1994;153:1921–35. [PubMed] [Google Scholar]
- 16.Satyaraj E, Rath S, Bal V. Induction of tolerance in freshly isolated alloreactive CD4+ T cells by activated T cell stimulators. Eur J Immunol. 1994;24:2457–61. doi: 10.1002/eji.1830241030. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield EA, Howard E, Paradis T, et al. B7.2 expressed by T cells does not induce CD28-mediated costimulatory activity but retains CTLA4 binding. Implications for induction of antitumor immunity to T cell tumors. J Immunol. 1997;158:2025–34. [PubMed] [Google Scholar]
- 18.Sidhu S, Deacock S, Bal V, Batchelor JR, Lombardi G, Lechler RI. Human T cells cannot act as autonomous antigen presenting cells, but induce tolerance in antigen-specific and alloreactive responder cells. J Exp Med. 1992;176:875. doi: 10.1084/jem.176.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis JH, Burden MN, Vinogradov DV, Linge C, Crowe JS. Interactions of CD80 and CD86 with CD28 and CTLA4. J Immunol. 1996;56:2700–9. [PubMed] [Google Scholar]
- 20.Verwilghen J, Lovis R, DeBoer M, Linsley PS, Haines GK, Koch AE, Pope RM. Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J Immunol. 1994;153:1378–85. [PubMed] [Google Scholar]
- 21.Blazar BR, Sharpe AH, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Korngold R, Vallera DA. Infusion of anti-B7.1 (CD80) and anti-B7.2 (CD86) monoclonal antibodies inhibits murine graft-versus-host disease lethality in part via direct effects on CD4+ and CD8+ T cells. J Immunol. 1996;157:3250–9. [PubMed] [Google Scholar]
- 22.Schreiber S, Heinig T, Thiele H-G, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–44. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 23.Van Deventer SJH, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Gastroenterology. 1997;113:383–9. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 24.Ebert EC. IL-10 enhances IL-2-induced proliferation and cytotoxicity by human intestinal lymphocytes. Clin Exp Immunol. 2000;119:426–32. doi: 10.1046/j.1365-2249.2000.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebert EC. Do the CD45RO+CD8+ intestinal intraepithelial T lymphocytes have the characteristics of memory cells? Cell Immunol. 1993;147:331–40. doi: 10.1006/cimm.1993.1073. [DOI] [PubMed] [Google Scholar]
- 26.Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–6. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 27.Crawford K, Stark A, Kitchens B, et al. CD2 engagement induces dendritic cell activation: implications for immune surveillance and T-cell activation. Blood. 2003;102:1745–52. doi: 10.1182/blood-2002-07-2206. [DOI] [PubMed] [Google Scholar]
- 28.Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958–67. doi: 10.4049/jimmunol.166.8.4958. [DOI] [PubMed] [Google Scholar]
- 29.Dalton HR, Dipaolo MC, Sachdev GK, Crotty B, Hoang P, Jewell DP. Human colonic intraepithelial lymphocytes from patients with inflammatory bowel disease fail to down-regulate proliferative responses of primed allogeneic peripheral blood mononuclear cells after rechallenge with antigens. Clin Exp Immunol. 1993;93:97. doi: 10.1111/j.1365-2249.1993.tb06503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeMaria R, Fais S, Silvestri M, Frati L, Pallone F, Santoni A, Testi R. Continuous in vivo activation and transient hyporesponsiveness to TcR/CD3 triggering of human gut lamina propria lymphocytes. J Immunol. 1993;23:3104–8. doi: 10.1002/eji.1830231209. [DOI] [PubMed] [Google Scholar]
- 31.Joseph NE, Fiocchi C, Levine AD. Crohn's disease and ulcerative colitis mucosal T cells are stimulated by intestinal epithelial cells: implications for immunosuppressive therapy. Surgery. 1997;122:809–16. doi: 10.1016/s0039-6060(97)90091-x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Geboes K, Colpaert S, D'Haens GR, Rutgeerts P, Ceuppens JL. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164:3608–15. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 33.Braunstein J, Qiao L, Autschbach F, Schurmann G, Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–20. doi: 10.1136/gut.41.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata Y, Foster A, Kurimoto M, et al. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFNγ-inducing factors but enhances NK cell production of IFNγ. J Immunol. 1998;161:4283–8. [PubMed] [Google Scholar]
- 35.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin 10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 36.Groux H, Bigler M, de Vries JE, Roncarolo M-G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–93. [PubMed] [Google Scholar]