Abstract
Alpha-fodrin, an intracellular organ-specific cytoskeleton protein is a recently identified autoantigen associated with Sicca- and Sjögren's syndrome (SS). SS frequently affects patients with Graves' ophthalmopathy (GO). We have therefore cloned and expressed the human recombinant 120-kDa fodrin-fragment. A sequential purification procedure was applied to isolate the recombinant protein. Using sera from patients with SS, the antigenicity of the purified fodrin fragment was demonstrated by immunoblotting. Sera from 144 patients with GO and 1200 blood donors were screened for the presence of anti-α-fodrin IgA and IgG antibodies by a newly developed ELISA using the human α-fodrin fragment as an autoantigen. In contrast to controls (<1% IgA only, P < 0·001) and to subjects with various autoimmune diseases (P < 0·001), α-fodrin antibodies were detected in 22% of patients with GO (n = 32). IgA and IgG antibodies were present in 21 (15%) and 14 (10%) GO subjects, respectively. A total of 45 patients with GO (31%) had at least one fodrin- or SS-antibody. GO patients with SS showed SS- and high titres of α-fodrin-antibodies. In GO patients, fodrin antibodies correlated with TPO- (P < 0·05) and SS-A (P = 0·002) antibodies. Thus, for the first time, antibodies reactive with fodrin are reported in patients with GO.
Keywords: autoantigen, fodrin, thyroid eye disease
Introduction
Fodrin, or a generalized form of erythroid spectrin, a heterodimer composed of an α and β subunit with a molecular weight of 240 and 235 kDa, respectively, is an abundant protein of eukaryotic cell membrane skeleton [1,2]. The α/β-fodrin dimers self-associate head-to-head into tetramers and serve as the basic structural element of the membrane skeleton. Both α- and β-fodrin share a homologous internal 106-AA repeating motif called spectrin repeat, and 22 and 17 spectrin repeats are included in α- and β-fodrin, respectively. β-fodrin has additional amino- and carboxy-terminal regions with no homology to α-fodrin. Fodrin associates with membrane ion channels and pumps and supports their composition of epithelial cells. Fodrin α-subunit is found at the periphery of chromaffin cells [1] and may be involved in secretion, whereas the β-subunit is involved in membrane attachment. Fodrin has binding sites for various proteins including actin, calmodulin and CD45. Proteolytic cleavage of α-fodrin resulting from the activation of a neutral calcium-activated protease (calpain) could be responsible for these conformational changes. Fodrin is cleaved in association with apoptosis and the 120-kDa fragment, which is found abundantly in the lacrimal gland, is a breakdown product of the fodrin α subunit [3,4].
Sera from an animal model of Sjögren's syndrome (SS) as well as those from SS patients specifically recognize the amino terminal fragments of α- and β-fodrin [5,6]. In addition, the 120-kDa α-fodrin fragment was detected in tissue homogenates of eyelid biopsies from patients with SS, but not in those from control individuals. In patients with SS, α-fodrin is also involved in the stimulation of peripheral blood T cells [7]. Based on these findings, the 120-kDa α-fodrin fragment is an important organ-specific autoantigen in both animal models of SS as well as in SS patients. SS is an autoimmune disease characterized by lymphocyte infiltrates and destruction of the lacrimal and salivary glands [8], and systemic production of autoantibodies to the ribonucleoprotein (RNP) particles SS-A and SS-B. SS of the lacrimal glands frequently affects patients with Graves' ophthalmopathy (GO). Therefore, the objectives of this study were (a) to clone and express the human recombinant 120-kDa fodrin-fragment, (b) to develop an enzyme-linked immunosorbent assay (ELISA) employing α-fodrin as autoantigen and (c) to test it in patients with GO and healthy controls. Autoantibodies reactive with fodrin have not been reported to date in GO.
Subjects and methods
Sera from 144 patients with GO and from 1200 blood donors, free of infectious diseases, were considered. Median age of the GO patients was 47 years, range 22–76 years. Of the patients 93 (65%) were female and 83 (58%) were smokers. Sixty-two (43%) had an inflammatory and congestive GO, 27 (19%) were on steroids (oral prednisolone starting with 1 mg/kg body weight), 35 (24%) had a GO of recent onset (<6 months) and 88 (61%) had a disease duration of more than 1 year. All patients with GO were either euthyroid or were receiving antithyroid drugs (methimazole 2·5–10 mg/day). Diagnosis of SS has been established when symptomatic dry eyes and/or dry mouth, due to insufficient secretion, plus objective examination for dryness, such as Shirmer's test (which measures the amount of wetting in 5 min) and/or ophthalmic confirmation of keratoconjunctivitis, and/or measurement of salivary flow were present. For comparison, sera from patients with Graves' disease without GO (GD), Hashimoto thyroiditis (HT), rheumatoid arthritis (RA), Crohn disease and systemic lupus erythematosus (SLE), respectively, were also tested. GD was diagnosed as presence of hyperthyroidism with TSH-receptor antibodies. HT was defined as hypothyroidism with high levels of thyreoperoxidase autoantibodies and typical patterns in thyroid ultrasound. RA and SLE were diagnosed according to the classification criteria for RA arthritis and SLE of the American Rheumatism Association 1987. RA was diagnosed if at least four of the seven following criteria were present: morning stiffness, arthritis of three or more joint areas/hand joints, symmetric arthritis, rheumatoid nodules, serum rheumatoid factor and typical radiographic changes. SLE was defined if at least four of the following 11 criteria were present: malar rash, discoid rash, photosensitivity, oral ulcers, arthritis, serositis, renal disorder, neurological disorder, haematological disorder, antinuclear antibody and/or other immunological disorder. Diagnosis of Crohn disease was confirmed by standard endoscopic, histological and radiological criteria. The patients gave informed consent to the investigation and the study was approved by the Medical Ethics Committee of the Gutenberg University.
Molecular cloning of α-fodrin
DNA manipulations were carried out according to Maniatis and Tasic [9]. Poly(A +)-RNA from human parotid gland was isolated by a mRNA Kit (Qiagen, Hilden, Germany). Screening of the library was performed by polymerase chain reaction (PCR), as described previously [10]. Primers corresponding to the 5′- and the 3′ regions of the α-fodrin gene [base pair (bp) 1–1784] were designed with additional recognition sites for the restriction enzymes. The obtained DNA fragments were further gel purified, digested with restriction enzymes and cloned into a pET blue vector (Novagen, Germany). The complete cDNA fragment (1794 bp) was sequenced by GENenterprise (Mainz, Germany). The cloning kit containing the pCRII vector was obtained from Invitrogen (Leele, the Netherlands); vector pTZ 18 U was from Pharmacia (Freiburg, Germany), the vector Kit containing the pET blue expression vector was from Novagen, Germany and the Ni2+ NTA agarose from Qiagen (Hilden, Germany). Restriction enzymes and modifying enzymes were purchased from New England Biolabs (Schwalbach, Germany), except for the Taq polymerase (Promega, Germany). The microcon 100 micro concentrators were from Amicon (Witten, Germany); the low melt agarose was purchased from Applichem (Heidelberg, Germany). Antibiotics and protease inhibitors were from Roche (Mannheim, Germany). Oligonucleotides were synthesized by Sigma, Germany. Chromatography columns for protein purification were purchased from Amersham Bioscience (Freiburg, Germany). The PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit was from Perkin Elmer Applied Biosystems (Langen, Germany).
Overexpression of fodrin and purification of recombinant protein
The coding sequence was amplified by PCR and cloned in pET32 (Novagen, Germany) to result in isopropylthio-β-D-galactoside (IPTG)-inducible expression of the α-fodrin gene product as a thioredoxin tagged fusion protein. The plasmid was used to transform the Escherichia coli strain BL21-CodonPlus™ (DE3)-RIL (Stratagene), and recombinant E. coli clones were selected on agar plates containing ampicillin and chloramphenicol. E. coli cells harbouring expression plasmids were grown at 30°C. Protein expression was induced at OD600 of 0·8 with 1 mm IPTG for 2 h. Cells were harvested by centrifugation and resuspended in a homogenization buffer according to [10]. Cell debris were removed by centrifugation and the supernatant loaded onto a Ni2+ NTA-column (Qiagen, Hilden, Germany). Unspecific bound proteins were removed using wash buffer (50 nm Na-phosphate pH 6·0; 150 nm Nacl; 10% glycerol (w/v); 0·1% NP-40; protease inhibitors as described in a homogenization buffer), and bound proteins were eluted using a gradient of 10–250 mm imidazole in wash buffer. Subsequent ion exchange chromatography (mono Q, Pharmacia, Freiburg, Germany) and gel filtration (HiLoad 16/60 Superdex 200 prep grade, Amersham Pharmacia Biotech, Germany) led to apparent homogeneity of α-fodrin. Purification was monitored by means of sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and cross-reactivity with defined human serum from patients with SS. SDS-PAGE with Coomassie or silver staining analysed the purity of the proteins. Western blot analysis of the eluted fodrin was performed with defined human serum from patients with SS and visualized by using the ECL Western blotting detection kit (Amersham Pharmacia Biotech). Molecular mass of α-fodrin was determined by means of a MALDI-TOF Bruker Reflex mass spectrometer equipped with a N2-UV laser (337 nm). The matrix was sinapic acid, and cytochrome c (bovine heart) was used as an internal standard for calibration.
Serological investigations
The anti-α-fodrin ELISA was produced by incubating microtitre plates (Costar, Germany) with target protein (0·5 µg/ml) diluted in phosphate-buffered saline (PBS) (100 µl per well) overnight at 4°C according to [11]. Flicking and slapping removed the antigen solution. Non-specific antibody binding was blocked by adding 200 µl/well of blocking buffer [PBS containing 1% bovine serum albumin (BSA) and 0·02% azide]. After 1 h at room temperature blocking buffer was removed, dried and plates were stored in bags at 4°C. Briefly, 100 µl aliquots of diluted sera from patients (1 : 100 diluted in a dilution buffer, PBS, Tween-20 0·1%), were incubated for 30 min on the corresponding ELISA plate, after three washing steps with dilution buffer, horseradish peroxidase-labelled goat antihuman either IgG- or IgA-specific antibody (Dianova, Hamburg, Germany) were added for 15 min. The substrate reaction was performed for 15 min after a second washing step. By addition of 100 µl of 1 m HCl, absorbance at 450 nm was determined using an ELISA reader (Rainbow Reader, Tecan, NY, USA). In all subjects with GO, anti-SS-A and anti-SS-B antibodies were also determined (ELISA, Orgentec, Mainz, Germany).
Statistics
Statistical analyses of the data were performed with spss/pc software for MS Windows, release 11·0 (SPSS Inc., Chicago, IL, USA). Differences in the incidences of present and absent IgA and IgG antibodies in patients versus controls were tested with Fisher's exact test. For quantitative variables, the criterion of normal distribution was tested with the Kolmogorov–Smirnov test. For either normally distributed or skewed data, group comparisons were performed by using parametric or non-parametric tests, respectively. The Mann–Whitney U-test was performed for group comparisons between patients and healthy controls. Spearman's correlation coefficients were applied in order to analyse associations of IgA or IgG antibody titres with fodrin or TPO antibody titres. In 14 patients, antibody titres for α-fodrin, SS-A and SS-B were measured repeatedly within 20 months. Changes between first and second measurements were compared with t-tests for paired samples. The level of significance (P) was taken as less than 0·05.
Results
Table 1 shows the characteristics of patients with GO as well as the relationships of these characters with anti-α-fodrin IgA- and IgG-antibodies.
Table 1.
Relationships between anti-α-fodrin IgA/IgG-antibodies and GO characteristics.
| IgA | IgG | |||||
|---|---|---|---|---|---|---|
| Character of GO | Negative | Positive | P1 | Negative | Positive | P1 |
| Activity | ||||||
| Inactive | 59 | 8 | 57 | 10 | ||
| Active | 67 | 6 | 1·000 | 62 | 11 | 0·576 |
| Duration | ||||||
| <6 months | 17 | 5 | 20 | 2 | ||
| >6 months | 101 | 16 | 0·329 | 105 | 12 | 1·000 |
| Steroid therapy | ||||||
| No | 94 | 12 | 90 | 16 | ||
| Yes | 31 | 2 | 1·000 | 28 | 5 | 0·519 |
| Smoking | ||||||
| No | 50 | 8 | 48 | 10 | ||
| Yes | 61 | 6 | 0·809 | 57 | 10 | 0·411 |
| TSH-R antibody | ||||||
| Negative | 65 | 5 | 60 | 10 | ||
| Positive | 39 | 5 | 0·444 | 35 | 9 | 0·505 |
| Tg antibody | ||||||
| Negative | 94 | 9 | 88 | 15 | ||
| Positive | 21 | 5 | 0·371 | 20 | 6 | 0·156 |
| TPO antibody | ||||||
| Negative | 51 | 1 | 43 | 9 | ||
| Positive | 66 | 13 | 0·810 | 67 | 12 | 0·008 |
Tg, thyroglobulin; TPO, thyroid peroxidase; TSH-R, TSH receptor.
Fisher's exact test.
Overexpression of α-fodrin and purification of recombinant protein
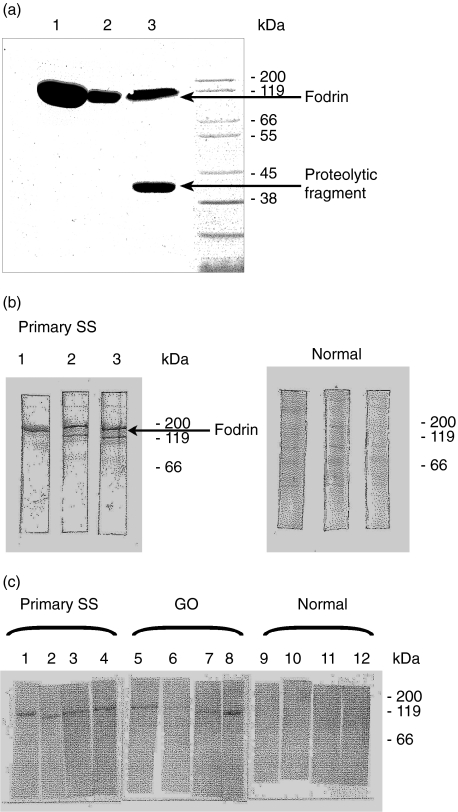
The120 kDa fodrin fragment was cloned, expressed and purified to determine whether sera of patients with GO cross react with the human α-fodrin fragment. Based on the published sequence, primers were designed to amplify the gene coding for α-fodrin fragment. The amplified fragment was cloned in the thioredoxin-tag expression vector pET. E. coli cells were collected and checked for the presence of α-fodrin by a small scale Ni-NTA purification and on SDS-PAGE. An aliquot of either BL21-CodonPlus™ (DE3)-RIL cells or BL21-Gold (DE3) cells, or E. coli M15 cells expressing the recombinant human α-fodrin gene, was induced at mid-logarithmic growth with 1 mm IPTG for 2 h. The expression of the protein in E. coli M15 resulted in a low yield of α-fodrin after induction with IPTG. Additional proteolytic cleavage of fodrin resulted in a fodrin migrating at a lower kDa value compared to the calculated molecular weight. Optimum yields of soluble protein were obtained after a transformation in E. coli BL21-CodonPlus host cells, growing at 30°C and an induction time of 2 h (Fig. 1a). A representative sera from a patient with SS bound to the 120 kDa protein in the three expression systems is shown as an immunoblot. A lower migrating degraded protein fragment was also seen in two expression systems not detected by Coomassie staining (Fig. 1b). Immunoblots incubated with sera from human healthy volunteers did not result in binding to the recombinant fodrin. Therefore, the 120 kDaA recombinant fodrin was purified by means of metal chelate affinity chromatography from a soluble extract of E. coli BL21-RIL CodonPlus host cells.
Fig. 1.
(a) Enhanced expression of recombinant human α-fodrin in BL21-CodonPlus™ cells: an aliquot of either BL21-CodonPlus™ (DE3)-RIL cells (lane 1 or far left) or BL21-Gold (DE3) cells (median lane 2), or E. coli M15 cells (right lane) expressing the recombinant human fodrin gene, was induced at mid-logarithmic growth with 1 mm IPTG for 2 h. After centrifugation each culture was homogenized in binding buffer, cleared by centrifugation and loaded on a Ni-NTA-resin. The eluted proteins were separated on a 10% acrylamide gel and stained with Coomassie® blue. (b) Immunoblot analysis with the recombinant alpha fodrin from experiment (a). Equal quantities of protein from the small scale Ni-NTA-purification of the above experiment were separated by electrophoresis, transferred to nitrocellulose membranes und probed with a 1: 200 dilution of a serum sample from a patient with a primary Sjögren syndrome or a serum sample from a human healthy volunteer. (c) Reactivities to recombinant alpha fodrin in GO sera. Purified protein was fractionated and transferred as in experiment (b) and probed with sera from the indicated humans.
Purity of α-fodrin was verified on SDS-PAGE and staining by Coomassie or the silver nitrate technique. Fodrin was found in a single band migrating with the expected size of 120 kDa. Although the Ni-column binds only His-tagged proteins, few other proteins with very low molecular weights were still detected after this purification step. Therefore an additional fractionation step by gel filtration was included. The final identity and purity was confirmed by mass spectroscopy analysis. The reactivity with sera of classified SS was used to confirm the structural integrity of the purified recombinant protein. Additional representative serum samples from patients with GO were also positive for this antigen under Western blot conditions, but not in control individuals (Fig. 1c).
Serological investigations
Subsequently, the presence of anti-α-fodrin antibodies was tested by a newly developed ELISA using the human α-fodrin fragment as autoantigen. Coefficients of variation (CV) were calculated for each of three anti-α-fodrin samples (taken from patients with low, moderate and high titres) based on the results of 24 determinations in a single run for intra-assay precision. Run-to-run precision was calculated from the results of six different runs with 24 determinations each. Intra-assay CV in the samples of the three samples was 4·0, 4·0 and 2·2% for mean concentrations of 15·9, 59·0 and 137·0 U/ml, respectively, whereas interassay CV was 2·9, 1·1 and 3·4% for mean concentrations of 15·7, 58·1 and 144·0 U/ml, respectively.
Antibodies against α-fodrin in patient sera were calculated using a cut-off defined as mean concentration in the sera of blood donors plus three standard deviations (s.d.). The lower detection limit for anti-α-fodrin IgG/IgA antibodies was determined at 1·0 U/ml. Means ± s.d. in sera for blood donors were 7·2 ± 3·8 U/ml and 9·9 ± 7·1 U/ml for IgA and IgG, respectively. In patients with GO, mean anti-α-fodrin titres were 11·7 ± 7·6 U/ml and 21·7 ± 10·0 U/ml for IgA and IgG antibodies, respectively. Patients had significantly higher levels of IgA and IgG (IgA: U = 7588·5; IgG: U = 20269·0; each, P < 0·001, Mann–Whitney U-test) than the healthy controls. In the patients with GO, a correlation (r = 0·3, P < 0·003) was noted between α-fodrin IgG and IgA antibodies. Anti-fodrin antibodies also correlated with SS-A antibodies (r = 0·3, P = 0·002).
In contrast to blood donors (<1% IgA only), α-fodrin antibodies were detected in 22% (n = 32) of patients with GO (P < 0·001; Fisher's exact test). In particular, IgA and IgG α-fodrin antibodies were present in 21 (15%) and 14 (10%) GO subjects, respectively. In comparison, antinuclear antibodies against the ribonucleoprotein (SS-A) and the La antigen (SS-B) were less prevalent in subjects with GO (n = 21 or 15%). A total of 45 patients with GO (31%) had at least one α-fodrin or SS-antibodies. GO patients with specific clinical signs of SS showed both SS- as well as high titres of α-fodrin antibodies. These subjects complained of abnormal lacrimal and salivary excretion ability. A negative correlation between gender and α-fodrin-IgA antibodies was noted (r = −0·27, P = 0·002) with higher titres of α-fodrin-IgA antibodies in males than in females (14·1 versus 11·1 U/ml). SS-A-60 antibody titres decreased strongly during steroid therapy in subjects with congestive GO (t = 3·21, P = 0·007, paired t-test). Furthermore, a trend for the α-fodrin-antibody titre to both correlate with the TSH-receptor antibody titre as well as to decrease in subjects with longer disease duration was noted. No significant relationship was noted between smoking, thyroid function and fodrin-antibodies.
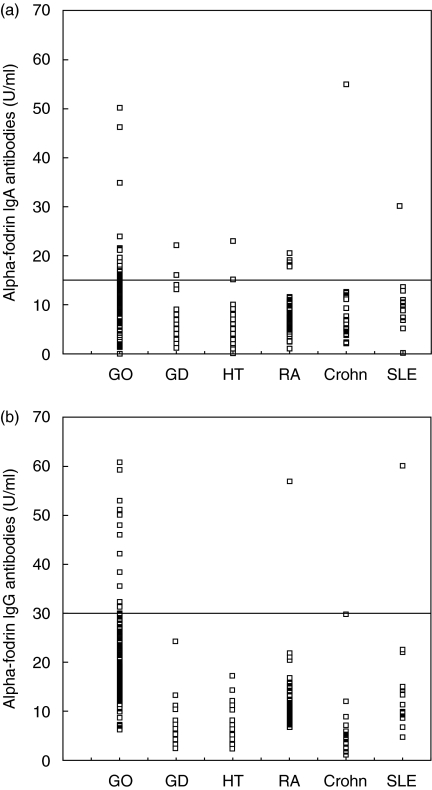
For comparison, prevalence and serum concentration of fodrin antibodies were also tested in subjects with various autoimmune diseases (Fig. 2). With respect to IgA antibodies, two of 55, four of 64, four of 52, one of 18 and one of 14 subjects with GD, HT, RA, Crohn disease and SLE, respectively, were positive. Regarding IgG antibodies, none of 55, one of 64, one of 55, none of 18 and one of 14 with GD, HT, RA, Crohn disease and SLE, respectively, were positive.
Fig. 2.
(a,b) Serum concentrations of anti-α-fodrin IgA and IgG autoantibodies. Cut-off values for IgA and IgG are shown as horizontal lines. GD: Graves' disease without manifest ophthalmopathy; GO: Graves' ophthalmopathy; RA: rheumatoid arthritis; Crohn: Crohn disease; HT: Hashimoto thyroiditis; SLE: systemic lupus erythematosus. Significant differences versus patients with GO for IgA: GD, P < 0·001; HT, P < 0·001; RA, P < 0·001; Crohn, P = 0·003 (Mann–Whitney U-tests). For IgG: GD, P < 0·001; HT, P < 0·001; RA, P < 0·001; Crohn, P < 0·001; SLE, P = 0·001.
Discussion
Clinical implications
In the last decades a large effort has been made to unravel the complex pathogenesis of GO. A key step in that process is identification of the orbital antigens responsible for triggering the local immune response. The TSH-receptor (R) is currently the best candidate autoantigen [12,13], but there remain uncertainties concerning the source of TSH-R in the orbit and its biological activity. Also, the orbital fibroblast remains the most likely target cell and the demonstration of TSH-R in the pre-adipocyte fibroblast population ties together the receptor and this cell type, but the non-pre-adipocyte fibroblast population could still express important antigens, with several studies demonstrating antibodies- and T cell-based responses suggesting that this is the case. In organ-specific autoimmune disease, target antigens are distinctively cell-specific proteins such as enzymes and receptors. Nevertheless, it is possible that local factors could somehow amplify an autoimmune response against one ubiquitous protein that, in other situations, does not lead to a significant change. In this paper, we have shown antibodies reactive against α-fodrin, a recently identified autoantigen associated with SS. In the sera of the GO patients, antibodies against this antigen correlated significantly with both TPO- as well as SS-antibodies. Because previous studies have shown that various antibodies are produced in the lacrimal gland of patients with SS, production of α-fodrin antibodies may indicate an inflammation of the lacrimal glands in GO patients more specifically. One may also consider this novel antibody as pathophysiologically associated with an orbital manifestation of Graves' disease. The fact that 22% only of the subjects with GO were positive for fodrin antibodies does not make α-fodrin a key autoantigen in this disease. Nevertheless, the presence of this antigen and probably its overexpression on the surface of lacrimal and orbital target cells may explain the frequent involvement of the lacrimal gland in patients with GO. In the congestive, early phase of the disease, this antigen may also enhance the autoimmune inflammatory reaction in the orbit through binding to autoreactive T cells with subsequent production of fodrin antibodies. Furthermore, determination of fodrin antibodies in Graves' patients may be predictive for development of SS in the future course of the disease.
Relationship between GO and fodrin
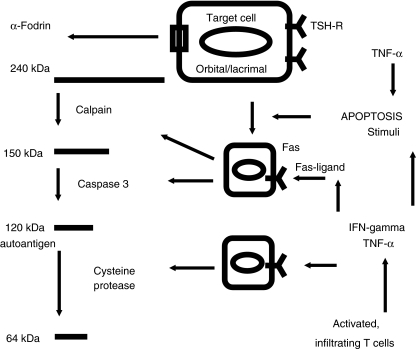
Fodrin undergoes a striking redistribution during apoptosis and relocate to the cell membrane of apoptotic cells. In subjects with GO and autoimmune involvement of the lacrimal gland, this alteration of fodrin structure may lead to antibody production. The fodrin α-subunit, which is cleaved in association with cytokine-mediated apoptosis of lacrimal- and adjacent orbital target cells, has binding sites for autoreactive T cells. The appearance of autoantigens, e.g. the TSH-R on the surface of induced cells, e.g. orbital fibroblasts could form the basis of a mechanism for antigen presentation, processing and antibody induction.
Furthermore, in patients with inflammatory, congestive GO, and most probably due to the expression of the TSH-R activated and infiltrating orbital lymphocytes, locally produce interferon (IFN)-γ and tumour necrosis factor (TNF)-α[14,15]. These proinflammatory Th-1 cytokines induce an increased expression of intracellular adhesion molecule-1 and HLA-DR on human lacrimal cells in vitro [7], which suggests that these cells are capable of antigen processing. These cytokines also induce Fas expression on human lacrimal and salivary gland cells in vitro [16]. Thus, autoantigens may be expressed both on lacrimal as well as on orbital target cells as a result of apoptosis induced by inflammatory cytokines, e.g. TNF-α (Fig. 3). In comparison, treatment of cultured primary thyroid cells with IFN-γ and TNF-α uniquely allowed the induction of Fas-mediated apoptosis [17–19]. In vivo, mice treated with IFN-γ and TNF-α showed significantly sustained lymphocytic infiltration in the thyroid compared with control animals. Furthermore, the number of apoptotic thyroid follicular cells was increased only in the thyroids from mice treated with the IFN-γ and TNF-α. These data demonstrate that this combination of inflammatory cytokines facilitates the apoptic destruction of thyroid follicular cells in experimental autoimmune thyroiditis, in a manner similar to that observed in Hashimoto's thyroiditis in humans.
Fig. 3.
Hypothetical role of α-fodrin in patients with GO. Presumed expression of the key autoantigen TSH-receptor on the surface of the various lacrimal gland epithelial- and orbital target cells leads to orbital infiltration of activated TH1 cells. Local secretion of proinflammatory cytokines, e.g. TNF-α and IFN-γ stimulates apoptosis of target cells via activation of the Fas–FasL pathway. Cytokine induced apoptosis leads to major structural changes of the target cells with subsequent expression of the α-fodrin subunit. Finally, Fas induced activation of caspases cleaves the fodrin subunit in a 120 kDa amino terminal fragment.
Hypothetical mechanism for the structural modification of fodrin in GO
Several proteins of the membrane skeleton, such as α-fodrin, were shown to be susceptible to caspases [20]. Cleavage of these molecules during apoptosis results in unstable cellular structures and subsequently in promoting processes in apoptosis. The glandular cell apoptosis in SS patients is mediated through a Fas/Fas ligand pathway [21,22]. Apoptosis facilitates entry of self-antigen into the cross-presentation pathway. A previously described cross-reactivity against the 120-kDa fodrin fragment suggests that altered distributions of α-fodrin in glandular epithelial cells may induce impaired secretory function [23]. Thus, expression of the antigenic fodrin fragment in lacrimal gland and orbital target cells may reflect an enhanced apoptotic cell death in patients with GO and SS. In our hands, anti-α-fodrin antibodies reacted with the 120-kDa fragment, but not to the intact molecule. It seems likely that the antigenic epitope within the amino-terminal region of fodrin is masked in the molecule containing the entire amino acid sequence even in its denaturated state [24]. Therefore, structural modification is required for expression of the antigenic epitope on fodrin recognized by antibodies in GO sera. Because antifodrin antibodies in GO sera reacted preferentially with the degradation product of fodrin lacking the spectrin repeats, rather than the intact fodrin, it is possible that the spectrin repeats, each of which folds into three αhelixes, interfere with antibody binding to the epitope located within the amino-terminal fodrin-specific region [25,26]. In our laboratory, the presence of both the intact fodrin molecule, as well as the amino terminal fragment, is being investigated in the tissue of GO subjects and in control samples.
Acknowledgments
We thank C. Biller, coworker of the thyroid research laboratory, for data collection.
References
- 1.Perrin D, Langley OK, Aunis D. Anti-α-fodrin inhibits secretion from permeabilized chromaffin cells. Nature. 1987;326:498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- 2.Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 3.Martin SJ, O'Brien GA, Nishioka WN, et al. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995;270:6425–8. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- 4.Vanags DM, Pörn-Ares MI, Copola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–85. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 5.Haneji N, Nakamura T, Takio K, et al. Identification of α-fodrin as a candidate autoantigen in primary Sjögren's syndrome. Science. 1997;276:604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 6.Kuwama M, Okano T, Ogawa Y, Kaburaki J, Kawakami Y. Autoantibodies to the amino-terminal fragment of β-fodrin expressed in glandular epithelial cells in patients with Sjögren's syndrome. J Immunol. 2001;167:5449–56. doi: 10.4049/jimmunol.167.9.5449. [DOI] [PubMed] [Google Scholar]
- 7.Yanagi K, Ishimaru N, Haneji N, Saegusa K, Saito I, Hayashi Y. Anti-120-kDa α-fodrin immune response with Th1-cytokine profile in the NOD mouse model of Sjögren's syndrome. Eur J Immunol. 1988;28:3336–45. doi: 10.1002/(SICI)1521-4141(199810)28:10<3336::AID-IMMU3336>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Sjögren H, Bloch K. Keratoconjunctivitis sicca and the Sjögren's syndrome. Surv Ophthalmol. 1971;16:145–59. [Google Scholar]
- 9.Maniatis T, Tasic B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature. 2002;418:236–43. doi: 10.1038/418236a. [DOI] [PubMed] [Google Scholar]
- 10.Rascher C, Pahl A, Pecht A, Brune K, Solbach W, Bang H. Leishmania major parasites express cyclophilin isoforms with an unusual interaction with calcineurin. Biochem J. 1998;334:659–67. doi: 10.1042/bj3340659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölschermann H, Rascher C, Oeschläger C, et al. Opposite regulation of tissue factor expression by calcineurin in monocytes and endothelia cells. J Immunol. 2001;169:7112–20. doi: 10.4049/jimmunol.166.12.7112. [DOI] [PubMed] [Google Scholar]
- 12.Weetman AP. Grave's disease 1835–2002. N Engl J Med. 2000;343:1236–48. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 13.Weetman AP, Kemp EH, Ridgway JN, Watson PF. Orbital autoantigens. In: Bahn RS, editor. Thyroid eye disease. Dordrecht, NL: Kluwer Academic Publishers; 2001. pp. 1–20. [Google Scholar]
- 14.Valyasevi RW, Jyonouchi SC, Dutton CM, Munsakul N, Bahn RS. Effect of tumor necrosis factor-alpha, interferon-gamma, and transforming growth factor-beta on adipogenesis and expression of thyrotropin receptor in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2001;86:903–8. doi: 10.1210/jcem.86.2.7188. [DOI] [PubMed] [Google Scholar]
- 15.Hiromatsu Y, Kaku H, Miyake I, Murayama S, Soejima E. Role of cytokines in the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12:217–21. doi: 10.1089/105072502753600160. [DOI] [PubMed] [Google Scholar]
- 16.McArthur C, Wang Y, Veno P, Zhang J, Fiorella R. Intracellular trafficking and surface expression f SS-A (Ro), SS-B (La), poly (ADP-ribose) polymerase and α-fodrin autoantigens during apoptosis in human salivary gland cells induced by tumor necrosis factor-α. Arch Oral Biol. 2002;47:443–8. doi: 10.1016/s0003-9969(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 17.Bretz JD, Rymaszewski M, Arscott PL, et al. Trail death pathway expression and induction in thyroid follicular cells. J Biol Chem. 1999;274:23627–32. doi: 10.1074/jbc.274.33.23627. [DOI] [PubMed] [Google Scholar]
- 18.Bretz JD, Arscott PL, Myc A, Baker JR. Inflammatory cytokine regulation in Fas-mediated apoptosis in thyroid follicular cells. J Biol Chem. 1999;274:25433–8. doi: 10.1074/jbc.274.36.25433. [DOI] [PubMed] [Google Scholar]
- 19.Wang SH, Bretz JD, Phelps E, et al. A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms non-destructive to destructive thyroiditis in experimental autoimmune thyroiditis. J Immunol. 2002;168:2470–4. doi: 10.4049/jimmunol.168.5.2470. [DOI] [PubMed] [Google Scholar]
- 20.Van de Water B, Tijdens IB, Verbrugge A, et al. Cleavage of the actin-capping protein α-adductin at Asp-Asp-Ser-Asp633-Ala by caspase-3 is preceded by its phosphorylation on serine 726 in cisplatin-induced apoptosis of renal epithelial cells. J Biol Chem. 2000;275:25805–13. doi: 10.1074/jbc.M001680200. [DOI] [PubMed] [Google Scholar]
- 21.Ishimaru N, Yoneda T, Saegusa K, et al. Severe destructive autoimmune lesions with aging in murine Sjögren's syndrome through Fas-mediated apoptosis. Am J Pathol. 2000;156:1557–64. doi: 10.1016/S0002-9440(10)65027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishimaru N, Yanagi K, Ogawa K, Suda T, Saito I, Hayashi Y. Possible role of organ-specific autoantigen for Fas ligand-mediated activation-induced cell death in murine Sjögren's syndrome. J Immunol. 2001;167:6031–7. doi: 10.4049/jimmunol.167.10.6031. [DOI] [PubMed] [Google Scholar]
- 23.Saegusa K, Ishimaru N, Yanagi K, et al. Prevention and induction of autoimmune exocrinopathy is dependent on pathogenic autoantigen cleavage in murine Sjögren's syndrome. J Immunol. 2002;169:1050–7. doi: 10.4049/jimmunol.169.2.1050. [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Tsubota K, Ono M, et al. Possible involvement of EBV-mediated α-fodrin cleavage for organ-specific autoantigen in Sjögren's syndrome. J Immunol. 2001;166:5801–9. doi: 10.4049/jimmunol.166.9.5801. [DOI] [PubMed] [Google Scholar]
- 25.Aki T, Yoshida KI, Fujimiya T. Phosphoinositide 3-kinase accelerates calpain-dependent proteolysis of fodrin during hypoxic cell death. J Biochem. 2002;132:921–6. doi: 10.1093/oxfordjournals.jbchem.a003305. [DOI] [PubMed] [Google Scholar]
- 26.Boulanger L, Sabatino DE, Wong EY, et al. Erythroid expression of the human α-spectrin gene promoter is mediated by GATA-1- and NF-E2-binding proteins. J Biol Chem. 2002;277:41563–70. doi: 10.1074/jbc.M208184200. [DOI] [PubMed] [Google Scholar]