Abstract
Migration of intraepithelial lymphocytes (IELs) into intestinal epithelium is not yet well understood. We established an IEL-cell line from ovalbumin (OVA) 23–3 transgenic (Tg) mice and investigated the effect of antigen stimulation on the dynamic process of IEL migration into small intestinal mucosa. The cell line was a T cell receptor (TCR) αβ+ CD4+ CD8– phenotype, expressing αEβ7 integrin in 90% of cells. Under intravital microscopy, the lined IELs adhered selectively to the microvessels of the intestinal villus tip of the Tg mice. The accumulation of IELs was significantly inhibited by an antibody against β7-integrin and MAdCAM-1. When IELs were stimulated with OVA, the accumulation was attenuated compared to that of resting cells, with decreased expression of αEβ7 integrin. In Tg mice fed with OVA, the number of IELs which migrated in the villus mucosa was significantly smaller than in the non-fed controls. The preferential migratory capacity of IELs to villus mucosa may be altered by specific antigen stimulations.
Keywords: adhesion molecules, cell trafficking, intraepithelial lymphocytes, ovalbumin, small intestine
Introduction
The intestinal epithelium is a region constantly exposed to intraluminal substances, such as intestinal flora and food antigens. It has been suggested that the functions of intraepithelial lymphocytes (IELs) are first-stage protection and maintenance of the epithelial layer. IELs consist of both αβ and γδ T cell receptor (TCR)-bearing cells with phenotypic and functional features distinct from those of cells in the peripheral lymphoid tissue [1]. Indeed, peripheral T cells and IELs have many different characteristics; for example, concanavalin A (ConA), which is mitogenic to peripheral T cells, cannot stimulate IELs [2]. These lymphocytes, mainly CD8+ T cells, are largely unresponsive to proliferative signals mediated via conventional stimulation of the CD3–TCR complex [3]. Most IELs express characteristic integrin αEβ7, which is hardly observed in peripheral T cells [4,5].
These T cells have diverse sources of origin: it is known that most peripheral T cells differentiate in the thymus, but a large population of IELs differentiate extrathymically. Some IELs may arise in situ in the gut epithelium [6]. Some may develop in the thymus, and yet others come from extraintestinal, extrathymic sources, and these lymphocytes reach the intestine. IEL precursors in the blood may gain access to the mucosal sites. It has been speculated that the γδ T cells present in the vaginal epithelium originate from the peripheral lymphoid organs [7]. There have been reports that injected peripheral T cells, under adequate conditions, can fill the epithelial compartments and acquire the characteristics of IELs [6,8]. However, there has been little information about the exact route and mechanisms by which IELs reach the microvessels of intestinal villi before they gain access to the epithelium.
There is evidence that the homing behaviour of lymphocytes can be altered profoundly by activation and differentiation. The migration properties of activated lymphocytes appear to be both more selective and more diverse than those of naive lymphocytes. Some migration properties of ‘memory’ lymphocytes resemble more closely those of activated lymphocytes [9–11]. Most memory and effector lymphocytes probably traffic through lymphoid organs, but unlike naive cells, they can also access and recirculate through extralymphoid immune effector sites such as the intestinal mucosa or inflamed skin and joints [11–13]. In humans, for example, CD4 cells that express both CLA and L-selectin preferentially accumulate in inflamed skin [14]. However, the effect of antigen-specific activation of IELs on their trafficking within microvessels supplying blood to the gut mucosa remains unclear.
Much remains to be clarified with regard to the functions and homing patterns of IELs and their molecular basis. One reason for the difficulty in studying IELs is the lack of appropriate cell lines established from these populations. The difficulty of establishing IEL cell lines is due to the poor proliferative capacity and a lack of knowledge about their adequate ligands. In the present study, we have established an antigen-specific IEL cell line from ovalbumin (OVA)23-3 mice expressing a transgenic (Tg) TCRαβ specific to OVA323-339 [15]. The majority of T cells in these mice expressed a TCR specific to this epitope, which gave us an advantage in establishing the antigen-specific cell line. Using this cell line, we analysed the functional characteristics of cells, such as their cytokine production and antigen-specific proliferation.
Recent in situ microscopy experiments with intestinal mucosa have demonstrated that lymphocyte homing involves organ-specific multi-step cascades of adhesion and signalling events in specialized blood vessels, termed high endothelial venules (HEV), as well as in the villus microvessels [16–18]. In this study, using the established IEL cell line we carried out an intravital microscopic procedure to monitor the dynamic process of lymphocyte migration in order to (1) investigate whether adhesion of IEL cells occurs in the villus mucosa of the small intestine and, if it does, examine a possible contribution of various adhesion molecules to this IEL–endothelial cell adhesive interaction, and (2) compare how recruitment of naive and antigen-specifically stimulated IEL cells differs in the villus mucosa of the small intestine.
Methods
OVA23-3 Tg mice and isolation of IEL
The process of establishing the OVA23-3 Tg mouse line we used has been described previously [15]. The mice carried a gene encoding TCR αβ (Vα3.1/Vβ15) derived from an OVA-specific CD4+ T cell clone, 7-3-7. The animals were housed and bred within animal facilities at the University of Tokyo. We obtained transgenic mice from the F1 generation of a cross between BALB/c (Clea Japan, Inc., Tokyo, Japan) and heterozygous transgenic mice. The care and use of laboratory animals were in accordance with the guidelines of the National Institute of Health.
IELs were isolated from Tg mice of both sexes, 8–24 weeks of age, by using modified procedures as described previously [19]. Briefly, an inverted intestine was cut into four segments and the segments were transferred into a 50-ml conical tube containing 45 ml of 5% fetal calf serum (FCS) in Ca2, Mg2-free Hanks's balanced salt solution (HBSS; Gibco Laboratories, Grand Island, NY, USA). The tube was shaken in an orbital shaker at 150 r.p.m. in the horizontal position for 45 min at 37°C. Cell suspensions were collected and passed through a glass-wool column to remove cell debris and adherent cells. Subsequently, the cells were suspended in 30% (wt/vol) Percoll (Pharmacia Biotech, Uppsala, Sweden) and centrifuged for 20 min at 600 g. After the centrifugation, the cells at the bottom of the solution were subjected to Percoll discontinuous-gradient centrifugation and IELs were recovered at the interface of 44% and 70% Percoll (>95% were CD3+). The obtained cell suspensions were washed and stored on ice in RPMI (pH 7·4) with 5% FCS until used.
Establishment of IEL cell line and antigen stimulation
IELs from OVA23-3 mice (106 cells/ml) were stimulated every week with mitomycin C-treated CD4– BALB/c splenocytes (2 × 106 cells/ml) in a culture medium with 5 mM OVA323-339 peptide and a 10% culture supernatant of ConA-stimulated rat splenocytes [20]. The method of mitomycin C treatment was as follows: 107 splenocytes/ml were incubated with 50 mg/ml of mitomycin C (Sigma) for 45 min at 37°C and washed with an RPMI-1640 medium (Nissui Pharmaceutical, Tokyo, Japan) three times. The medium for the cell culture was RPMI-1640 containing 100 U/ml penicillin, 100 mg/ml streptomycin, 5 × 10−5m 2-mercaptoethanol, and 10% FCS (Cansera International, Rexdale, Canada). Several weeks after the initiation of the culture, the growing cells were expanded and then an antigen-specific IEL line from the OVA23-3 mice was established. The IEL cell line was induced to rest by changing only the culture medium without antigen-presenting cells once a week for 4 weeks after the final antigen stimulation.
An antigen-stimulated IEL cell line was obtained by removing CD4– splenocytes by magnetic cell sorting (MACS) soon after the final antigen stimulation. In brief, the IELs (1 × 107) with CD4– splenocytes were suspended in 90 µl of phosphate buffered saline (PBS) containing 0·5% bovine serum albumin and 5 m M ethylenediaminetetra acetic acid (EDTA) and incubated in 10 µl of antimouse CD4 (L3T4)-labelled MACS microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) for 15 min at 6°C. After that, the treated IELs were passed through a separation column (type MS, Miltenyi Biotech) placed in the magnetic field of a MACS separator. The magnetically labelled CD4+ IEL cells were retained in the column, while the unlabelled CD4– splenocytes ran through. After removing the column from the magnetic field, the retained CD4+ IEL cells could be eluted as an antigen-stimulated IEL cell line. The CD4+ IEL cells were washed and, until used, resuspended in RPMI-1640 with 5% FCS stored on ice.
Cell proliferation and cytokine production assay
The cell line was plated in 96-well plates at 1 or 2 × 105 cells/well with OVA or ConA (10 mg/ml) and at 4 × 105 cells/well with antigen-presenting cells (APC) (mitomycin C-treated BALB/c splenocytes) in a total volume of 200 ml. After 24 and 48 h, 0·5 mCi of [3H]thymidine was added to each well. The cells were harvested 20 h later and the [3H]thymidine incorporation was measured by scintillation counting. Cytokines in the culture supernatants were detected using a two-site sandwich enzyme-linked immunosorbent assay (ELISA) as described previously [21]. Briefly, for assays of interleukin (IL)-4, IL-5 and interferon (IFN)-γ, Maxisorp immunoplates (Nunc Roskilde, Denmark) were coated with a rat antimouse IL-4 (BVD4–1D11, PharMingen, San Diego, CA, USA), a rat antimouse IL-5 and a rat antimouse IFN-γ antibody (XMG1.2). This was followed by incubation with alkaline phosphatase-streptavidin. A substrate (p-nitrophenyl phosphate) was added and the colour development was stopped by the addition of 5 N NaOH. The absorbance was determined at 405 nm.
Flow cytometry
Cells (2 × 105/sample) were washed with HBSS containing 5% FCS and 0·2% NaN3 (the flow cytometry buffer) and centrifuged at 4°C, 400 g for 5 min. Each antibody was diluted appropriately with the flow cytometry buffer and then 25 µl was added to each cell preparation. The cells were stained on ice for 20 min, with mixing every 5 min. If the used antibodies were conjugated to biotin, the cells, after having been washed twice, were further stained with streptavidinylated fluorochrome. The samples were washed again, and 500–700 µl of flow cytometry buffer were added for analysis. Flow cytometry was performed using a FACSort machine (Becton Dickinson Immunocytometry Systems, Mountain View, CA, USA). Aggregated cells were excluded from the analysis by gating a forward and a side scatter.
The MoAbs used in this study were as follows: an anti-pan TCR β chain (H57-597) conjugated to biotin and anti- Thy 1·2 (30-H12) conjugated directly to R-phycoerythrin (R-PE) were purchased from PharMingen, FITC-anti-CD4 (YTS191.1.2) and R-PE-anti-CD8α (53.6.7) were purchased from Gibco BRL (Gaithersburg, MD, USA); streptavidin-R-PE was purchased from Gibco BRL, anti-αEβ7 (2E7) was presented by Lefrancois et al. (22), and FITC-antihamster IgG (H + L) was purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL, USA). Antibodies against mouse L-selectin (MEL-14, rat IgG2a), α4-integrin (R1-2, rat IgG2b), αE-integrin (M290), β7-integrin (FIB27), CD11a (M17/4) and CD3 (145-2C11, hamster IgG) were purchased from PharMingen.
Lymphocyte labelling with carboxyfluorescein diacetate succinimidyl ester (CFSE)
CFDSE (Molecular Probes, Eugene, OR, USA) was dissolved in dimethylsulphoxide to 15·6 mm and a small aliquot (300 µl) was stored in a cuvette sealed with argon gas at – 80°C until the experiments were conducted. Lymphocytes (1 × 107) were incubated in CFSE solution (20 µl of stock solution was diluted with 20 ml of RPMI-1640) for 30 min at 37°C. The labelled lymphocytes were centrifuged immediately through a cushion of heat-inactivated fetal bovine serum and washed twice with a cold suspension medium. The cells were resuspended in 0·2 ml of the medium and used within 30 min.
Intravital observation of lymphocyte migration in intestinal mucosa
The intestinal villi were observed from the mucosal surface and lymphocyte migration was also observed. After an intraperitoneal injection of pentobarbital sodium (50 mg/kg), the abdomen was opened via a midline incision. A 7-cm ileal segment ending at the caecal valve was gently extended onto a plate and a longitudinal incision of about 2 cm was made in the middle of the segment by microcautery along its antimesenteric border. The intestine was kept warm and moist by continuous superfusion with physiological saline warmed to 37°C. The adjacent intestinal segment and mesentery were covered with absorbent cotton soaked with Krebs–Ringer solution.
Suitable areas of villus tips were observed from the mucosal surface by an inverted fluorescence microscope (Diaphot TMD-2S, Nikon, Tokyo, Japan) and the observation was recorded by using a videotape recording system. The same area of ileal mucosa was always examined throughout the observation period. The behaviour of fluorescently labelled lymphocytes was visualized on a television monitor by using a fluorescence microscope equipped with a silicon intensified target image tube (SIT) camera with a contrast-enhancing unit (C-2400–08, Hamamatsu Photonics Co., Shizuoka, Japan) according to a method described previously [16,18]. In this setting, the tip of each villus was observed as an oblique circle, and archade microvessels in the villi were also observed. In another set of experiments, a 5-cm ileal segment ending at the caecal valve was chosen for observation of Peyer's patches. Two small incisions in the bowel wall were made and the luminal pressure of the gut loop was maintained at 10 cmH2O with physiological saline. The microcirculation in Peyer's patches was observed through the serosa by microscope. Epi-illumination was achieved by using filters for excitation at 470–490 nm and for emission at 520 nm. Lymphocytes (1 × 107 dissolved in 1 ml) were injected into a jugular vein of the recipient mice for 3 min. The cell kinetics of the infused lymphocytes, their interaction with microvascular beds and their accumulation in the villus mucosa or Peyer's patches were monitored and recorded continuously on S-VHS videotapes for the first 20 min and then, at 10-min intervals, for 40 min. Lymphocytes adhering to the microvessels of the villus mucosa or Peyer's patches and remaining in the same position without movement for more than 30 s were defined as ‘sticking’ lymphocytes. The number of sticking lymphocytes was determined in a 1-mm2 area observed in a video image.
Histological examination
The localization of infused IEL cells to intestinal mucosa was assessed immunohistochemically by using the labelled streptavidin–biotin (LSAB) method. Forty minutes after infusion of CFSE-labelled IELs, the small intestine was removed and fixed in a periodate, lysine–paraformaldehyde (PLP) solution. The samples were embedded in OCT compound (Miles, Elkhart, IN, USA) before being frozen in dry ice and acetone. Cryostat sections were reacted with MoAb of factor VIII (rabbit polyclonal; Dako, Carpinteria, CA, USA) or CD34 (RAM34; PharMingen, San Diego, CA, USA) overnight at 4°C after they were incubated in 5% normal goat serum in PBS. Sections were incubated with a second antibody, rhodamine-conjugated antirat IgG antibody (Chemicon International, Temecula, CA, USA) for 1 h at room temperature. The fluorescent preparations were examined using a laser-scan microscope (Carl Zeiss, Jena, Germany) at 488 nm for CFSE and 543 nm for rhodamine.
Administration of antibodies and OVA feeding
In some experiments lymphocytes were preincubated with MoAbs, which functionally block adhesion molecules. Antibodies against αE-integrin (M290, rat IgG2a), β7-integrin (FIB27, rat IgG2a) and CD11a (M17/4, rat IgG2a) were purchased from PharMingen Co. (San Diego, CA, USA) and 1 × 107 cells were incubated in 100 µg/ml of MoAbs for 30 min before the infusion of T lymphocytes. In other experiments, anti-MAdCAM-1 MoAb (MECA367, PharMingen, 2 mg/kg) dissolved in 0·2 ml of saline was infused from a jugular vein at 30 min before the injection of T lymphocytes. As controls, isotype-matched irrelevant antibodies were also used under the same conditions.
In another set of experiments, OVA 23–3 Tg mice were fed with OVA at a dose of 200 mg/day for 3 days and the alteration of migration patterns of IEL cell lines (stimulated and unstimulated) in the villus microvessels was compared with that in unfed mice.
Statistics
All results are expressed as means ± s.d. The differences among groups were evaluated by one-way analysis of variance (anova) and Fisher's post-hoc test. The cut-off for statistical significance was set at P < 0·05.
Results
Expression of surface antigens and characteristics of the IEL cell line
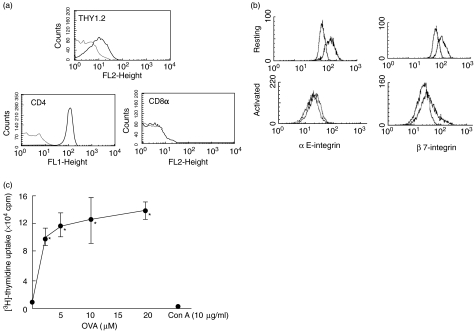
IELs from OVA 23–3 mice were stimulated with antigen-presenting cells and OVA323-339 peptide every week. One IEL line from the OVA23-3 mice was obtained several weeks after the initiation of the culture, and the growing cells were expanded and then used for analysis. Figure 1 shows the expression of surface markers of the IEL cell line obtained by flow cytometry. The cell line expressed TCRαβ and CD4 but not CD8α. We also detected a Thy1 molecule, which is a pan-T cell marker. Expression of various adhesion molecules (L-selectin, α4-integrin, αE-integrin, β7-integrin and CD11a) on the surface of the IEL-line cells was determined. As shown in Fig. 1b, the IEL line cells always showed a strong expression of αE- and β7-integrin molecules on their surface. There was also expression of CD11a. However, there was no expression of L-selectin and α4-integrin molecules in the IEL cell line. However, the extent of the expression of αE- and β7-integrin molecules was decreased significantly when these cells were stimulated with a specific antigen, OVA.
Fig. 1.
Expression of surface antigens on intraepithelial lymphocyte (IEL) cell line established from ovalbumin (OVA)23-3 transgenic mice; 2 × 105 lymphocytes were first incubated with antimouse monoclonal antibodies against Thy1·2 (30-H12), pan T cell receptor (TCR) β chain (H57-597), CD4 (YTS191·1.2), CD8a (53.6.7), αE-intergin (M290), β7-integrin (Fib27), l-selectin (MEL-14) and CD11a (M17/4). They were then incubated with 1 ml of fluorescein isothiocyanate (FITC)-labelled antirat IgG and antihamster IgG. Flow cytometric analysis was performed using FACSort (Becton Dickinson). Data on viable cells, as determined by forward light-scatter intensity, were obtained using consort software. Representative data from at least four individual measurements are shown. (a) Thy 1·2, CD4 and CD8α expression on resting IEL cell line (b); αE and β7 expression on resting and activated IEL cell line. For IEL cell line activation, cells were stimulated by a specific antigen, OVA (20 µm), for 20 h. (c) Antigen-specific and mitogenic proliferation of IEL cell line. Uptake of [3H]-thymidine was examined as described in Materials and methods. Proliferation of IEL cell line in response to different concentrations of ovalbumin (OVA, 1–20 µm) and concanavalin A (ConA, 10 µg/ml) was determined. Values are means ± s.d. from six experiments. *P < 0·05 versus OVA 0 µm.
The cell line was cultured with intact OVA and ConA in the presence of mitomycin C-treated BALB/c splenocytes. Figure 1c shows the proliferation of this cell line in response to OVA and ConA as determined by [3H]-thymidine uptake. OVA stimulation showed a significant proliferation of the IEL cell line at concentrations greater than 3 µ M. In contrast, ConA did not induce such proliferation even at a concentration of 10 µg/ml, by which the proliferation of splenocytes is usually strongly stimulated. The unresponsiveness of the IEL line to ConA was also confirmed by microscopic observation (data not shown). Next we analysed the cytokine production by the cell line. Secretion of three different cytokines (IFN-γ, IL-4 and IL-5) was determined after stimulation with OVA. ELISA of the culture supernatants showed that the cell line produced only IL-5 (3·0 ± 0·60 ng/ml; n = 5) without OVA stimulation. On the other hand, antigen stimulation induced all of these cytokines, and the IEL cell line secreted 88 ± 9·2 U/ml IFN-γ (n = 4), 170 ± 18 pg/ml IL-4 (n = 4) and 11 ± 2·1 ng/ml IL-5 (P < 0·05 versus without OVA; n = 4) after stimulation with OVA at a concentration of 10 µ M.
Migration of IELs in villus mucosa
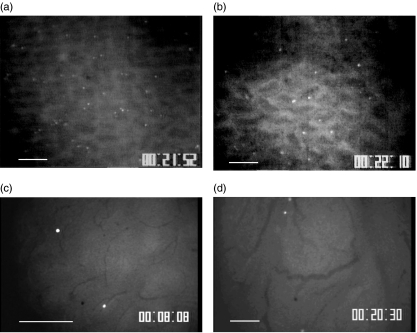
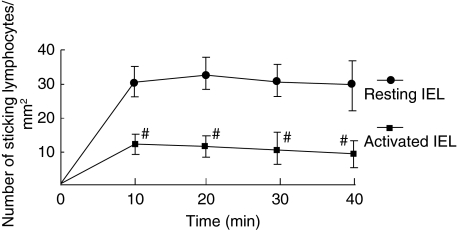
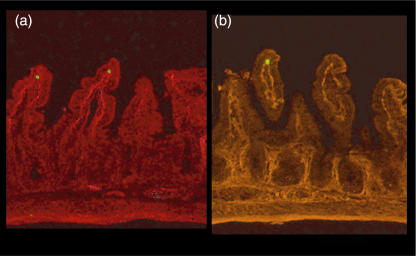
We investigated the migration of IEL line cells to the villus mucosa by observation from the mucosal side. Figure 2 shows a microscopic picture of T lymphocytes adhered to the archade microvessels of a villus tip in the ileal mucosa. As shown in Fig. 2a and c, a significant number of IEL line cells accumulated in the microvessels of the lamina propria of the villi 20 min after injection. IELs adhered to the microvessels of the villus mucosa without rolling. Some IEL cells were also observed at the base of crypt, but few IELs were present inside the submucosal venules (data not shown). On the other hand, in Peyer's patches only a few IEL cells showed ‘rolling’ behaviour (less than 5%), and almost no IEL cell adherence was observed in postcapillary venules of Peyer's patches during the observation, as shown in Fig. 2d. Figure 3 illustrates the time–course change in the number of sticking IEL line cells with and without activation in the villus mucosa of the OVA23-3 mice. The number of IEL cells accumulated in the microvessels of the villus tips increased rapidly, especially within the first 10 min, reached a maximum at 20 min, and then showed no significant change during the observation. When the IEL cells were activated with OVA, the number of adherent cells was significantly smaller compared to that of resting cells at any given time. However, the total number of IEL cells that had entered villus microvessels did not differ significantly between with and without activation in the OVA23-3 mice (control, 22·4 ± 3·1/min; with activation, 19·1 ± 3·8/min). To confirm whether injected IEL cells were within the epithelium of the villi, we examined the tissue section of intestinal mucosa 40 min after the administration of CFSE-labelled cells. Figure 4 shows the adhesion site of infused IELs and the location of Factor-VIII+ or CD34+ microvessels. It has been demonstrated that these cells coincided well with the lamina propria microvessels of ileal villi.
Fig. 2.
Representative images of the distribution of a carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled resting intraepithelial lymphocyte (IEL) cell line in control mice (a) and in ovalbumin (OVA)-fed mice (b) Following adherance to the microvessels of a villus tip of the ileal mucosa at 20 min after infusion ( × 10). Bar represents 100 µm. (c) Higher magnification image of labelled IEL cell line in control mice adhered to arcade microvessels of villus tips ( × 20). Bar represents 100 µm. (d) Observation of CFSE-labelled resting IEL cell line postcapillary venules of Peyer's patches 20 min after infusion. There were few sticking IELs in this area ( × 10).
Fig. 3.
Time–course change in the number of sticking intraepithelial lymphocyte (IEL) line cells to villus mucosa in control ovalbumin (OVA)23-3 mice. The number of cells accumulated in the microvessels of villus tips is compared between unstimulated (resting) and activated (stimulated with OVA, 20 µm) IEL cell lines. The lymphocytes located in the 1-mm2 observation field were counted. #P < 0·05, compared with resting IELs. Values are means ± s.d. for six animals.
Fig. 4.
Representative pictures of simultaneous observation of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled intraepithelial lymphocyte (IEL) (green) and factor VIII-positive (a) or CD34-positive (b) microvessels (red fluorescence) in small intestinal villi as determined by immunohistochemistry. The lysine–paraformaldehyde (PLP)-fixed sections 40 min after IEL infusion were observed (× 100).
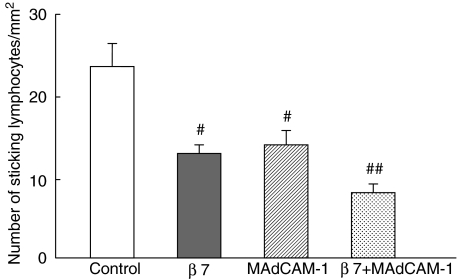
Figure 5 shows the inhibitory effect of the function-blocking of adhesion molecules on the sticking of unstimulated IEL line cells to archade microvessels of the villus mucosa at 20 min. The number of sticking lymphocytes in the control group was 24·0 ± 2·1 cells/mm2, but this number decreased significantly as a result of pretreatment with MoAb, which blocks β7-integrins. Preinfusion of an anti-MAdCAM-1 antibody into the mice also significantly inhibited this cell interaction. However, the inhibitory effect of these antibodies was found to be partial, and more than 50% of lymphocytes remained adherent after administration of antiβ7 and anti-MAdCAM-1, respectively. Moreover, the combined blocking of β7-integrin with MAdCAM-1 further attenuated the sticking of IELs in this area, although it only partially blocked the IEL adhesion. On the other hand, antibodies against either αE-integrin or CD11a did not significantly inhibit the IEL accumulation.
Fig. 5.
The inhibitory effect of function-blocking of adhesion molecules on the sticking of resting intraepithelial lymphocyte (IEL) line cells to microvessels of villus mucosa at 20 min. The effect of monoclonal antibody against β7-integrin (Fib27) and MAdCAM-1 (MECA367)-treatment on sticking of IELs was investigated. IELs were treated with monoclonal antibody (100 µg/ml) against β7-integrin before infusion. In some experiments, the animals were pretreated (30 min before lymphocyte infusion) with a monoclonal antibody against MAdCAM-1 (2 mg/kg). The combined effect of functional blocking of β7-integrin and MAdCAM-1 molecules was also examined. #P < 0·05, compared with controls. ##P < 0·05, compared with β7-integrin- and MAdCAM-1-blocking alone. Values are means ± s.d. for six animals.
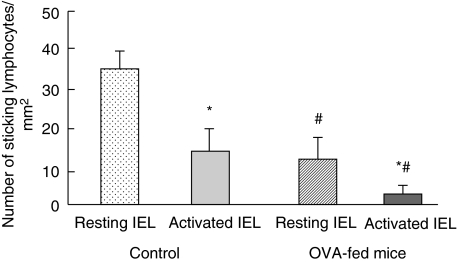
Figure 6 compares the number of IEL cells that adhered to the microvessels of the villus tips in the OVA23-3 control mice to that in the OVA-fed mice 20 min after the injection.Figure 2b shows a microscopic picture of resting IELs adhered to the microvessels of the villi in the ileal mucosa of the OVA-fed mice. Although the number of resting IEL cells increased gradually in the microvessels of the OVA-fed mice, showing a time–course change similar to that in the control mice (data not shown), the number of sticking cells was significantly lower than that in the control transgenic mice. Similarly, in the case of stimulated IELs, only a small number of activated IELs were observed in the microvessels of the OVA-fed mice and this number did not increase significantly during the observation (Fig. 6).
Fig. 6.
Comparison of the number of intraepithelial lymphocyte (IEL) line cells adhered to the microvessels of villus tips in ovalbumin (OVA)23-3 control mice with that in OVA-fed mice, 20 min after injection. OVA 23–3 transgenic mice were fed with OVA at a dose of 200 mg/day for 3 days (OVA-fed mice), and the number of adhered IEL cell lines (resting and activated) was compared with that in unfed mice (control). #P < 0·05, compared with unfed controls. Values are means ± s.d. for six animals. *P < 0·05, compared with resting IELs.
Discussion
In the present study, we established an Ag-specific IEL line from OVA23-3 TCR-Tg mice. To our knowledge, this is the first report on the establishment of an antigen-specific IEL line. This is because there has been no suitable method to induce expansion of IEL clones, which would respond to a specific antigen, as no appropriate antigens could be found to stimulate IELs. In this study we used Tg mice whose TCR restriction and specific ligands are well known and we also used, instead of an intact antigen, an OVA323-339 peptide which can cause significant stimulation of the culture at an early stage. The cell line established from OVA23-3 expressed Thy1·2, TCR αβ and CD4, but not CD8α. In our preliminary study, we also observed that CD4+ IELs freshly isolated from the Tg mice proliferated more strongly than CD4– IELs. These results suggest that in OVA23-3 mice, CD4+ CD8 IELs have the capacity to proliferate selectively for a long time. This preferential selection of CD4+ IELs may be due to the TCR in these Tg mice being restricted to I-Ad, major histocompatibility complex (MHC) class II molecules [15].
The cell line expressed TCR αβ and CD4 in a way similar to that of peripheral T cells, and did not express TCRγδ and CD8αα, which are the more common phenotypes of IELs. However, the cell line also expressed αEβ7 integrin, which is a characteristic adhesion molecule expressed in 80–90% of IELs. We also showed that the cell line could not respond to ConA, in spite of its proliferative capacity to specific Ag. These observations suggest that the IEL cell line we established does, in fact, possess the characteristics specific to IELs and that these characteristics differ from those of peripheral T cells. Note also that we used a cell line with some characteristics of mature IELs for demonstrating possible mechanisms of localization to the intestinal villi, whereas we do not know what the characteristics of the circulating IEL precursor is. Therefore, there is a possibility that the circulatory precursor could express undiscovered adhesion molecules which are down-regulated on mucosal entry.
The ELISA of culture supernatants showed that this cell line can produce IFN-γ, IL-4 and IL-5. The secretion of these cytokines was dose-dependently stimulated by a specific antigen. CD4+ T cells have largely been grouped into two distinct subsets, Th1 cells producing IL-2 and IFN-γ, and Th2 cells producing IL-4 and IL-5 [23]. It appears that IEL cell lines produce both types of cytokines, suggesting that these cell lines consist of a mixed population of Th1 and Th2 helper T cells. This is in accordance with the previous finding by Fujihashi et al., who reported that CD4+ IELs produced IL-4, IL-5 and IFN-γ[24].
In the present study, we have demonstrated that there is a significant accumulation of IEL line cells in the villus mucosa of the small intestine, not in the HEVs of Peyer's patches. We have reported previously that freshly isolated IELs from the intestinal mucosa of BALB/c mice showed very little interaction with Peyer's patch–HEVs, due possibly to a lack of l-selectin and weak expression of α4-integrin molecules [18], which is similar to the present results. In this study, accumulation of IEL line cells was observed along the archade microvessels near the epithelial cells of villus tips. This adhesion was significant, and was abrogated partially by antiβ7-integrin and by anti-MAdCAM-1 antibodies. Because the involvement of α4-integrin in IEL migration was almost negligible in this study, we do not know the exact reason for the paradoxical finding that migration is not inhibited by an anti-αE integrin, but is inhibited by anti-MAdCAM-1. Several studies have shown that αEβ7 is not a ligand for MAdCAM-1, and αEβ7 is not believed to participate in lymphocyte–endothelial cell interaction in the vascular endothelium [25,26]. Instead it has been shown that αEβ7 mediates adhesion of lymphocytes to epithelial cells [4,5], and it is also speculated that this integrin may be involved in the migration of lymphocytes to epithelial sites [27]. Therefore, there is a possibility that β7 and MAdCAM-1 could be functioning separately in this situation, although the counter ligands for β7-integrin or MAdCAM-1 in IEL interaction with the villus mucosa remain to be identified. An additive inhibitory effect of antiβ7-integrin and anti-MAdCAM-1 antibody on IEL adhesion could support this possibility, although there is another possibility that each antibody was not completely neutralizing. Moreover, it should be also noted that even the combination of both antiβ7 integrin and anti-MAdCAM-1 antibodies reduced the IEL adherence to microvessels of villi to only about 65% of what it was in the controls, suggesting that other mechanisms account for lymphocyte migration in this site. These other mechanisms may include the G-protein-independent mechanisms, such as capillary plugging.
In this study, we demonstrated that the pattern of IEL migration to the villus mucosa changed significantly after stimulation by a specific antigen. When the IEL line cells were activated with OVA, the adhesion of the antigen-stimulated IELs to the villus mucosa was significantly attenuated compared to that of the resting cells, with decreased surface expression of both αE- and β7-integrins. Because we found that an antiβ7-integrin antibody decreased the adherence of IEL line cells to villus microvessels, while an anti-αE antibody did not, the decreased cell surface expression of β7-integrin might be responsible for the decreased migration of antigen-stimulated IELs to the intestinal mucosa. One can speculate that changes in the expression of adhesion molecules could also interfere with the IEL interaction and migration to intestinal epithelial cells. We also demonstrated that in Tg, OVA-fed mice, the number of IEL line cells that accumulated in the villus mucosa was significantly lower than that in the non-fed control mice both in terms of resting and activated cells. In particular, in the case of antigen-activated IELs, the cell migration into the villus mucosa in the OVA-fed mice was almost completely eliminated. The exact mechanism of this inhibition is not known, but there is a possibility that factors other than adhesion molecules, including neurohumoral factors and chemokines, can also be involved in this inhibition. The immune hyporesponsiveness following an oral administration of an antigen is dose-dependent, and high-dose (200 mg) feeding of OVA in Tg-mice could lead to clonal anergy or clonal deletion [28,29], although in a Tg mouse model T cells specific to certain determinants on a self-antigen have been found to be less susceptible to tolerance [30]. The reduction of antigen-reactive T cells in lymphoid tissues was accompanied by a marked increase in the percentage of apoptotic cells following an antigen feeding [28]. These shut-down effects of antigen-activated IEL migration to the antigen-sensitized villus mucosa might be due to the early elimination of these infused cells from the circulating population. However, we found that the total influx of IEL line cells to the villus mucosa did not decrease compared to that in the controls.
In this study we have shown decreased migration of IELs to the intestinal mucosa after antigen activation, which can alter the population of these antigen-reactive T cells in the villus mucosa. The significance of the decreased migration of antigen-activated IELs and their exact role in allergic conditions and oral tolerance are subjects for future investigation.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology and by grants from the Keio University School of Medicine, the National Defense Medical College and the University of Tokyo. This study was also supported by Funds for Food Allergy from the Japanese Ministry of Health, Labour and Welfare.
References
- 1.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Curr Opin Immunol. 1993;5:247–52. doi: 10.1016/0952-7915(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 2.Dillon SB, MacDonald TT. Functional characterization of ConA-responsive Lyt2-positive mouse small intestinal intraepithelial lymphocytes. Immunology. 1986;59:389–96. [PMC free article] [PubMed] [Google Scholar]
- 3.Mosley RL, Whetsell M, Klein JR. Proliferative properties of murine intestinal intraepithelial lymphocytes (IEL): IEL expressing TCR αβ or TCR γδ are largely unresponsive signals mediated via conventional stimulation of the CD3–TCR complex. Int Immunol. 1991;3:563–9. doi: 10.1093/intimm/3.6.563. [DOI] [PubMed] [Google Scholar]
- 4.Cepak KL, Parker CM, Madara JL, Brenner MB. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–70. [PubMed] [Google Scholar]
- 5.Roberts K, Kilshaw SJ. The mucosal T cell integrin αM290β7 recognizes a ligand on mucosal epithelial cell lines. Eur J Immunol. 1993;23:1630–5. doi: 10.1002/eji.1830230735. [DOI] [PubMed] [Google Scholar]
- 6.Reinmann J, Rudolphi A. Co-expression of CD8α in CD4+ T cell receptor αβ+ T cells migrating into the murine small intestinal epithelial layer. Eur J Immunol. 1995;25:1580–8. doi: 10.1002/eji.1830250617. [DOI] [PubMed] [Google Scholar]
- 7.Rakasz E, Rigby S, de Andres B, et al. Homing of transgenic γδ T cells into murine vaginal epithelium. Int Immunol. 1998;10:1509–17. doi: 10.1093/intimm/10.10.1509. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey PJ, Charrier K, Horovitz DA, Fletcher FA, Watson JD. Analysis of the intra-epithelial lymphocyte compartment in SCID mice that received co-isogenic CD4+ T cells. Evidence that mature post-thymic CD4+ T cells can be induced to express CD8αin vivo. J Immunol. 1995;154:2678–86. [PubMed] [Google Scholar]
- 9.Bradley LM, Watson SR. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr Opin Immunol. 1996;8:312–20. doi: 10.1016/s0952-7915(96)80118-x. [DOI] [PubMed] [Google Scholar]
- 10.Hamann A, Rebstock S. Migration of activated lymphocytes. Curr Top Microbiol Immunol. 1993;184:109–24. doi: 10.1007/978-3-642-78253-4_9. [DOI] [PubMed] [Google Scholar]
- 11.Mackey CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–7. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 12.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 13.Salmi M, Andrew DP, Butcher EC, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181:137–49. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picker LJ, Martin RJ, Trumble A, et al. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur J Immunol. 1994;24:1269–77. doi: 10.1002/eji.1830240605. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Sasahara T, Nakamura Y, et al. Naive T cells can mediate delayed-type hypersensitivity response in T cell receptor transgenic mice. Eur J Immunol. 1994;24:1512–16. doi: 10.1002/eji.1830240708. [DOI] [PubMed] [Google Scholar]
- 16.Miura S, Tsuzuki Y, Kurose I, et al. Endotoxin stimulates lymphocyte–endothelial interactions in rat intestinal Peyer's patches and villus mucosa. Am J Physiol. 1996;271:G282–92. doi: 10.1152/ajpgi.1996.271.2.G282. [DOI] [PubMed] [Google Scholar]
- 17.Hokari R, Miura S, Fujimori H, et al. Altered migration of gut-derived T lymphocytes after activation with concanavalin A. Am J Physiol. 1999;277:G763–72. doi: 10.1152/ajpgi.1999.277.4.G763. [DOI] [PubMed] [Google Scholar]
- 18.Koseki S, Miura S, Fujimori H, et al. In situ demonstration of intraepithelial lymphocyte adhesion to villus microvessels of the small intestine. Int Immunol. 2001;13:1165–74. doi: 10.1093/intimm/13.9.1165. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa H, Li Y, Abeliovich A, Yamamoto S, Kaufmann SHE, Tonegawa S. Cytotoxic and interferon γ-producing activities of γδ T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci USA. 1993;90:8204–8. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisatsune T, Enomoto A, Nishijima K, et al. CD8+ suppressor T cell clone capable of inhibiting the antigen- and anti-T cell receptor-induced proliferation of Th clones without cytolytic activity. J Immunol. 1990;145:2421–6. [PubMed] [Google Scholar]
- 21.Yoshida T, Hachimura S, Kaminogawa S. The oral administration of low-dose antigen induces activation followed by tolerization, while high-dose antigen induces tolerance without activation. Clin Immunol Immunopathol. 1997;82:207–15. doi: 10.1006/clin.1996.4319. [DOI] [PubMed] [Google Scholar]
- 22.Lefrancois L, Barrett TA, Havran WL, Puddington L. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur J Immunol. 1994;24:635–40. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Fujihashi K, Yamamoto M, McGhee JR, Kiyono H. Alpha beta T cell receptor-positive intraepithelial lymphocytes with CD4+, CD8– and CD4+, CD8+ phenotypes from orally immunized mice provide Th2-like function for B cell responses. J Immunol. 1993;151:6681–91. [PubMed] [Google Scholar]
- 25.Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with β7 integrins and memory differentiation. J Immunol. 1996;156:3727–36. [PubMed] [Google Scholar]
- 26.Strauch UG, Lifka A, Goßlar U, Kilshaw PJ, Clements J, Holzmann B. Distinct binding specificities of integrins α4β7 (LPAM-1), α4β1 (VLA-4), and αIELβ7. Int Immunol. 1994;6:263–75. doi: 10.1093/intimm/6.2.263. [DOI] [PubMed] [Google Scholar]
- 27.Parker CM, Cepek KL, Russell GJ, et al. A family of β7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci USA. 1992;89:1924–8. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 29.Gonnella PA, Chen Y, Inobe J, Komagata Y, Quartulli M, Weiner HL. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J Immunol. 1998;160:4708–18. [PubMed] [Google Scholar]
- 30.Cibotti R, Kanellopoulos JM, Cabaniols JM, et al. Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci USA. 1992;89:416–20. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]