Abstract
The effects of immunosuppressive agents on T cell function have been well characterized but virtually nothing is known about the effects of renal transplantation on human dendritic cells (DCs). With the use of flow cytometry, we studied the kinetics of myeloid and plasmacytoid DCs in peripheral blood of 24 kidney allograft recipients before and after transplantation, and in 23 donors before and after kidney donation. All patients were treated with tacrolimus, mycophenolate mofetil and prednisone. Surgery resulted in a strong decline in the number of myeloid and plasmacytoid DCs, both in kidney donors and in their recipients. However, in donors this effect was transient, as the numbers of both DC subsets had normalized completely by the third postoperative month. In contrast, the recovery of myeloid DC counts in kidney transplant recipients was only incomplete at the end of the 3-month follow-up, despite tapering of immunosuppression. The seven patients who required additional immunosuppressive treatment because of acute rejection experienced an even more marked decrease in DC counts in the early postoperative period compared with patients who remained rejection-free. Surgical procedures markedly affect the numbers of circulating myeloid and plasmacytoid DCs. Immunosuppressive drugs have important additional in vivo effects on this cell type and impair the reconstitution of the myeloid DC subset in peripheral blood after renal transplantation.
Keywords: dendritic cells, flow cytometry (FACS), human studies, renal transplantation, steroids/ glucocorticoids/ corticosteroids
Introduction
Dendritic cells (DCs) are antigen-presenting cells (APCs) that can initiate and regulate T-, B- and natural killer lymphocyte immunity against foreign & aberrant antigens [1]. Physiologically, DCs are important for the protection against invading pathogens and tumour surveillance. In addition, DCs are thought to maintain tolerance to self, possibly by inducing the differentiation of regulatory T cells [2–4].
DCs originate from bone marrow progenitors and travel via blood to non-lymphoid tissues, where they reside as immature DCs that have a high capacity for phagocytosis. Antigen uptake in combination with an appropriate inflammatory stimulus causes DCs to ‘mature’, switching these cells into an immunostimulatory mode. This maturation (or activation) process is characterized by the loss of their ability to capture antigens and an increased expression of distinct chemokine receptors, major histocompatibility complex (MHC) classes I and II and diverse adhesion and costimulatory molecules. This enables DCs to traffic to secondary lymphoid tissue and stimulate effector cells from both the innate and adaptive immune system [1,5].
For transplantation medicine, DCs are of special interest. First, the direct or indirect presentation of donor antigens by DCs is critical for the occurrence of antidonor responses [6]. Secondly, DCs are considered to be the key to the development of allograft acceptance [7,8]. Finally, the high incidence of infectious complications and malignancies after transplantation may be explained partly by the interference of immunosuppressive drug treatment with normal DC function.
In recent years, the effects of immunosuppressive drugs on specific DC functions, such as antigen uptake, maturation, migration and T cell stimulation, have been characterized extensively in vitro (reviewed in [9]). However, only few studies have addressed the actions of immunosuppressive agents on DC number and function in human organ transplant recipients [10,11]. A better understanding of DC biology in vivo is essential if we are to exploit the potential of DCs to induce tolerance in the clinical setting. Therefore, the aim of the present study was to characterize the DC kinetics in patients receiving a renal transplant and who were treated with a ‘standard’ immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil (MMF) and prednisone. More specifically, we determined the numbers of the two main DC subsets present in human peripheral blood, myeloid (m) and plasmacytoid (p) DCs [12,13], before and after transplantation. In addition, the activation status and homing potential of these cells was determined by studying their expression of CD83 and the chemokine receptor CCR7. CD83 is a cell surface molecule that is up-regulated upon antigen capture and functional maturation of DCs [14]. Like CD83, the expression of CCR7 increases upon activation and allows the DC to respond to the CCR7 ligands CCL19 and CCL21, enabling the cell to traffic to secondary lymphoid organs [5,15,16].
Materials and methods
Patients
Twenty-four adult patients (≥18 years) who were admitted to our hospital to undergo deceased or living-donor kidney transplantation were asked to participate in the study. After obtaining informed consent, blood was drawn on the day of hospital admission (usually the day before transplantation) and on day 7 (±1 day), day 30 (±5 days) and day 90 (±21 days) after renal transplantation for flow cytometric analysis. Twenty-three living kidney donors [seven males and 16 females; mean age 51 years (range 23–75 years)] served as controls for transplant recipients. In this group, blood was drawn on the day before donation, on the day of hospital discharge (usually the third day after surgery) and 90 days (±21 days) after donation. Nineteen healthy volunteers [seven males and 12 females; mean age 34 years (range 22–59 years)] served as a second control group. In these subjects all the parameters of interest were measured and this was repeated 3 months later.
Immunosuppressive regimen
All renal transplant recipients were treated initially with triple immunosuppression consisting of tacrolimus, MMF and corticosteroids. Tacrolimus was dosed twice daily aiming at target predose concentrations of 15–20 ng/ml during the first 14 days, 10–15 ng/ml between weeks 3 and 7, and 5–10 ng/ml hereafter. MMF was started 2 days after renal transplantation with a dose of 1000 mg twice daily. After 14 days the MMF dose was tapered to 750 mg twice daily in patients with a body weight below 90 kg. All patients were treated with 100 mg prednisolone intravenously on the first 3 postoperative days, and were subsequently given prednisone orally, according to body weight (20 mg once daily for a patient with a body weight between 50 and 70 kg). The prednisone dose was tapered over time and discontinued at month 3 after transplantation. Cytomegalovirus (CMV) seronegative patients who received a kidney from a CMV-positive donor were treated with CMV hyperimmunoglobulins (Megalotect) according to local protocol.
Antibodies for staining
Fluorescence-activated cell sorter (FACS) analysis was performed using the following mouse antihuman monoclonal antibodies: fluorescein isothiocyanate (FITC)-conjugated CD34 (clone 8G12), FITC-conjugated lineage cocktail 1 [containing CD3 (SK7), CD14 (mΦP9), CD16 (3G8), CD19 (SJ25C1), CD20 (L27) & CD56 (NCAM16·2)], phycoerythrin (PE)-conjugated anti-interleukin (IL)-3 receptor α chain (CD123) (9F5), allophycocyanin (APC)-conjugated CD11c (S-HCL.3), peridinin chlorophyll protein (PerCP)-conjugated anti-HLA-DR (L243) (all purchased from Becton-Dickinson Biosciences, San Jose, CA, USA), PE-conjugated CD83 (HB15A17·11; DPC, Serotec, Oxford, UK), PE-conjugated anti-CCR7 (CDw197) (150503; R&D Systems Europe, Abingdon, UK) and PE-conjugated IgG2a (X39) and IgG2b (27–35) isotype control monoclonal antibodies (Becton-Dickinson).
Immunofluorescence staining and flow cytometric analysis
Analysis of DC numbers was performed as described recently [11]. In brief, peripheral venous blood samples were collected in standard lithium-heparinized tubes and processed within 3 h after collection. Blood from patients undergoing haemodialysis was collected immediately before the start of a dialysis session. Whole blood samples were first incubated with the above-mentioned monoclonal antibodies for 30 min in the dark at room temperature. Next, erythrocytes were lysed by incubating the samples for 10 min with FACS lysing solution (Becton-Dickinson). Cells were then washed twice using FACS flow and subsequently 300 000 events were measured on a FACScalibur flow cytometer using cellquest pro software (Becton Dickinson). As illustrated in Fig. 1, cells that stained negative for the lineage cocktail (Lin–) and positive for HLA-DR were gated and analysed for CD11c and CD123 expression. mDCs and pDCs were identified as Lin–, HLA-DR+, CD11c+ and CD123–/low and Lin–, HLA-DR+, CD11c– and CD123high, respectively. Absolute counts of mDCs and pDCs were then obtained by multiplying the proportion of each DC subset within the total leucocyte population by the absolute number of white blood cells as determined on an automated microcell counter (Sysmex F-300, TOA Medical Electronics Co. Ltd, Kobe, Japan). For the expression of CD83 and CCR7 on mDCs and pDCs, CD123PE was replaced by CD83PE or CCR7PE. The Lin–, HLA-DR+, CD11c+ cells were then considered as the mDC population and within this subset the percentage of CD83 or CCR7-positive cells was determined by comparison to their respective isotype control antibodies. The same was performed for the pDC population (Lin–, HLA-DR+, CD11c– cells). Determination of the percentage CD83 and CCR7-positive mDCs and pDCs was performed only if a minimum of 100 events was acquired for each DC subset.
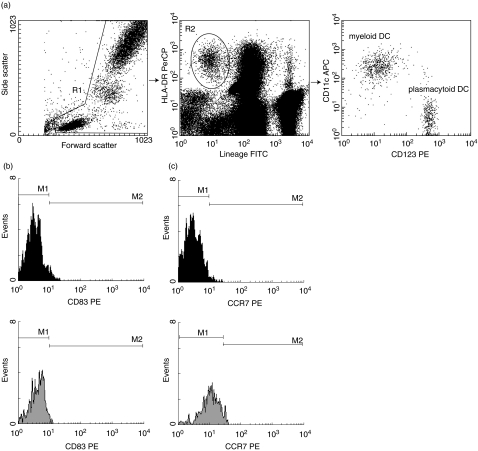
Fig. 1.
Typical example illustrating the peripheral blood dendritic cell (DC) composition of a healthy volunteer. First, leucocytes were gated using their forward and side-scatter characteristics (Fig. 1a, left panel). Next, DCs were identified as leucocytes that stained negative for lineage and positive for HLA-DR (a, middle panel). The myeloid and plasmacytoid DC subpopulations were then distinguished based on their expression of CD123 & CD11c (a, right panel). The maturation status of the myeloid (filled histograms) and plasmacytoid (grey histograms) DC subsets was determined by analysing the expression of CD83 (b) and CCR7 (c).
Statistical analysis
All values are presented as mean ± standard deviation (s.d.), unless stated otherwise. To achieve a normal distribution, the percentage of CD83 and CCR7-positive mDCs and pDCs, as well as the mDC to pDC ratio, were logarithmically transformed for statistical analysis. For comparison between different groups at a single time-point, the t-test, Mann–Whitney U-test, one-way analysis of variance (anova) or Kruskal–Wallis test were used as appropriate. Post-hoc analysis was performed using Bonferroni's test for multiple comparisons or Mann–Whitney U-test. For comparisons within groups, the Wilcoxon matched pairs test or repeated measurements anova was performed. Repeated measurements anova was also used to compare longitudinal changes between groups. P-values at α = 0·05 were considered statistically significant.
Results
Baseline
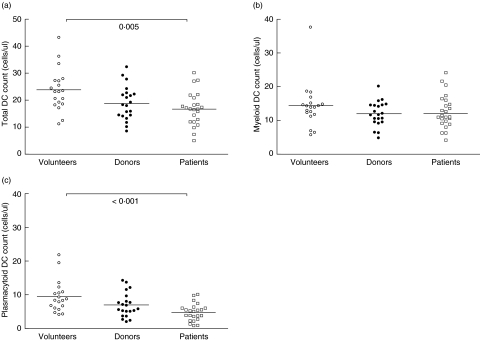
The clinical and demographic characteristics of the transplant recipients are depicted in Table 1. At baseline, total leucocyte counts were comparable between the three study groups (median (range)): 6·0 (4·3–9·7) versus 6·2 (3·7–13·6) versus 5·2 (3·5–13·6) × 109 cells/l for healthy volunteers, living kidney donors and patients with chronic kidney disease (CKD), respectively (P = 0·17). There existed a significant difference in mean total DC counts at baseline between volunteers, donors and patients before transplantation: 23·7 ± 7·8 versus 18·8 ± 6·3 versus 16·7 ± 6·3 cells/µl, respectively (overall: P = 0·005) (Fig. 2a). This overall difference was caused by a difference in plasmacytoid, but not myeloid, DC numbers between healthy volunteers and patients with CKD (Fig. 2b,c). As a result, the median mDC to pDC ratio was highest in patients with CKD and significantly different from that observed in donors (2·9 versus 1·9, respectively; P = 0·036) and healthy volunteers (2·9 vversus 1·6, respectively; P = 0·002). Total DC numbers and mDC and pDC counts were comparable between patients treated with haemodialysis (n = 8), peritoneal dialysis (n = 12) and patients not receiving renal replacement therapy before transplantation (n = 4) (data not shown).
Table 1.
Characteristics of 24 patients undergoing kidney transplantation.
| No. of patients | |
|---|---|
| Patients (male/female) | 24 (15/9) |
| Age (years) (range) | 51 (19–72) |
| Previous renal replacement therapy | |
| ″Haemodialysis | ″8 |
| ″Peritoneal dialysis | ″12 |
| ″None | ″4 |
| Primary kidney disease | |
| ″Polycystic kidney disease | ″5 |
| ″Hypertensive nephropathy | ″3 |
| ″Diabetic nephropathy | ″2 |
| ″Obstructive nephropathy | ″2 |
| ″Nephrolithiasis | 2 |
| ″Glomerulonephritis | 1 |
| ″Other/unknown | 9 |
| Transplantation type | |
| ″Living related | ″16 |
| ″Living unrelated | ″5 |
| ″Deceased donor | ″3 |
| Transplantation number | |
| ″First | ″ 19 |
| ″Second | ″5 |
| Number of HLA mismatches | |
| ″HLA-A | 0·92 ± 0·12 |
| ″HLA-B | 1·08 ± 0·10 |
| ″HLA-DR | 0·96 ± 0·14 |
| Recipient negative for CMV, donor positive | ″2 |
| Patients with PRA > 5% | 3 |
| Duration of cold ischaemia (h) (median and range) | 0·5 (0·2–25·8) |
PRA, panel reactive antibodies.
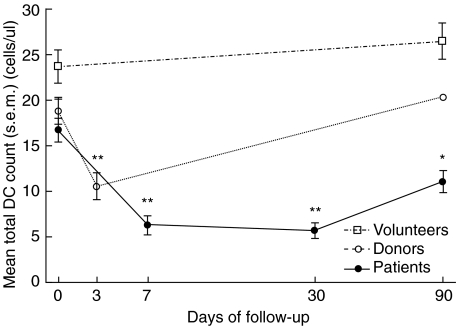
Fig. 2.
Absolute counts of total dendritic cells (DCs) (a), myeloid (m) DCs (b) and plasmacytoid (p) DCs (c) in peripheral blood of healthy volunteers, living kidney donors and patients with chronic kidney disease. Bars represent mean values; P-values indicate the difference between volunteers and patients.
We found no evidence for DC activation in the circulation as demonstrated by the low numbers of mDCs expressing CD83 (percentage of CD83+ mDCs (median): 8·4%versus 9·0%versus 7·9% for volunteers, donors and patients, respectively; P = 0·64) or CCR7 (8·7%versus 8·9%versus 7·1%; P = 0·29). The majority of pDCs expressed CCR7 at a low level (‘dim’; Fig. 1) [16]: percentage of CCR7+ pDCs (median): 56·4%versus 55·0%versus 64·3%. However, not once did we observe markedly elevated CCR7 expression. Again, the number of CCR7+ pDCs was comparable between the study groups (P = 0·52). Analysis of CD83 expression on pDCs demonstrated a significant difference in the percentage of positively staining cells. The highest percentage of CD83+ DCs was observed in patients with CKD (5·3%), which was significantly different from that observed in volunteers (2·1%; p = 0·001) and donors (2·8%; P = 0·026). However, the absolute counts of CD83+ pDCs were not significantly different between the three study groups (data not shown). Taken together, our observations indicate that CKD is associated with a lower number of circulating (p)DCs, but that renal failure or dialysis treatment does not cause marked DC activation in peripheral blood.
After kidney transplantation
During the first 3 months after surgery, immunosuppression was tapered gradually. Patients were receiving an average daily dose of 9·7 mg tacrolimus, 2000 mg MMF and 23·9 mg prednisone at day 7, which was reduced to 9·5 mg tacrolimus, 1705 mg MMF and 18·0 mg prednisone at day 30. At day 90, the mean tacrolimus, MMF and prednisone doses were reduced further to 6·4, 1450 and 5·9 mg/day, respectively. Tacrolimus predose concentrations decreased over time to 15·2, 11·7 and 8·3 ng/ml for days 7, 30 and 90, respectively. Throughout the initial postoperative period many of the 24 patients who received a renal transplant experienced one or more complications, which included acute rejection (n = 7), bacterial and viral infections (n = 13), acute tubular necrosis (n = 3) and drug toxicity (n = 6). Two patients died during the 3-month follow-up (one patient because of sepsis, another due to myocardial infarction).
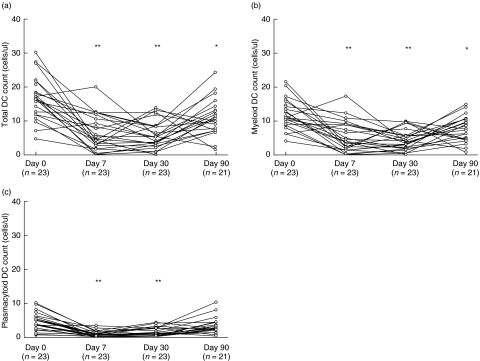
However, despite the clinically highly variable post-transplant course, the DC kinetics displayed a remarkably uniform pattern. Total DC counts declined sharply to 6·3 ± 5·1 cells/µl at day 7 after transplantation and stabilized at 5·7 ± 4·0 cells/µl at month 1. Three months after transplantation, the total DC count had increased to 11·1 ± 5·5 cells/µl, which was significantly different from baseline (P < 0·01; Fig. 3a). This pattern of initial decrease, followed by stabilization and partial recovery, was observed in both DC subsets (Fig. 3b,c). Myeloid DC counts decreased from 12·0 ± 4·4 cells/µl at baseline to 5·4 ± 4·5 cells/µl at day 7, stabilized at 4·2 ± 2·9 cells/µl on day 30, and increased to 7·7 ± 3·8 cells/µl at month 3 after transplantation. Plasmacytoid DC numbers decreased from 4·7 ± 2·6 cells/µl before transplantation to 0·9 ± 0·9 & 1·5 ± 1·3 cells/µl at days 7 and 30 after transplantation, respectively. The pDC count observed at month 3 (3·4 ± 2·4 cells/µl) was numerically lower compared with baseline, but this difference was not statistically significantly different (P = 0·07). Although the numbers of both the myeloid and the plasmacytoid DC subsets decreased after transplantation, the initial lowering of pDC numbers was more pronounced, resulting in a relative increase of the mDC percentage from 73% at day 0 to 85% at week 1 (P = 0·001). The percentage mDC normalized at month 1 (74%) and decreased slightly at month 3 (69%) (P = 0·025, compared with baseline).
Fig. 3.
Individual total dendritic cell (DC) counts (a), myeloid DC counts (b) and plasmacytoid DC counts (c) of 24 patients undergoing renal transplantation. **P < 0·001, *P < 0·01; both compared with baseline.
Next, we analysed if kidney transplantation resulted in maturation of DCs in the circulation. Comparison of the percentage of CD83 expressing mDCs over time demonstrated a small increase in the percentage of CD83+ cells from day 0 to day 7 (median): 7·9%versus 11·1% (P = 0·020). After week 1, the percentage of CD83 expressing cells returned to baseline levels: 9·3% and 7·5%, for days 30 and 90, respectively. A trend towards a similar pattern was observed for CCR7 expression: the percentage of CCR7 expressing mDCs increased from 7·1% (day 0) to 9·9% (day 7) and decreased to 8·5% and 7·4% at months 1 and 3, respectively (overall: p = 0·057). Because of the limited number of pDCs present in the circulation during the early postoperative period, CD83 and CCR7 expression on this DC subset could be reliably measured (i.e. >100 events in a substantial number of patients) only at baseline and at month 3 after transplantation. No significant differences existed between these two time-points for both CD83 and CCR7 expression. These observations suggest that renal transplantation causes substantial quantitative changes in DC numbers in peripheral blood, but that it does not result in a marked change in the percentage of activated DCs in this compartment.
Dendritic cells in relation to clinical events
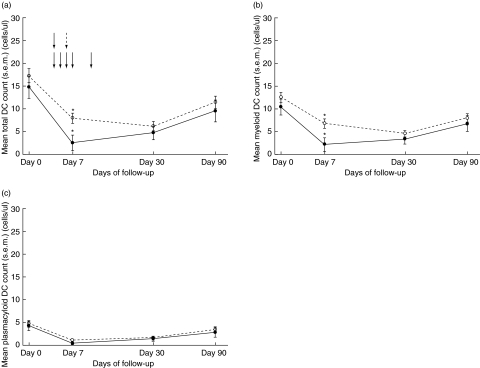
Throughout the 3-month follow-up seven patients were treated for acute rejection (AR), which was confirmed histologically in five individuals. Anti-AR therapy consisted of methylprednisolone for 3 consecutive days in case of a tubulo-interstitial rejection, and rabbit (r) ATG in case of vascular rejection. As depicted in Fig. 4a, mean DC counts of patients treated with anti-AR therapy were significantly lower throughout the 3-month follow-up period when compared with kidney transplant recipients who did not receive anti-AR treatment (P = 0·045). When these two groups were compared at the separate time-points, this difference in total DC numbers was significant only at day 7 (AR-treatment versus no AR-treatment (mean ± s.e.m.): 2·6 ± 1·7 versus 8·0 ± 1·1 cells/µl; P = 0·016). The difference in total DC numbers was caused by a lower mDC number at day 7 (AR-treatment versus no AR-treatment (mean ± s.e.m.): 2·2 ± 1·5 versus 6·8 ± 1·0 cells/µl; P = 0·019, Fig. 4b), whereas pDC numbers were comparable between the two groups (Fig. 4c). When only patients who received methylprednisolone were included, a similar trend towards lower mDCs at day 7 in the treatment group was observed (P = 0·052, data not shown). At no time-point was there a significant difference in the percentage of CD83 and CCR7 expressing mDCs and pDCs between the two groups.
Fig. 4.
Effect of anti-rejection therapy on total dendritic cell (DC) count (a), myeloid DC count (b) and plasmacytoid DC count (c). The dotted curve represents the mean (± s.e.m.) DC number of patients not treated for acute rejection (n = 17), whereas the straight curve represents patients who received treatment for acute rejection (n = 7). Arrows indicate the start of anti-rejection therapy (straight arrows indicate methylprednisolone treatment, whereas the dotted arrow indicates the start of rATG administration). *P < 0·05.
During the 3-month follow-up, four patients experienced clinical CMV syndrome. However, DC counts in these patients were never different from patients who did not have symptomatic CMV infection. Similarly, DC numbers of patients who underwent deceased-donor kidney transplantation (n = 3) were never different from living-donor kidney transplant recipients.
After kidney donation
The donor kidney explantation procedures were uncomplicated, except for one donor who required blood transfusion and re-laparotomy and was later treated for pneumonia. As in the patients, total DC counts decreased significantly from 18·8 ± 6·3 cells/µl at baseline to 10·6 ± 5·5 cells/µl on day 3, and 20·3 ± 5·1 cells/µl on day 90 after surgery (Fig. 5). This decrease was apparent in the mDC subset [11·9 ± 3·7 (day 0) versus 6·6 ± 4·4 (day 3) versus 12·7 ± 4·4 cells/µl (day 90)], as well as in the pDC subset [6·9 ± 3·6 (day 0) versus 4·0 ± 2·1 (day 3) versus 7·7 ± 2·9 cells/µl (day 90)]. However, in contrast to renal transplant recipients, DC numbers had normalized completely at month 3 after donation and the mDC to pDC ratio was not affected by the surgical procedure. In addition, kidney donation did not result in a significant change in the percentage of CD83 or CCR7 expressing mDCs and pDCs (data not shown). Finally, when comparing open with laparoscopic kidney donation we found no difference in the magnitude of the decrease in mDC or pDC counts.
Fig. 5.
Mean (± s.e.m.) total dendritic cell counts in healthy volunteers (n = 19; squares), living kidney donors (n = 23; circles) and kidney transplant recipients (n = 24; solid circles) throughout the 3-month follow-up. **P < 0·001, *P < 0·01; both compared with baseline.
Discussion
In the current study, we investigated the effects of kidney transplantation and kidney donation on the absolute number of mDCs and pDCs, as well as the effects of surgery on their maturation status. Numbers of circulating pDCs were significantly lower in patients with CKD compared with healthy volunteers, confirming the decreased DC counts that were reported recently in haemodialysis patients [17]. Chronic kidney disease is a well-known cause of impaired immunity. Patients with CKD frequently suffer from infectious complications and in general have low responses to vaccination [18,19]. Several explanations for this immunodeficient state have been described, including low numbers of circulating lymphocytes [20] and a decreased antigen-presenting capacity of monocytes [21]. The present data suggest that decreased numbers of pDCs may also contribute to the immunodeficiency of CKD because immature pDCs are a major source of interferon-α and thus important for the defence against viruses, whereas terminally differentiated pDCs are potent APCs [22].
The cause of the low numbers of circulating pDCs in patients with CKD is at present unclear. An increased susceptibility to apoptosis, that was demonstrated in haemodialysis patients for other leucocytes of lymphoid lineage, could explain the decreased pDC counts reported here [23]. An alternative explanation is the difference in age which existed between the volunteer and patient groups. In contrast to mDC counts, the number of pDCs decreases slowly with age [24]. However, analysis of covariance allowing for age showed that the difference in pDC counts between healthy volunteers and patients with CKD remained statistically significantly different (P = 0·03), indicating that renal failure itself affects circulating pDC numbers.
Interestingly, donor DC numbers resembled those of patients awaiting renal transplantation, although donor DC counts were not significantly different from those of healthy volunteers. It is tempting to speculate that anxiety for the upcoming surgery influenced circulating DC numbers. An increased sympathic tone and high plasma levels of catecholamines and cortisol can dramatically affect leucocyte function and redistribution, but no data on the relationship between the acute stress response and DC counts have been published [25].
Kidney transplantation and kidney donation resulted in a sharp decrease in the number of mDCs and pDCs in peripheral blood. These changes in DC numbers are in line with the findings of Ho et al. [26], who observed a temporary depression of absolute DC counts on the second and third days after cholecystectomy. In addition, Athanassopoulos et al. [11] have described recently a decline in DC numbers, comparable to that in this study, during the first week after heart transplantation. An issue that requires further investigation is the cause of these changes in DC counts in response to surgery. A first explanation is that DC numbers decreased secondary to blood loss. However, we feel that this is an unlikely possibility because blood loss during most of the surgical procedures was minimal.
Secondly, DC numbers in peripheral blood may have decreased as a result of tissue redistribution. Animal experiments have demonstrated that surgical injury or exposure to an inflammatory stimulus results in a rapid recruitment of DCs at the site of tissue damage followed by migration of these cells to regional lymph nodes [27,28]. One of the possible mechanisms underlying this DC redistribution are the actions of glucocorticoids. Surgery leads, among others, to an increased secretion of endogenous glucocorticoids and catecholamines [29,30]. These alterations in hormone levels result in turn in granulocytosis and lymphopenia in peripheral blood, mobilization of granulocytes to the site of tissue damage and redistribution of lymphocytes to lymphatic tissue [25,29,30]. Although the effects of surgery on human DC trafficking have not been investigated, DCs may respond in a similar way to surgical stress. Further evidence for the implication of glucocorticoids comes from the study by Shodell et al. [31], who showed that administration of steroids to patients or healthy volunteers resulted in a decrease of circulating pDCs. In the current study, the recovery of DC counts in transplant recipients, but not in kidney donors, was incomplete by the third postoperative month. In contrast to kidney donors, in which cortisol levels probably normalized within the first week after surgery, renal transplant recipients were treated continuously with prednisone. Moreover, the decrease in DC counts was more pronounced in patients who received anti-AR therapy compared with patients who were not treated for rejection.
A third explanation for the postoperative changes in DC counts is an impaired generation of DCs at the bone marrow level with or without increased (peripheral) DC turnover (apoptosis). Again, these processes are likely to have been influenced by immunosuppression. In vitro, glucocorticoids strongly inhibit the generation of human immature DCs from either monocytes or from CD34+ bone marrow progenitors by inducing apoptosis of DC precursors and by blocking their differentiation into immature DCs [32–34]. In contrast to glucocorticoids, tacrolimus and MMF do not affect DC generation [34,35].
Finally, no marked DC activation was observed in the circulation in response to surgery or during rejection episodes. We anticipated that inflammation would cause up-regulation of CD83 and CCR7 on the DC surface. However, mature DCs can enter draining lymphatics, which probably remained largely intact in kidney donors after surgery. In contrast, the afferent lymphatics of the transplanted kidney are severed during explantation and the only remaining exit route from the (rejecting) graft would be via the blood [36]. Nevertheless, the lymphatics may have been the exit route from the allograft for mature DCs after all. Classic experiments in dogs revealed that lymphatic regeneration occurs as early as day 3 following kidney transplantation and after 3 weeks the lymphatics of the transplant were almost indistinguishable from normal [37]. Recent evidence shows that lymphatic neoangiogenesis also occurs in human kidney transplants but it is unknown how soon after transplantation this takes place [38]. Alternative explanations for the lack of DC maturation in the blood are limitations of the detection technique (due to the very small number of circulating DCs after surgery) or interference with DC maturation by immunosuppressive agents. Glucocorticoids can block DC maturation both in vitro and ex vivo[32,39,40]. MMF can also impair murine DC maturation in vitro[35], whereas tacrolimus does not markedly influence phenotypic DC activation [32].
In summary, surgical procedures in living kidney donors and their recipients result in a marked decrease of myeloid and plasmacytoid DCs in peripheral blood. In donors this effect was transient, whereas in transplant recipients the recovery of myeloid DCs was far from complete. These findings, together with our observations in patients treated for rejection, suggest an important additional effect of immunosuppression.
References
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2000;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 5.Förster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 6.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roelen DL, Schuurhuis DH, van den Boogaardt DE, et al. Prolongation of skin graft survival by modulation of the alloimmune response with alternatively activated dendritic cells. Transplantation. 2003;76:1608–15. doi: 10.1097/01.TP.0000086340.30817.BA. [DOI] [PubMed] [Google Scholar]
- 8.Mirenda V, Berton I, Read J, et al. Modified dendritic cells coexpressing self and allogeneic major histocompatability complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15:987–97. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 9.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 10.Mazariegos GV, Zahorchak AF, Reyes J, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003;3:689–96. doi: 10.1034/j.1600-6143.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 11.Athanassopoulos P, Vaessen LM, Maat AP, Balk AHHM, Weimar W, Bogers AJ. Peripheral blood dendritic cells in human end-stage heart failure and the early post-transplant period: evidence for systemic Th1 immune responses. Eur J Cardiothorac Surg. 2004;25:619–26. doi: 10.1016/j.ejcts.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 15.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–6. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 17.Verkade MA, van de Wetering J, Klepper M, Vaessen LMB, Weimar W, Betjes MGH. Peripheral blood dendritic cells and GM-CSF as an adjuvant for hepatitis B vaccination in hemodialysis patients. Kidney Int. 2004;66:614–21. doi: 10.1111/j.1523-1755.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 18.Allon M, Depner TA, Radeva M, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol. 2003;14:1863–70. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 19.Fabrizi F, Martin P. Hepatitis B virus infection in dialysis patients. Am J Nephrol. 2000;20:1–11. doi: 10.1159/000013548. [DOI] [PubMed] [Google Scholar]
- 20.Deenitchina SS, Ando T, Okuda S, et al. Cellular immunity in hemodialysis patients: a quantitative analysis of immune cell subsets by flow cytometry. Am J Nephrol. 1995;15:57–65. doi: 10.1159/000168802. [DOI] [PubMed] [Google Scholar]
- 21.Meuer SC, Hauer M, Kurz P, Meyer zum Buschenfelde KH, Kohler H. Selective blockade of the antigen-receptor-mediated pathway of T cell activation in patients with impaired primary immune responses. J Clin Invest. 1987;80:743–9. doi: 10.1172/JCI113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 23.Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204–12. doi: 10.1681/ASN.V131204. [DOI] [PubMed] [Google Scholar]
- 24.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–21. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 25.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–64. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho CS, Lopez JA, Vuckovic S, Pyke CM, Hockey RL, Hart DN. Surgical and physical stress increases circulating blood dendritic cell counts independently of monocyte counts. Blood. 2001;98:140–5. doi: 10.1182/blood.v98.1.140. [DOI] [PubMed] [Google Scholar]
- 27.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–6. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfield JG, Wang Y, Li S, et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 1999;56:1759–69. doi: 10.1046/j.1523-1755.1999.00741.x. [DOI] [PubMed] [Google Scholar]
- 29.Toft P, Svendsen P, Tønnesen E, Rasmussen JW, Christensen NJ. Redistribution of lymphocytes after major surgical stress. Acta Anaesthesiol Scand. 1993;37:245–9. doi: 10.1111/j.1399-6576.1993.tb03708.x. [DOI] [PubMed] [Google Scholar]
- 30.Toft P, Tønnesen E, Helbo-Hansen HS, Lillevang ST, Rasmussen JW, Christensen NJ. Redistribution of granulocytes in patients after major surgical stress. Apmis. 1994;102:43–8. doi: 10.1111/j.1699-0463.1994.tb04843.x. [DOI] [PubMed] [Google Scholar]
- 31.Shodell M, Siegal FP. Corticosteroids depress IFN-alpha-producing plasmacytoid dendritic cells in human blood. J Allergy Clin Immunol. 2001;108:446–8. doi: 10.1067/mai.2001.117928. [DOI] [PubMed] [Google Scholar]
- 32.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;30:1807–12. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Woltman AM, Massacrier C, de Fijter JW, Caux C, van Kooten C. Corticosteroids prevent generation of CD34+-derived dermal dendritic cells but do not inhibit Langerhans cell development. J Immunol. 2002;168:6181–8. doi: 10.4049/jimmunol.168.12.6181. [DOI] [PubMed] [Google Scholar]
- 34.Woltman AM, de Fijter JW, Kamerling SW, et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood. 2001;98:174–80. doi: 10.1182/blood.v98.1.174. [DOI] [PubMed] [Google Scholar]
- 35.Mehling A, Grabbe S, Voskort M, Schwarz T, Luger TA, Beissert S. Mycophenolate mofetil impairs the maturation and function of murine dendritic cells. J Immunol. 2000;165:2374–81. doi: 10.4049/jimmunol.165.5.2374. [DOI] [PubMed] [Google Scholar]
- 36.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mobley JE, O'Dell RM. The role of lymphatics in renal transplantation. Renal lymphatic regeneration. J Surg Res. 1967;7:231–3. doi: 10.1016/0022-4804(67)90057-1. [DOI] [PubMed] [Google Scholar]
- 38.Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 39.Rea D, van Kooten C, van Meijgaarden KE, Ottenhoff TH, Melief CJ, Offringa R. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood. 2000;95:3162–7. [PubMed] [Google Scholar]
- 40.Verhoeven GT, Van Haarst JM, De Wit HJ, Simons PJ, Hoogsteden HC, Drexhage HA. Glucocorticoids hamper the ex vivo maturation of lung dendritic cells from their low autofluorescent precursors in the human bronchoalveolar lavage: decreases in allostimulatory capacity and expression of CD80 and CD86. Clin Exp Immunol. 2000;122:232–40. doi: 10.1046/j.1365-2249.2000.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]