Abstract
CD4+ CD25+ regulatory T (Treg) cells play a critical role in the maintenance of peripheral tolerance and the prevention of autoimmunity. In the present study, we have explored the characteristics of CD4+ CD25+ Treg cells in patients with rheumatoid arthritis (RA). The frequency and phenotype of CD4+ CD25+ T cells in paired samples of synovial fluid (SF) and peripheral blood (PB) from patients with RA and PB from normal controls were analysed. An increased frequency of CD4+ cells T cells expressing CD25 was detected in SF compared to PB from patients with RA. No significant difference was observed in the numbers of CD4+ CD25+ T cells in PB from patients and controls. SF CD4+ CD25+ T cells expressed high levels of CTLA-4 (both surface and intracellular), GITR and OX40, as well as Foxp3 transcripts. Functionally, SF CD4+ CD25+ T cells were impaired in their proliferative responses and could suppress the proliferation of their CD4+ CD25– counterparts. In conclusion, these data demonstrate that CD4+ CD25+ Treg cells, with the potential to regulate the function of effector T cells and antigen-presenting cells, accumulate in the synovium of patients with RA.
Keywords: human, regulatory T cells, rheumatoid arthritis
Introduction
CD4+ CD25+ regulatory T (Treg) cells play a pivotal role in peripheral tolerance and the prevention of autoimmunity [1–3]. They constitute a minority of the CD4+ T cell population in peripheral blood (PB) [4–8] and appear to arise in the thymus, probably through a specific altered negative selection of T cells expressing T cell receptors (TCR) with intermediate affinity to major histocompatibility complex (MHC)–peptide complexes [9]. The forkhead/winged helix transcription factor Foxp3 has been shown to be essential for the development as well as for the suppressive function of CD4+ CD25+ Treg cells [10–12]. Humans with a mutation in the Foxp3 gene develop a severe autoimmune/allergy disorder, IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) characterized by global immune dysregulation [13]. Foxp3 has been suggested to be specific for the CD4+ CD25+ Treg cells [10–12]. However, it appears that Foxp3 expression coupled to suppressive function can also be induced on peripheral CD4+ CD25– T cells upon activation [14], either suggesting that CD4+ CD25+ Treg cells also develop in the periphery or that Foxp3 is not specific for thymus-derived CD4+ CD25+ Treg cells [15,16].
Treg cells are anergic cells that upon TCR activation inhibit interleukin (IL)-2 transcription and the proliferation of naive and memory CD4+ and CD8+ T cells [4–8,17]. Treg cells can also induce suppressive activity in their target T cells [18,19]. Furthermore, Treg cells directly regulate the function of antigen-presenting cells (APC) and B cells [20–23]. Suppressive function of CD4+ CD25+ Treg cells is known to depend on direct cellular contact with the target cells [4–8,17]. The characterization of Treg cells has been complicated by the lack of a specific surface marker to distinguish them from the recently activated CD25+ T cells. Treg cells constitutively express several activation-related molecules, such as cytotoxic lymphocyte-associated antigen 4 (CTLA-4), a homologue of CD28, glucocorticoid-induced tumour necrosis factor receptor family-related gene (GITR), OX40, CD45RO and HLA-DR [5–8,24,25]. In humans, the regulatory function associates with CD4+ T cells expressing CD25 at high levels [4,24]. So far, the expression of Foxp3 has been suggested to best identify the population of CD4+ CD25+ T cells with the regulatory function [10–12].
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects mainly the peripheral joints, causing bone and cartilage damage [26]. Activated/memory CD4+ T cells recruited in the RA synovium have been thought to drive the synovial inflammation by stimulating the production of proinflammatory cytokines by macrophages and fibroblasts, activating B cells and promoting the formation of osteoclasts [26]. The majority of CD4+ T cells in RA synovium are CD45RO+, express a variety of activation markers, such as CD69 and HLA-DR, and seem to display a Th1 effector phenotype [26–28]. However, some features of synovial T cells do not seem to be in accordance with the suggested effector function of these cells [26]. Namely, T cell-derived cytokines are found at scanty concentrations in the synovium. Furthermore, synovial T cells are profoundly hyporesponsive to TCR-ligation. The compromised activation state of synovial T cells has been attributed previously to chronic exposure to tumour necrosis factor (TNF)-α, among others [29]. However, the role of CD4+ CD25+ Treg cells or other types of immunoregulatory T cells in controlling effector T cell functions and inflammatory processes in RA remain elusive.
Any autoimmune disease could follow from impaired development or function of Treg cells. We undertook this study to explore the presence and characteristics of CD4+ CD25+ Treg cells in patients with RA. We show that although CD25+ cells represent a minority of the CD4+ population in synovial fluid (SF), the frequency of CD4+ T cells expressing CD25 is increased significantly compared to the PB of the patients. No significant difference was observed in the numbers of CD4+ CD25+ T cells in PB from patients and controls. Phenotypically, SF CD4+ CD25+ T cells express high levels of surface and intracellular CTLA-4, GITR and OX40, the strongest expression of these molecules found on CD4+ CD25high T cells. Importantly, isolated SF CD4+ CD25+ cells showed the classic functional characteristics of Treg cells and expressed high levels of Foxp3 transcripts. These data demonstrate that CD4+ CD25+ Treg cells with the potential to regulate the function of effector T cells and APC accumulate in RA synovium.
Materials and methods
Patients
A total of 18 patients with RA (14 women, four men), as determined by the criteria of the American College of Rheumatology (formerly the American Rheumatism Association) [30], were enrolled in this study. The median age of the patients was 56 years (range 22–80) and the median duration of the disease was 14 years (range 0·3–28). Nine patients had a seronegative disease. All but one of the patients were receiving treatment: 14 were treated with disease-modifying antirheumatic drugs, 10 with corticosteroids, and 12 with non-steroidal anti-inflammatory drugs. Informed consent of the patients was obtained. SF samples from the inflamed knee joints were collected by needle aspiration into heparinized tubes. PB samples were taken concurrently. Control PB was obtained from healthy volunteers at the Department of Medical Microbiology. This study was approved by the joint ethical committee of Turku University and Turku University Central Hospital.
Reagents
Following mouse antihuman and unspecific control antibodies were used for the FACS-stainings: fluorecein isothiocyanate (FITC)-conjugated anti-CD4, -CD25 and control mouse IgG (mIgG), phycoerythrin (PE)-conjugated anti-CD4, -CD69, -CD154, -CD134 (OX-40), -IL-10 and mIgG, allophycocyanin (APC)-conjugated CTLA-4 (CD152) and mIgG and peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 and mIgG were all obtained from BD Pharmingen (San Diego, CA, USA). PE-conjugated anti-GITR was purchased from R&D Systems (Minneapolis, MN, USA). Agonistic antihuman CD28 was purchased from Pharmingen, and antihuman CD3 from Becton-Dickinson (San Jose, CA, USA). The reagents and capillaries for Lightcycler polymerase chain reaction (PCR) were purchased from Roche Molecular Diagnostics (Mannheim, Germany). Reagents for magnetic activated cell sorting (MACS) were purchased from Miltenyi Biotec (Auburn, CA, USA).
Cell preparations
SF samples were treated with bovine testicular hyaluronidase (10 µg/ml; Type IV-S, Sigma, Steinheim, Germany) for 15 min at 37°C. Synovial fluid mononuclear cells (SFMC) and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. MACS (Miltenyi Biotec, Auburn, CA, USA) was used to sort the cell populations. SF CD4+ cells and control PB CD4+ cells were isolated using CD4 Multisort Kit (Miltenyi). CD25 Microbeads (Miltenyi) were used to further isolate CD25+ and CD25– cells from SF CD4+ cell preparations. The purity of the cell preparations was always analysed using FACS. Cells obtained using this method were typically >98% CD3+ CD4+ cells. The purity of CD25+ fraction was 40–70%. CD25 depleted cells contained less than 3% of contaminating CD25+ cells.
Flow cytometry
SFMC and PBMC were stained by direct immunofluorescence for three- or four-colour flow cytometry. To analyse cell surface molecule expression, the cells were washed with phosphate-buffered saline (PBS), 2% fetal calf serum (FCS) and stained for 30 min at 4°C. After the staining cells were washed again and analysed with a FACSCalibur flow cytometer (Becton Dickinson) using the CellQuest software (Becton Dickinson). To analyse the expression of intracellular molecules, Fix & Perm® Cell Permeabilization Kit from Caltaq Laboratories, Inc. (Burlingame, CA, USA) was used according to the manufacturer's staining protocol.
Proliferation assays
CD4+ CD25+ and CD4+ CD25– SF T cells (5–7·5 × 104/well) were cultured in triplicate in 200 µl IMDM (Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat inactivated AB-serum (Finnish Red Cross, Helsinki, Finland), 0·1 m m 2-mercaptoethanol (Sigma, St Louis, MO, USA), 10 m m HEPES (Gibco BRL) and 100 µg/ml gentamicin in flat-bottom 96-well microtitre plates (Costar® 3599, Costar, Cambridge, MA, USA) for 4 days. Either anti-CD3 (1 µg/ml) + anti-CD28 (10 µg/ml) MoAbs or phytohaemagglutinin (PHA) (5 µg/ml) were added in the beginning of the culture. Tritiated thymidine (Du Pont, Boston, MA, USA) (1·0 µCi/well) was added 16–18 h before terminating the culture. The cells were harvested to glass fibre filters (Wallac, Turku, Finland) with a 96-well harvester (Tomtec, Orange, CT, USA). The radioactivity was measured with a 1450 Microbeta Plus liquid scintillation counter (Wallac). Assays analysing the inhibitory capacity of CD4+ CD25+ SF T cells were performed under the same conditions. In these assays 5 × 104 CD4+ CD25– SF T cells were cultured in triplicate with or without 1·25 × 104 or 2·5 × 104 CD4+ CD25+ SF T cells and anti-CD3+ anti-CD28 MoAbs in U-bottomed 96-well microtitre plates (Nunclon®, InterMed/Nunc, Denmark).
Total RNA isolation and reverse transcription
Total RNA was extracted from both SFCD4+ CD25+ and CD4+ CD25– cells, and control PB CD4+ cells TRIZOL®Reagent (Life Technologies) according to the manufacturer's instructions. Total RNA was reverse transcribed using avian myeloblastosis virus (AMV) [first-strand cDNA Synthesis Kit for reverse transcription-polymerase chain reaction (RT-PCR); Roche Diagnostics, Mannheim, Germany] in a reaction volume of 20 µl according to the manufacturer's instructions. AMV reverse transcriptase was omitted from the control RT reactions. Each reaction was incubated at 42°C for 1 h using a DNA thermal cycler (Perkin-Elmer, Norwalk, CT, USA). A heat inactivation step at 99°C for 5 min was performed to denaturate reverse transcriptase, followed by a cooling step at 4°C for 5 min.
Real-time quantitative PCR
Real-time quantitative PCR (LightCycler™, Roche Molecular Diagnostics, Mannheim, Germany) was used to study Foxp3 mRNA expression. LightCycler-FastStart DNA Master SYBR Green 1 kit (Roche) was used according to the manufacturer's instructions. Two µl of cDNA dilutions were used for amplification in a 20 µl reaction volume. MgCl2 was added to achieve the optimal final concentration of 2 m m. Housekeeping β-actin was amplified from the same pool of cDNA. First the reactions were heated to 95°C for 10 min to activate the FastStart Taq DNA polymerase enzyme. Amplification steps (β-actin: 15 s at 95°C, 5 s at 67°C and 8 s at 72°C; Foxp3: (touchdown-PCR was used) 10 s at 95°, 5 s at 70–63°C (temperature lowered 0·5°C/cycle) and 14 s at 72°C) were repeated 50 times. Melting curves were performed by lowering the temperature to 65°C, then raising the temperature by 0·1°C/s to 99°C and measuring the fluorescence continuously. Analysis was made using the second derivative maximum method. The standard curve was constructed using cDNA from control CD4+ PB T cells in four dilutions (5 × 10−1, 10−1, 10−2 and 10−3). Foxp3 and β-actin mRNA expression in the samples was quantified on the basis of the standard curves and Foxp3 mRNA levels were normalized to the β-actin mRNA levels. After each run, the presence of amplification products was also verified on 1·5% agarose gels (SeaKem, FMC Bioproducts, Rockland, ME, USA). Specific primers used were: β-actin (sense 5′-AGCCTCGCCTTTGC CGA-3′, antisense 5′- CTGGTGCCTGGGGCG-3′) [31] and Foxp3 (sense 5′-AAGGAGAAGCTGAGTGCCAT-3′, antisense 5′-GTCCATGTTGTGGAGGAACT-3′).
Statistical analyses
Statistical significance was tested using Wilcoxon's signed-rank test for paired samples and Mann–Whitney U-test for unpaired data.
Results
RA synovial fluid contains increased frequency of CD4+ CD25+ T cells with the phenotypic characteristics of regulatory T cells
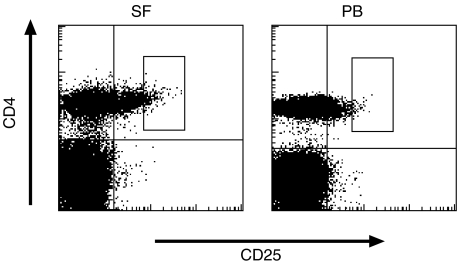
We first compared the expression of CD25 between paired SF and PB samples from patients with RA. As shown in Table 1, 5–20% (mean 12%) of freshly isolated SF CD4+ T cells expressed CD25. Compared to SF, the frequency of CD4+ T cells expressing CD25 was significantly lower in PB from the patients (mean 5·5%). Notably, the proportion of CD4+ T cells expressing CD25 at high levels appeared to be increased in SF compared to PB, which was also demonstrated by the significantly higher mean fluorescence intensity ratio of CD25 expression in SF CD4+ cells (Table 1). A typical pattern of CD25 expression in SF and PB CD4+ T cells is illustrated in Fig. 1. No significant difference was observed in the frequency CD25+ cells between PB CD4+ cells from the patients and healthy controls (mean 6·7%) (Table 1).
Table 1.
The expression of CD25 on CD4+ T cells from synovial fluid (SF) and peripheral blood (PB) from patients with rheumatoid arthritis (RA) and PB from normal controls.*
| CD25 expression | |||
|---|---|---|---|
| n | % | MFIR | |
| RA SF CD4+ | 10 | 12·0 ± 4·8†‡ | 1·6 ± 0·2§¶ |
| RA PB CD4+ | 10 | 5·5 ± 4·1 | 1·3 ± 0·2 |
| Normal PB CD4+ | 9 | 6·7 ± 5·0 | 1·3 ± 0·3 |
Percentages (%) and mean fluorescence intensity ratios (MFIR) of CD25 expression on gated CD4+ cells are shown. MFIR is the MFI of the staining with the anti-CD25 MoAb divided by the MFI of the negative control antibody. Results are shown as mean ± s.d.
P = 0·009 and
P = 0·013 when compared with RA PB CD4+ T cells.
P = 0·034 and
P = 0·041 when compared with normal PB CD4+ T cells.
Fig. 1.
Synovial fluid (SF) CD4+ T cells express increased levels of CD25. Representative FACS stainings of SF and peripheral blood mononuclear cells (PB) from one patient with rheumatoid arthritis (RA) is shown. Cells with light-scatter characteristics of lymphocytes were gated. Double stainings with anti-CD4 and anti-CD25 MoAbs are shown. Gates define the cells expressing CD25 at high levels (CD4+ CD25high).
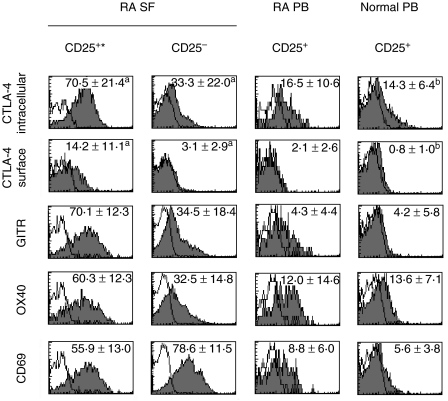
A number of markers have been suggested to define CD4+ CD25+ Treg cells. For example, constitutive expression of intracellular CTLA-4, GITR and OX40 has been linked to the phenotype of these cells [5–8,24,25]. To characterize more effectively the SF CD4+ CD25+ T cells, we analysed their expression of these markers compared to CD4+ CD25– cells. Both surface and intracellular CTLA-4 was associated strongly with the CD4+ CD25+ phenotype (Fig. 2). CD4+ CD25– cells expressed hardly any surface CTLA-4 and the intracellular CTLA-4 was expressed at significantly higher levels on CD4+ CD25+ cells. In addition, CD4+ CD25+ SF T cells expressed significantly higher levels of GITR and OX40 than their CD4+ CD25– counterparts, the majority of CD25+ cells being positive. Importantly, CD4+ CD25high phenotype seemed to associate with the highest expression of CTLA-4, GITR and OX40 (Table 2). We also compared the expression of CD69 early activation antigen, CD154 (CD40 ligand) and intracellular IL-10 in SF CD4+ CD25+ and CD4+ CD25– T cells. Both CD4+ CD25+ and CD4+ CD25– cells expressed negligible levels of CD154 (0·5 ± 0·6% (mean ± s.d., n = 6) and 0·8 ± 0·6%, respectively, and were negative for intracellular IL-10 (data not shown). A proportion of CD4+ CD25+ SF T cells expressed CD69 but at significantly lower levels than their CD25– counterparts.
Fig. 2.
Synovial fluid (SF) CD4+ CD25+ T cells from patients with rheumatoid arthritis (RA) express increased levels of markers associated with the regulatory T cell phenotype. CD4+ mononuclear cells with light scatter characteristics of lymphocytes were gated. The expression of markers was analysed on gated SF CD25+ and CD25– cells and peripheral blood (PB) CD25+ cells from the corresponding patients as well as normal controls. Numbers indicate the percentages (mean ± s.d.) of positive cells. Number of samples analysed was six, except for (a) 10 and (b) nine. Representative stainings from one patient and one control are illustrated. *P < 0·05 when compared to SF CD4+ CD25– cells and RA PB or normal PB CD4+ CD25+ cells.
Table 2.
Comparison of the phenotypes of synovial fluid (SF) CD4+ CD25high and CD4+ CD25intermediate T cells from patients with rheumatoid arthritis (RA).*
| CD25high | CD25intermediate | |
|---|---|---|
| CTLA-4s | 12·3 ± 7·8† | 6·3 ± 5·0 |
| CTLA-4i | 70·0 ± 19·0† | 52·9 ± 16·7 |
| OX-40 | 65·1 ± 12·2† | 49·1 ± 15·9 |
| GITR | 78·4 ± 8·9† | 55·5 ± 14·6 |
| CD69 | 50·6 ± 11·6 | 53·1 ± 16·9 |
CD4+ SF mononuclear cells with light scatter characteristics of lymphocytes were gated. The expression of markers was analysed on gated CD25high (the intensity of CD25 expression exceeding that of negative control ∼ 10-fold; see Fig. 1 for representative gating) and CD25intermediate cells (the remaining CD25+ cells). Results are shown as percentages (mean ± s.d.) of positive cells. Number of samples analysed was six.
P < 0·05 when compared to CD4+ CD25intermediate cells. CTLA-4s = CTLA-4 surface, CTLA-4i = CTLA-4 intracellular.
The expression of these selected markers was also compared on CD4+ CD25+ cells from SF and PB from the patients and normal controls. As demonstrated in Fig. 2, SF CD4+ CD25+ differed more from PB CD4+ CD25+ cells than from their CD4+ CD25– counterparts in SF. The expression of CTLA-4 (surface and intracellular), OX-40, GITR and CD69 was significantly higher in SF cells when compared to PB cells from both sources. PB CD4+ CD25+ T cells from the patients did not differ significantly from the corresponding cells in PB from normal controls. Interestingly, GITR was expressed by only a minority of PB CD4+ CD25+ both from the patients and the controls.
CD4+ CD25+ SF T cells express Foxp3 transcripts
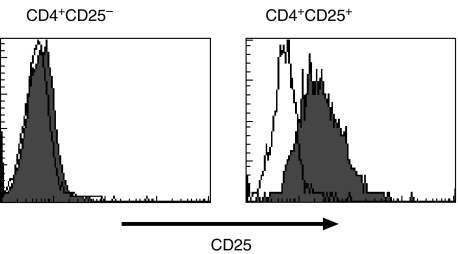
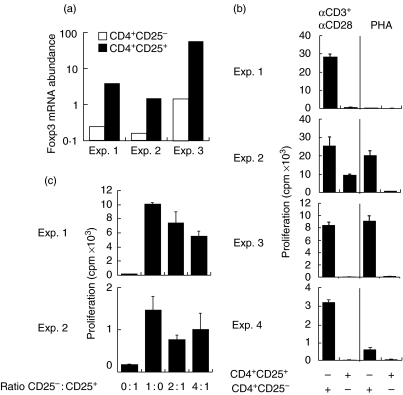
For the time being, the most specific marker/gene suggested to identify CD4+ CD25+ Treg cells is Foxp3 [10–12]. Therefore, we sorted CD4+ CD25+ and CD4+ CD25– SF T cells using MACS and analysed the expression of Foxp3 mRNA using real-time quantitative PCR. A typical pattern of CD25 expression on CD4+ CD25+ and CD4+ CD25– preparations is shown in Fig. 3. Foxp3 transcripts were detectable in both populations but the level of mRNA expression was significantly higher in CD4+ CD25+ cells, exceeding the level of CD25– cells 10–40-fold (Fig. 4a).
Fig. 3.
Typical pattern of CD25 expression on sorted CD4+ CD25– and CD4+ CD25+ SF T cell fractions used for functional studies. Representative stainings of one patient sample are shown.
Fig. 4.
(a) Synovial fluid (SF) CD4+ CD25+ T cells express high levels of Foxp3 trancripts. Relative expression of Foxp3 mRNA in CD4+ CD25+ and CD4+ CD25– T cell populations in SF from three patients with rheumatoid arthritis was studied by real-time quantitative polymerase chain reaction (PCR). β-actin was used as a reference gene. Normalized Foxp3 mRNA abundance was derived from the ratio of Foxp3 mRNA to β-actin mRNA expression in each sample. (b) SF CD4+ CD25+ T cells are hyporesponsive. SF CD4+ CD25+ and CD4+ CD25– cells (5–7·5 × 104 cells/well) were stimulated with either anti-CD3 (1 µg/ml) + anti-CD28 (10 µg/ml) MoAbs or phytohaemagglutinin (PHA) (5 µg/ml) and the proliferative response was measured by tritiated thymidine incorporation after 4 days of culture. (c) SF CD4+ CD25+ T cells are able to suppress the proliferation of their SF CD4+ CD25– counterparts. CD4+ CD25– cells (5 × 104 cells/well), CD4+ CD25+ cells (5 × 104 cells/well) and co-cultures of both CD4+ CD25– (5 × 104 cells/well) and CD4+ CD25+ cells (2·5 × 104 or 1·25 × 104 cells/well) from two patients were stimulated with anti-CD3 (1 µg/ml) and anti-CD28 (10 µg/ml) MoAbs for 4 days.
CD4+ CD25+ SF T cells are hyporesponsive and inhibit the proliferation of CD4+ CD25 – cells
Hyporesponse to TCR-mediated stimulation and mitogens is one characteristic of CD4+ CD25+ Treg cells [4–8]. Therefore, we studied the proliferation of sorted CD4+ CD25+ and CD4+ CD25– cells in response to anti-CD3+ anti-CD28 MoAbs or PHA. Significant, although variable, proliferative responses of CD4+ CD25– cells were observed, whereas the proliferation of CD4+ CD25+ cells was almost undetectable in three of four samples studied and reduced significantly in relation to that of CD4+ CD25– cells in all the samples (Fig. 4b). As one of the main functions of Treg cells is to inhibit the activation of T cells we studied whether enriched CD4+ CD25+ SF cells are able to inhibit the proliferation of their CD4+ CD25– counterparts. As shown in Fig. 4c, the presence of CD4+ CD25+ cells clearly reduced the proliferative response of CD4+ CD25– SF cells to anti-CD3+ anti-CD28 MoAbs in the two samples tested. The degree of suppression was not clearly cell ratio-dependent, propably because the CD4+ CD25+ preparations also contain activated, conventional T cells. These data confirm that CD4+ CD25+ SF T cells show the in vitro functional activities of Treg cells.
Discussion
Present study shows that, compared to PB, an increased frequency of CD4+ CD25+ T cells, with the phenotypic and functional properties of Treg cells, has accumulated in the SF from patients with RA. The suppressive function of SF CD4+ CD25+ T cells was confirmed by the observations that these cells were hyporesponsive and inhibited the proliferation of SF CD4+ CD25– T cells after polyclonal activation. Importantly, SF CD4+ CD25+ T cells were also shown to express high levels of Foxp3 transcripts. While this work was under preparation, Cao et al. [32] published the first report showing, in accordance with our study, that SF from patients with RA contain an increased frequency of CD4+ CD25+ Treg cells. Recently, consistent data were also shown by van Amelsfort et al. [33]. Cao et al. showed that the cells with suppressive function reside mainly in the CD4+ CD25high population of SF T cells, and suggested that CD25intermediate cells represent the recently activated cells [32]. Typical enrichment of CD4+ T cells expressing CD25 at high intensity in SF was observed in both our study and that of Cao et al. Our results, together with those of Cao et al. and van Amelsfort et al., provide evidence that CD4+ CD25+ T cells with immunoregulatory function are present in the joints during the progression of RA.
According to our results the number of CD4+ CD25+ cells in the PB from RA patients was comparable to that from healthy controls, suggesting that there is no significant alteration in the size of the circulating CD4+ CD25+ Treg cell population in RA and that CD4+ CD25+ Treg cells accumulate specifically into the joints of patients with RA. However, recently Cao et al. analysed samples from a total of 135 patients with RA and observed that the frequency of CD4+ CD25high cells was significantly lower in PB from the patients than normal controls, while in the great majority of patients the frequencies were much higher in SF than PB, further supporting active recruitment of Treg cells into the synovium [34]. It has been demonstrated that CD4+ CD25+ Foxp3+ T cells with a regulatory function can be induced upon activation of CD4+ CD25– Foxp3– cells [14]. Therefore, the high number of CD4+ CD25+ Treg cells in SF may also be due to local generation in response to synovial environment, as well as due to recruitment. Our finding that CD4+ CD25– T cells express low levels of Foxp3 trancripts also may support the local generation.
Consistent with previous reports showing that CD4+ CD25+ Treg cells constitutively express CTLA-4, GITR and OX40 [5–8,24,25], we demonstrate that CD4+ CD25+ SF T cells and, especially the CD4+ CD25high cells, express high levels of these receptors. The intensity of GITR and CTLA-4 expression has been correlated to the suppressive capacity of the Treg cells [24]. Signalling through CTLA-4 has been suggested to be essential for the suppressive function of Treg cells, whereas GITR may deliver a negative signal to Treg cells [25,35,36]. Compared to PB CD4+ CD25+ T cells, the expression of CTLA-4 (both surface and intracellular), GITR and OX40 was very high on SF CD4+ CD25+ T cells, suggesting that SF cells represent activated Treg cells. This is supported further by the far higher expression of CD69 on SF CD4+ CD25+ T cells. Although CD69 has not been shown to separate Treg cells from conventional T cells, the expression CD69 has been shown to be up-regulated in Treg cells after activation [8]. Knowledge on human Treg cells is, to a great extent, based on the observations on resting/in vitro-activated PB Treg cells. Our findings suggest that the phenotype of Treg cells in inflammatory tissues is distinct from that of the circulating counterparts in PB.
Defining the position of Treg cells in the pathogenesis of RA is a challenge. The depletion of CD4+ CD25+ T cells, well before disease induction, results in the increased severity of collagen-induced arthritis (CIA) [37], supporting the conclusion that CD4+ CD25+ Treg cells play an important role already in the induction phase of the disease. Furthermore, the attenuation of CIA after mucosal administration of Escherichia coli heat-labile enterotoxin B subunit has been shown to involve the activity of CD4+ CD25+ Treg cells [38]. Very recently it has been shown that while CD4+ CD25+ Treg cells isolated from PB of patients with RA suppress the proliferation of effector T cells in vitro, they are unable to suppress proinflammatory cytokine production from activated T cells and monocytes, as well as to convey suppressive function to effector CD4+ CD25– cells [39]. Most importantly, it was shown that the suppressive capacity was restored after treatment with anti-TNF-α therapy. These data suggest that RA may be characterized by a functionally compromised Treg cell compartment, and that agents that expand the number of Treg cells or restore the function of these cells may prove to be highly effective in the treatment of RA. Interestingly, compromised function of PB Treg cells has also been observed recently in some other autoimmune diseases, including multiple sclerosis [40].
Many inflammatory responses in the synovium have been suggested to be T cell driven [26]. However, the number of CD4+ T cells in ST and the score for knee pain have been observed to negatively correlate [41]. Furthermore, the treatment of RA patients with depleting anti-CD4 antibodies has been shown reduce the number of accumulating T cells without a marked clinical improvement [42]. These results may be interpreted to reflect the crucial participation of Treg cells in disease processes. As Treg cells have the capacity to render target T cells anergic, general hyporesponsiveness of synovial T cells may be due in part to the presence of Treg cells in the synovium. It would seem logical that CD4+ CD25+ T cells limit the inflammatory processes in the synovium and the progression of RA. However, considering that the anergic state of T cells may play a role in the chronicity of synovial inflammation [30], a possibility remains that the presence of Treg cells in the synovium may actually hinder the clearance of inflammation.
Treg cells have been shown to directly modulate the function of APCs by down-regulating the expression of co-stimulatory molecules and stimulating IL-10 production [20,23]. Indoleamine 2,3-dioxygenase, an enzyme that catabolizes tryptophan and thereby inhibits T cell proliferation, is one of the mechanisms of APCs to maintain peripheral tolerance. Interestingly, it was first demonstrated that CTLA-4-immunoglobulin fusion protein (CTLA-4Ig) stimulates dendritic cells (DC) to produce IDO [43], and thereafter shown that Treg cells expressing CTLA-4 perform the same function [22]. However, we did not observe any increase in the expression of IDO mRNA in CD14+ SF macrophages after CTLA-4Ig stimulation (unpublished observations). Although the evidence is indirect, these data suggest that the regulation of IDO production by SF macrophages is not the principal function of Treg cells. It is possible that CD80/CD86-mediated induction of IDO is a feature of a certain subset of DC only, as demonstrated previously [44].
In this study we have confirmed the presence of CD4+ CD25+ Treg cells in RA SF. The identification of CD4+ CD25+ Treg cells in the synovium provides new insight into the regulation of T cell homeostasis and synovial inflammation in RA. Increasing evidence suggests that CD4+ CD25+ Treg cells play a crucial role in RA as well as other autoimmune diseases. However, further studies are needed to reveal their exact role in the pathogenesis of RA and to identify the factors regulating their function and accumulation in the synovium.
Acknowledgments
Dr Elli Veistinen is acknowledged for her help and advice with the Lightcycler-analyses. Anna Karvonen and Jasperiina Mattsson are acknowledged for their expert technical assistance. This study was supported by the Academy of Finland. Dr Milja Möttönen is a recipient of a training grant from the Turku Graduate School of Biomedical Sciences.
References
- 1.Bach FJ. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–98. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Control of immune responses by naturally arising CD4+ regulatory T cells that express Toll-like receptors. J Exp Med. 2003;197:397–401. doi: 10.1084/jem.20030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Boehmer H. Dynamics of suppressor T cells: in vivo veritas. J Exp Med. 2003;198:845–9. doi: 10.1084/jem.20031358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory T cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 5.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4(+) CD25(+) cells. a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 14.Walker MR, Kasprowich DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roncarolo M-G, Levings MK, Traversari C. Differentiation of T-regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–12. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD4+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin-10-producing, contact-independent type 1-like regulatory T cells. J Exp Med. 2002;196:247–53. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 22.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 23.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 24.Levings MK, Sangregorio R, Sartirana C, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin-10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+) CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 26.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 27.Cush JJ, Lipsky PE. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988;31:1230–8. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- 28.Nanki T, Lipsky PE. Cytokine, activation marker, and chemokine receptor expression by individual CD4+ memory T cells in rheumatoid arthritis synovium. Arthritis Res. 2000;2:415–23. doi: 10.1186/ar120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cope AP. Studies of T-cell activation in chronic inflammation. Arthritis Res. 2002;4:S197–211. doi: 10.1186/ar557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 31.Kreuzer KA, Lass U, Landt O, et al. Highly sensitive and specific fluorescence reverse transcription-PCR assay for the pseudogene-free detection of beta-actin transcripts as quantitative reference. Clin Chem. 1999;45:297–300. [PubMed] [Google Scholar]
- 32.Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 33.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 34.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmström V. CD25 bright CD4+ regulatory cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory T cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 37.Morgan ME, Sutmuller RPM, Witteveen HJ, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 38.Luross JA, Heaton T, Hirst TR, Day MJ, Williams NA. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 2002;46:1671–82. doi: 10.1002/art.10328. [DOI] [PubMed] [Google Scholar]
- 39.Ehrestein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tak PP, Smeets TJM, Daha MR, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–25. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 42.Tak PP, van der Lubbe PA, Cauli A, et al. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995;38:1457–65. doi: 10.1002/art.1780381012. [DOI] [PubMed] [Google Scholar]
- 43.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 44.Mellor AL, Baban B, Chandler P, et al. Induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;177:1652–5. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]