Abstract
Many studies concerning the role of T cells and cytokines in allergy have been performed, but little is known about the role of natural killer (NK) cells. Accordingly, the expression of co-stimulatory, inhibitory and apoptosis receptors, cytokine profiles and their effect on immunoglobulin isotypes were investigated in polyallergic atopic dermatitis (AD) patients with hyper immunoglobulin E (IgE) and healthy individuals. AD patients showed significantly decreased peripheral blood NK cells compared to healthy individuals. Freshly isolated NK cells of polyallergic patients spontaneously released higher amounts of interleukin (IL)-4, IL-5, IL-13 and interferon (IFN)-γ compared to healthy individuals. NK cells were differentiated to NK1 cells by IL-12 and neutralizing anti-IL-4 monoclonal antibodies (mAb), and to NK2 cells by IL-4 and neutralizing anti-IL-12 mAb. Following IL-12 stimulation, NK cells produced increased levels of IFN-γ and decreased IL-4. In contrast, stimulation of NK cells with IL-4 inhibited IFN-γ, but increased IL-13, production. The effect of NK cell subsets on IgE regulation was examined in co-cultures of in vitro differentiated NK cells with peripheral blood mononuclear cells (PBMC) or B cells. NK1 cells significantly inhibited IL-4- and soluble CD40-ligand-stimulated IgE production; however, NK2 cells did not have any effect. The inhibitory effect of NK1 cells on IgE production was blocked by neutralization of IFN-γ. Except for CD40, NK cell subsets showed different expression of killer-inhibitory receptors and co-stimulatory molecules between the polyallergic and healthy subjects. These results indicate that human NK cells show differences in numbers, surface receptor and cytokine phenotypes and functional properties in AD.
Keywords: allergy, IgE, IgG4, KIR receptors, natural killer cells
Introduction
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease characterized by typically distributed eczematous skin lesions [1–4]. Most patients with AD are polyallergic to food and/or airborne allergens and some of them show allergic rhinoconjunctivitis and asthma. Several lines of evidence suggest the contribution of immunological mechanisms in the pathogenesis of AD. Most of the patients with AD show very high concentrations of total and allergen-specific immunoglobulin E (IgE). Activation of peripheral blood T cells and their preferential secretion of Th2 cytokines has been reported repeatedly [5,6]. Numerous studies demonstrated increased frequency of allergen-specific Th2 cells producing increased interleukin (IL)-4, IL-5 and IL-13 in the peripheral blood of AD patients [7,8]. However, the role of natural killer (NK) cells in allergic diseases has so far gained little attention.
NK cells are one component of the innate immune system and have the ability to both lyse target cells and serve as regulators of immune responses by releasing a variety of cytokines, such as interferon (IFN)-γ, tumour necrosis factor (TNF)-α, granulocyte macrophage-colony stimulating factor (GM-CSF), IL-5 and IL-8 [9]. It has been shown that human NK cells are able to polarize in vitro into two functionally different subsets NK1 or NK2, analogous to T cell subsets Th1 or Th2. NK1 cells produce IFN-γ but also produce IL-10, whereas NK2 cells produce IL-5 and IL-13 [10]. Recently, the in vivo existence of human NK1 and NK2 cell subsets was demonstrated in freshly purified IFN-γ secreting and IFN-γ non-secreting NK cell subsets from peripheral blood of healthy individuals [11].
Human NK cells can be divided into two subsets based on their cell surface density of CD56. The majority of human NK cells have low density expression of CD56 (CD56dim) and express high levels of CD16 (FcγRIII) [12]. Freshly isolated CD56bright cells are capable of producing large quantities of type I and type II cytokines, whereas CD56dim cells produce substantially less cytokines [13].
NK cells can be distinguished from other lymphocytes with the lack of the T cell receptor and surface immunoglobulin, and many NK cell-specific surface molecules with different functions. Human NK cells express structurally and functionally two distinct families of major histocompatibility complex (MHC) class I receptors: killer cell immunoglobulin-like receptors (KIR) and lectin-like receptors. These receptors are also divided into two families that include activatory and inhibitory receptors [14,15]. The ligands for many, but not all, of these inhibitory receptors are MHC class I molecules, which are expressed by almost all nucleated cells and are often down-regulated in viral infected and cancer cells [16]. Activatory receptors on NK cells recognize structures that are present on both harmful target cells and normal cells, but the influence of the inhibitory pathways dominates when class I MHC is recognized [17]. In addition to their cytolytic activity, NK cells participate either directly or indirectly in the regulation of antibody response [18]. The role of NK cells in the modulation of B cell response and antibody production has been attributed to their ability to interact directly with B cells and/or produce cytokines that regulate B cell differentiation and isotype swicthing [19].
In this study, expression of co-stimulatory, killer inhibitory, and apoptosis receptors, cytokine profiles and their effect on immunoglobulin isotypes in freshly purified and in vitro differentiated NK cells were investigated in AD patients and compared to that of healthy individuals.
Materials and methods
Study population
Thirty-two patients with AD (mean age, 30 years), who fulfilled the criteria of Hanifin and Rajka were selected for the study [1]. Fifteen patients had allergic conjunctivitis and none had asthma. All patients were polyallergic and had positive cutaneous tests to at least three aeroallergens. Patients showed specific IgE antibodies at radioallergosorbent test class ≥2 and high amounts of serum total IgE of > 400 IU/ml (11·230 ± 2104 IU/ml; mean ± s.d.). Thirty-one healthy individuals (mean age, 31 years) with no history of atopy were included in the study as a normal control group. Their mean serum total IgE levels were 57·0 ± 16·7 IU/ml. The study was approved by the ethical committee of Davos, Switzerland.
Antibodies and reagents
Fluorescent-labelled monoclonal antibodies (mAbs) for flow cytometric analyses were purchased from Beckmann Coulter Corp. (Hialeh, FL, USA), Immunotech Ltd (Marseilles, France), PharMingen (San Diego, CA, USA), Alexis (Alexis Biochemicals, Canada) and Dako (Dako A/S, Denmark). IL-2 and IL-4 and neutralizing anti-IFN-γ (45–15) and anti-IL-4 (8F12) were from Novartis (Basel, Switzerland). IL-12 was from R&D Systems (Abingdon, UK), and anti-IL-12 and anti-IL-13 (JES 10–5A2) was from PharMingen (San Diego, CA, USA). Soluble (s) CD40L was produced from transfected cell line 8-40-1, originally generated by Dr P. Lane [20] and cultured for 3 days in CG medium (Vitromex, Vilshofen, Germany). It was standardized according to maximal IgE-inducing capacity after 12 days of peripheral blood mononuclear cells (PBMC) culture in the presence of 25 ng/ml IL-4 [21]. Supernatants from the corresponding untransfected cell line J558L (kindly provided by Dr M. Reth, University of Freiburg, Germany) were used as control.
Cell purification
PBMC were obtained from heparinized blood by density gradient centrifugation over Ficoll (Sigma Chemical Co., St Louis, MO, USA). NK cells were purified by magnet-activated cell separation (MACS, Miltenyi Biotec AG, Bergisch Gladbach, Germany). Briefly, NK cells were isolated from PBMC by immunomagnetic depletion of T cells, B cells, monocytes and other myeloid cells such as basophils and dendritic cells, according to expression of CD3, CD4, CD19 and CD33. The purity of NK cells was > 98% as assessed by flow cytometric analysis of cells stained with fluoroscein isothiocyanate (FITC)-labelled anti-CD16, rhodamine-labelled anti-CD56 and phycoerythrin-Texas Red-X (ECD)-labelled anti-CD3 (EPICS XL, Coulter Corp., Hialeh, FL, USA). CD3 contamination in purified NK cells was < 1%. B cells were purified by microbead-conjugated anti-CD19 following depletion of monocytes by microbead-conjugated anti-CD14 (Miltenyi Biotec AG). The purity of B cells was > 94% as assessed by FITC-labelled anti-CD19 mAb by flow cytometry.
NK cell cultures
Freshly purified NK cells were washed and resuspended in RPMI-1640 medium supplemented as described previously [22]. For the in vitro differentiation, 2·5 × 105 NK cells and 3000 rad gamma-irradiated autologous NK cell-depleted 1·25 × 105 PBMC and 1·25 × 105 BuB1 cells were cultured in 48-well tissue culture plates (Costar Corp., Cambridge, MA, USA) in the presence of 50 U/ml IL-2 and 5 µg/ml phytohaemagglutinin (PHA, Sigma Chemical Co.) [11]. BuB1 is an in-house Epstein–Barr virus-transformed B cell line that effectively stimulates NK cells. NK cells were differentiated to NK1 cells in the presence of 10 ng/ml IL-12 and 10 µg/ml neutralizing anti-IL-4 mAb; and to NK2 cells in presence of 25 ng/ml IL-4 and 10 µg/ml neutralizing anti-IL-12 for 10 days.
Flow cytometric analysis
After purification, 5 × 104 cells were stained with anti-CD16-FITC, anti-CD56-PE, anti-CD40-PE, anti-CD45RA-FITC, anti-CD45RO-PE, anti-CD95-FITC (Immunotech Ltd), anti-CD3-ECD (Coulter Corp.), anti-CD95L-FITC (Alexis Biochemicals) anti-CD154-PE (Ancell Corp, MN, USA) and anti-ICOS-FITC (F44, gift from Dr R. Kroczek, Robert-Koch Institute, Berlin, Germany). Fluorescent-labelled inhibitory and activatory KIR receptors anti-EB6 (CD158a, KIR2DL1 and KIR2DS1)-PE, anti-GL183 (CD158b, KIR2DL2,3 and KIR2DS2)-PE and inhibitory anti-NKAT-2 (CD158.b, p58.2, KIR2DL3)-FITC and unlabelled inhibitory lectin-like receptor mAb anti-NKG2A and inhibitory KIR receptor anti-p70 (KIR3DL1) were used. FITC-labelled goat anti-human Ig was used as a second antibody (Sigma Chemical Co.). Stained cells were fixed in 2% paraformaldehyde. The controls were FITC-, ECD-, PE- or RD-conjugated mouse IgG1 (Coulter Corp.). Flow cytometric analysis was performed with an EPICS XL (Coulter Corp.).
Intracytoplasmic cytokine staining
Freshly purified NK cells were stimulated for 12 h by a combination of PHA (5 µg/ml), phorbol ester (PMA, 50 ng/ml), Ca2+ ionophore (ionomycin, 250 ng/ml) (all from Sigma Chemical Co.). The combination of the three stimuli was used to achieve the strongest stimulus for intracytoplasmic cytokine detection. Monensin (Sigma Chemical Co.) was added at final concentration of 1 µ M during the last 10 h. The cells were washed with phosphate buffered saline (PBS), then fixed and permeabilized with paraformaldehyde/saponin solution (Ortho Permeafix, Ortho Diagnostic Systems Inc, Raritan, NJ, USA). After washing with PBS containing 5% fetal calf serum, 1·5% bovine serum albumin (Sigma Chemical Co.) and 0·0055% EDTA (Fluka Chemie AG, Buchs, Switzerland), the cells were stained with PE- or FITC-conjugated isotype control antibody, anti-IL-4, anti-IL-5, anti-IL-13 and anti-IFN-γ mAbs (all from PharMingen) for 30 min at 4°C and analysed by flow cytometry. The specificity of the intracytoplasmic cytokine staining was confirmed by inhibition of the staining by using excess amounts of cytokines added during the staining procedure or by using cell lines that are negative for these cytokines.
NK cell stimulation and quantification of cytokines
Freshly purified NK cells and in vitro differentiated NK1 and NK2 cells were stimulated for 72 h with or without PHA (5 µg/ml) for investigation of the cytokine profile. After 72 h, supernatants were harvested and IL-5, IL-4, IL-13 and IFN-γ levels were determined by enzyme-linked immunosorbent assay (ELISA).
The solid phase sandwich ELISAs for IL-5, IL-4, IL-13 and IFN-γ were performed as described previously [23,24]. The sensitivity of IFN-γ ELISA was ≤ 10 pg/ml (mAbs and IFN-γ standard were provided by Dr C. H. Heusser, Novartis, Basel, Switzerland). The detection limit of the IL-5 ELISA was 50 pg/ml (mAbs and IL-5 standard were from PharMingen). The detection limit of IL-13 ELISA was 300 pg/ml. The sensitivity of IL-4 ELISA was < 20 pg/ml (mAbs were from PharMingen and standards were from PeproTech, Rocky Hill, NJ, USA).
Induction of IgE and IgG4 production
PBMC (4·5 × 105) were cultured with 0·5 × 105in vitro differentiated NK cells in 48-well flat-bottomed plates in 500 µl in duplicate and 5 × 104 B cells were cultured with 5 × 103 freshly isolated and in vitro differentiated NK cells in 96-well flat-bottomed plates in 200 µl in triplicate. The RPMI-1640 medium contained additional 4 µg/ml bovine insulin and 40 µg/ml human transferrin (both from Sigma Chemical Co.). Cells were stimulated with 25 ng/ml IL-4 and 20% sCD40L containing 8-40-1 cell supernatant, as described previously [21]. Neutralizing anti-IFN-γ, anti-IL-4 and control antibody were used at 10 µg/ml, and anti-IL-13 at 5 µg/ml in some cultures. IgE, IgG4 and IgM were determined in supernatants taken after 12 days [25].
Determination of IgE, IgG4 and IgM
Total IgE was determined using anti-ɛ mAb 14–41 for coating and biotinylated mAb 6–7 for detection (Novartis AG, Basel, Switzerland) [7,23]. The detection limit was 0·2 ng/ml of human IgE (Behringwerke AG, Marburg, Germany). Mouse anti-IgG4 mAb RJ4 and peroxidase-conjugated anti-human IgG (Sigma Chemical Co.) were used to quantify IgG4. The detection limit of total IgG4 was 0·6 ng/ml of World Health Organization (WHO) reference serum 67–97 [23]. Total IgM was measured using anti-human IgM (Boehringer-Mannheim, Mannheim, Germany) for coating and peroxidase-conjugated rabbit antihuman IgM (Dako AG, Wiesentheid, Germany) for detection. The detection limit was 1 ng/ml of human IgM standard (Behringwerke AG, Marburg, Germany).
Statistical interpretation
Data are expressed as means ± s.d. Statistical analysis was performed by Student's t- and Mann–Whitney U-tests.
Results
Human NK cell subsets express different receptors for co-stimulation, apoptosis, KIR and lectin-like receptors
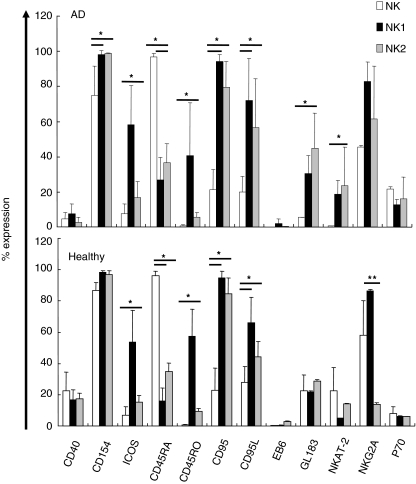
We investigated whether changes in numbers and subsequent cytokine profile may influence several apoptosis and co-stimulatory receptors on NK cells. Activatory or inhibitory signals play an important role in NK cell functions, which are regulated by MHC class I-specific receptors on NK cells. Therefore, freshly isolated and in vitro differentiated NK1 and NK2 cells from AD and healthy individuals were investigated for the expression of apoptosis, co-stimulatory and NK cell receptors. Cells were stained with mAbs to CD40, CD154, ICOS, CD45RA, CD45RO, CD95 and CD95L, EB6 (CD158a, KIR2DL1 and KIR2DS1), GL183 (CD158b, KIR2DL2,3 and KIR2DS2), NKAT-2 (CD158b, p58.2, KIR2DL3), NKG2A, p70 (KIR3DL1) and analysed by flow cytometry (Fig. 1).
Fig. 1.
Co-stimulatory, apoptosis and natural killer (NK) receptor expressions in NK cell subsets. Freshly purified NK cells and in vitro differentiated NK1 and NK2 cells from atopic dermatitis (AD) and healthy individuals stained with fluorescent-labelled or unlabelled co- stimulatory, apoptosis and NK cell receptor monoclonal antibodies (mAbs) and analysed by flow cytometry. Unlabelled antibodies detected with a FITC-conjugated goat anti-human Ig. Results of co-stimulatory and apoptosis are shown as mean ± s.d. of eight AD and six healthy individuals, and NK receptor results are shown as mean ± s.d. of nine individuals in each group. *P≤ 0·001 or P≤ 0·01 and **P ≤ 0·05.
In vitro differentiated NK cells up-regulated some of the co-stimulatory and apoptosis receptors. In AD patients, CD154, CD95 and CD95L expressions were found to be higher in NK1 and NK2 cells compared to freshly isolated NK cells (P ≤ 0·01, P ≤ 0·001 and P ≤ 0·01, respectively). However, ICOS expression was significantly increased in NK1 cell subset compared to NK and NK2 cells (P ≤ 0·01 and P ≤ 0·001, respectively). In healthy subjects, ICOS, CD95 and CD95L expressions were increased on NK1 and NK2 cell subsets compared to NK cells (P ≤ 0·001, P ≤ 0·001 and P ≤ 0·01, respectively). CD95 and CD95L were highly expressed on NK1 and NK2 cells compared to NK cells in AD and also healthy subjects. CD45RA was significantly high in NK cells compared to NK1 and NK2 cells (P ≤ 0·001); however, CD45RO expression was higher in NK1 cell subsets compared to NK and NK2 cells in both AD and healthy individuals (P ≤ 0·001). CD40 expression was significantly low on NK, NK1 and NK2 cells of AD patients compared to healthy subjects (P ≤ 0·05).
In AD group, NKAT-2 and GL183 expressions were significantly higher in NK2 cells compared to NK cells (P ≤ 0·01) (Fig. 1). The analysis of NKG2A, EB6 and p70 did not show any difference between NK subsets. In healthy individuals, only NKG2A expression was found to be significantly higher in NK1 cells compared to NK2 cells (P ≤ 0·05). NKG2A expression was significantly increased in NK2 cell subset of AD patients compared to healthy subjects (P ≤ 0·05). However, EB6 expression of NK2 cells of AD was significantly decreased compared to healthy subjects (P ≤ 0·05).
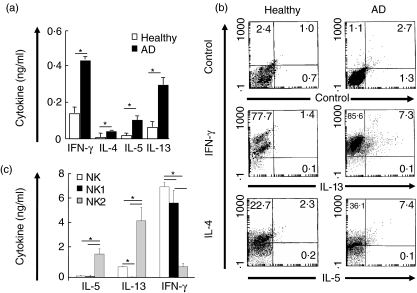
Cytokine profile of NK cells and NK cell subsets in AD
Heterogeneous functional properties for NK cells have been reported, suggesting that NK cells do not constitute a unique functional cell type [26]. We analysed the spontaneous cytokine release and intracytoplasmic IFN-γ, IL-4, IL-5 and IL-13 production of NK cells in healthy and polyallergic atopic individuals. Freshly purified NK cells were cultured from five AD patients and five healthy controls without any stimulation. Spontaneously released IL-4, IL-5, IL-13 and IFN-γ were determined from 72 h supernatants by ELISA. As shown in Fig. 2a, NK cells from AD patients spontaneously released significantly more IL-4, IL-5, IL-13 and IFN-γ than those of controls, demonstrating an in vivo activation of NK cells in atopic patients (P < 0·001). To determine their full capacity to synthesize these cytokines, NK cells were stimulated with PHA, PMA and ionomycin combination and intracytoplasmic cytokines were stained. In this case, significantly high percentages of IL-5 and IL-13 producing NK cells were detected in allergic patients, whereas very few IL-5 and IL-13-producing NK cells were found in healthy individuals (P < 0·001) (Fig. 2b). Accordingly, we analysed the cytokine profile of NK cell subsets in AD patients. Freshly isolated NK cells, NK1 and NK2 cell subsets from AD patients were cultured with PHA stimulation. IL-5, IL-13 and IFN-γ levels were determined from 72 h supernatants by ELISA. As shown in Fig. 2c, IL-5 and IL-13 release were increased in NK2 cells compared to NK1 and NK cells (P < 0·01). In contrast, IFN-γ was higher in NK1 cell subsets compared to NK and NK2 cells (P < 0·01). NK1 cells did not show any IL-13, but released significantly high amounts of IFN-γ. The above results suggest that NK cells might influence the overall inflammatory network in allergy by increased IL-4, IL-5, IL-13 and IFN-γ release.
Fig. 2.
Cytokine profile of natural killer (NK) cells from healthy individuals and atopic dermatitis (AD) patients. Purified NK cells were cultured for 72 h without any stimulation. Spontaneously secreted cytokines were determined by enzyme-linked immunosorbent assay (ELISA). Results show mean ± s.d. of five AD and five healthy individuals (a). Purified NK cells were stimulated with PHA, PMA and ionomycin for 12 h. Intracytoplasmic cytokines were determined by flow cytometry. Results are from representative donors of three AD patients and three healthy individuals tested (b). NK1 and NK2 cell subsets were differentiated from freshly isolated NK cells of AD patients. Cytokines were determined from supernatants 3 days after PHA stimulation. Results are shown as mean ± s.d. of six experiments (c). *P < 0·01.
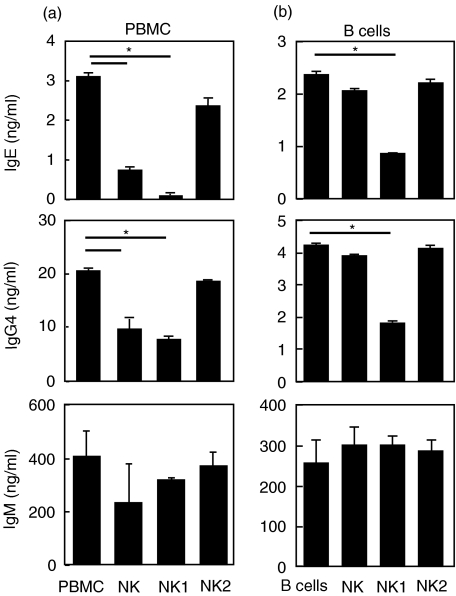
NK1 cells inhibit IgE production by B cells via IFN-γ
The functional capacity of in vitro differentiated NK1 and NK2 cells to regulate IgE, IgG4 and IgM synthesis was further analysed. To include the entire system of peripheral blood immune cells, NK1 and NK2 subsets and freshly isolated NK cells from AD patients were co-cultured with autologous PBMC. The cells were stimulated with IL-4 and sCD40L, as a strong stimulus that induces IgE [21,25]. As shown in Fig. 3, NK1 cells abolished IgE synthesis (P < 0·05). There was also a significant decrease in IgG4 production, whereas IgM did not change (P < 0·05). Interestingly, NK2 cells did not influence IL-4- and sCD40L-stimulated IgE and IgG4 synthesis. These findings were confirmed by experiments with IL-4- and sCD40L-stimulated B cell and NK cell co-cultures. Again, NK1 cells showed a significant inhibition of IgE and IgG4 but not of IgM (P < 0·05). These results demonstrate that NK1 cells may play an IgE counter-regulatory role in allergy. The direct toxicity of NK1 cells on B cells was principally eliminated, because IgM production was not affected by NK cells.
Fig. 3.
Natural killer (NK) cells inhibit immunoglobulin E (IgE) production by B cells. Peripheral blood mononuclear cells (PBMC) were co-cultured with in vitro differentiated NK1 and NK2 cells, stimulated with IL-4 and sCD40L for 12 days and IgE, IgG4 and IgM levels were determined by enzyme-linked immunosorbent assay (ELISA) (a). Purified B cells were co-cultured with in vitro differentiated NK1 and NK2 cells, stimulated with IL-4 and sCD40L for 12 days and IgE, IgG4 and IgM levels were determined by ELISA (b). The results are shown as mean ± s.d. of triplicate cultures. The same results were obtained in two other experiments. *P < 0·05.
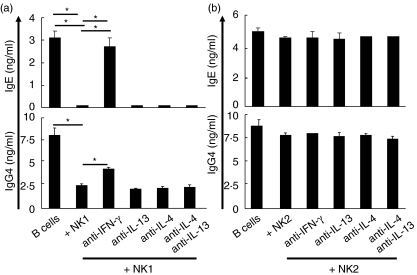
To investigate the mechanism of IgE inhibition by NK1 cells, in vitro differentiated NK1 cells were co-cultured with purified B cells in the presence of neutralizing anti-IFN-γ, anti-IL-4 and anti-IL-13 mAbs. As shown in Fig. 4a, inhibition of IgE and IgG4 by NK1 cells was significantly blocked by neutralization of IFN-γ (P < 0·001 and P < 0·02, respectively).
Fig. 4.
Suppression of immunoglobulin E (IgE) production by natural killer (NK) NK1 cells is mediated by interferon (IFN)-γ. Purified B cells were co-cultured with in vitro differentiated NK1 cells in the presence of IL-4 and sCD40L for 12 days. Neutralizing anti-IFN-γ, anti-IL-4 and anti-IL-13 monoclonal antibodies (mAbs) were added to cultures from the start. IgE and IgG4 levels were determined by enzyme-linked immunosorbent assay (ELISA) (a). Mean ± s.d. of triplicate cultures is shown. The same results were obtained in two other experiments. Purified B cells were co-cultured with in vitro differentiated NK2 cells in the presence of interleukin (IL)-4 and sCD40L for 12 days. Neutralizing anti-IFN-γ, anti-IL-4 and anti-IL-13 mAbs were added to cultures from the start. IgE and IgG4 levels were determined by ELISA (b). Mean ± s.d. of triplicate cultures is shown. The same results were obtained in two other experiments. *P < 0·001 or P < 0·02.
There was no influence in neutralization of IL-4 and IL-13 on NK1 cell-mediated IgE suppression. Unlike Th2 cells, NK2 cells did not induce IgE in B cell co-cultures (Fig. 4b). In addition, there was no difference in IgE regulation in NK1 and NK2 subsets between healthy and allergic individuals (data not shown). Accordingly, it appears that there is no intrinsic defect on NK cell differentiation between allergic and healthy individuals.
Discussion
Analysis of the expression of co-stimulatory, NK cell and apoptosis receptors and demonstrate a considerable heterogeneity in immune regulation in polyallergic atopic patients with regard to their NK cell subsets. The decreased percentage of CD16+CD56+ NK cells in polyallergic patients [27] initiates questions for further characterization of NK cell subsets, because they may play a role in skewed peripheral Th2 response in polyallergic patients and healthy individuals.
Although there was no clear difference for the expression of co-stimulatory molecules, except CD40, between the polyallergic and healthy subjects, these molecules were expressed differently in NK cell subsets. The decreased expression of CD40 in NK, NK1 and NK2 cell subsets of polyallergic patients might be related to immunoglobulin regulation. The in vivo relevance of low CD40 expression on NK cells of AD patients remains to be elucidated. Although it was low in all three subsets, it has to be noted here that only NK1 cells showed a significant anti-IgE effect, whereas NK2 cells did not influence IgE production. There was no difference for the expression of CD45RA, CD45RO and ICOS on NK cell subsets between the polyallergic and healthy subjects. However, CD45RA expression was higher in freshly isolated NK cells compared to NK1 and NK2 cells; in contrast, CD45RO and ICOS expression was higher in NK1 cells compared to NK and NK2 cells in both polyallergic and healthy subjects.
Different studies have shown that NK1 cells express higher levels of cell surface CD95 antigen than NK2 cells and are more sensitive to antibody or chemically induced apoptosis. However, CD95 mRNA expression did not differ in NK1 and NK2 cells, suggesting a post-translational regulation [28]. Increased levels of CD95 surface expression in NK2 cells were shown in remission of multiple sclerosis patients [29]. In the present study, CD95 and CD95L expressions were found to be higher in NK1 and NK2 cells compared to NK cell subsets in AD patients and also healthy subjects. These findings showed that freshly isolated NK cells might be protected from apoptosis, whereas NK1 and NK2 cell subsets might be more sensitive to apoptotic mechanisms. It has been reported that the Th1 compartment of activated memory/effector T cells selectively undergoes activation-induced cell death, skewing the immune response toward surviving Th2 cells in AD patients [30].
Although unactivated NK cells lyse a variety of cells, target recognition is governed by specific receptors that either activate or inhibit NK cell activity. The success in cloning of human NK cells has been basic for the demonstration that NK cells display a clonal heterogeneity in their ability to recognize determined HLA class I molecules [15,31]. In AD patients, KIR, NKAT-2 and GL183 receptor expressions were significantly higher in NK2 cells compared to NK cells.
In healthy individuals, only lectin-like inhibitory receptor NKG2A expression was found to be significantly higher in NK1 cells compared to NK2 cells. NKG2A expression was significantly increased in NK2 cell subset of AD patients compared to healthy subjects. HLA-E complexes can bind to NKG2A receptor, which is present on NK cells in a complex with the cell surface molecule CD94. Whereas CD94/NKG2A receptors through HLA-E control the global status of class I expression, KIR molecules monitor the loss of expression of single class I molecules [32]. However, EB6 expression of NK2 cells of AD was significantly decreased compared to healthy subjects. EB6 or EB6-like mAbs can react both KIR2DL1 (inhibitory) and KIR2DS1 (activatory), and GL183 or GL183-like mAbs can react KIR2DL2 (inhibitory), KIR2DL3 (inhibitory) and KIR2DS2 (activatory) [33]. Given the high degree of homology between different KIRs, most of the KIR-specific mAbs recognize epitopes shared not only by two or more inhibitory KIRs, but also by their activating counterpart.
These results suggest that NK1 and NK2 cell subsets show differences in KIR and lectin-like receptor expression, and the relevance of increased NKAT-2 and GL183 expressions in NK2 cells in atopic individuals require further studies.
The present study shows that NK cells comprise distinct cytokine producing subsets similar to Th1 and Th2 cells in humans and these subsets interfere with IgE regulation. NK cells from polyallergic AD patients spontaneously released higher amounts of IL-4, IL-5, IL-13 and IFN-γ compared to healthy donors. Upon stimulation, NK cells of allergic patients displayed IL-5- and IL-13-producing subsets. Although T cells constitute a large population of cellular infiltrate in AD, a dysregulated cytokine mediated response of the immune system appears to be an important pathogenic factor [3,34]. Apparently, spontaneous cytokine release in negatively selected, freshly purified NK cells refers to in vivo activation and may suggest the involvement of NK cells in unbalanced cytokine network in allergic inflammation. In AD, circulating allergen-specific memory/effector T cells expressing the skin-specific homing receptor, the cutaneous lymphocyte-associated antigen, have been demonstrated to be activated in vivo[7,8]. Similar to NK cells in the present study, cutaneous lymphocyte-associated antigen-bearing T cells in AD spontaneously released higher IL-5 and IL-13 and regulated IgE and eosinophilia compared to healthy controls [7,8,35].
It has been shown that NK cells from cord blood grown in cultures favouring naive T cells to Th1 cell differentiation condition differentiate into NK1 cells producing IFN-γ, whereas NK cells grown in a Th2 differentiation condition differentiate into NK2 cells producing predominantly IL-5 and IL-13 [10]. The in vivo existence of human NK cell subsets, similar to Th1 and Th2 cells, was also shown in freshly isolated IFN-γ-secreting and IFN-γ non-secreting NK cells [11].
Although some studies have reported that NK cells inhibit B cell differentiation and suppress antibody production [36,37], others have demonstrated that NK cells promote B cell growth and increase IgM and IgG antibody production by activated as well as resting B cells [38,39]. The functional immunoglobulin isotype regulatory capacity of NK cells was investigated by co-culturing in vitro differentiated NK cell subsets with purified B cells. Although NK2 cells produce IL-4 and IL-13 that are known to induce IgE class switch [40,41], their co-culture with purified B cells induced very little or undetectable IgE. For this reason the cells were stimulated with IL-4 and sCD40L, mimicking Th2 cell activation that helps B cells for IgE production as demonstrated repeatedly in allergic diseases [7,8,21,42–45]. In both PBMC and B cell cultures, NK1 cells showed a significant anti-IgE effect, whereas NK2 cells did not influence the IL-4 and sCD40L-induced IgE production. The anti-IgE effect of NK1 cells was inhibited by blocking of IFN-γ, demonstrating the important role of this cytokine on inhibition of IgE synthesis. IFN-γwas demonstrated previously as an inhibitor of IgE in several studies. IFN-γ down-regulates IgE synthesis by human B cells [21,46,47] and IFN-γ treatment decreases serum IgE levels in hyper-IgE syndrome [47].
In conclusion, the present study demonstrates that human NK cells comprise distinct receptor-expressing and cytokine-producing subsets similar to Th1 and Th2 cells. These subsets of NK cells show differences in surface KIR receptors and co-stimulatory receptors and interfere with immunoglobulin regulation.
Acknowledgments
This work was supported by the Swiss National Foundation (32·105865 and 32·100266), Deutsche Forschungsgemeinschaft (DFG, SFB 571 to C.S.F) and the Research Fund of the University of Istanbul. Project number: T-780/0703200.
References
- 1.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermol Venerol. 1980;92:44–7. [Google Scholar]
- 2.Novak N, Bieber T, Leung DY. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112:128–39. doi: 10.1016/j.jaci.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–7. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;51:1715–21. doi: 10.1016/S0140-6736(97)12082-7. [DOI] [PubMed] [Google Scholar]
- 5.Walker C, Virchow J-C, Bruijnzeel PLB, Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991;146:1829–35. [PubMed] [Google Scholar]
- 6.Robinson DS, Hamid QA, Ying S, et al. Predominant Th2-like bronchoalveolar T lymphocyte population in atopic asthma. N Eng J Med. 1992;326:298–306. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 7.Akdis M, Akdis CA, Weigl L, Disch R, Blaser K. Skin homing, CLA+ memory T cells are activated in atopic dermatitis and regulate IgE by an IL-13-dominated cytokine pattern. IgG4 counter-regulation by CLA– memory T cells. J Immunol. 1997;159:4611–9. [PubMed] [Google Scholar]
- 8.Akdis M, Simon HU, Weigl L, Kreyden O, Blaser K, Akdis AC. Skin homing CLA+ CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol. 1999;163:466–75. [PubMed] [Google Scholar]
- 9.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–4. [PubMed] [Google Scholar]
- 11.Deniz G, Akdis M, Aktas E, Blaser K, Akdis AC. Human NK1 and NK2 subsets determined by purification of IFN-γ secreting and IFN-γ non-secreting NK cells. Eur J Immunol. 2002;32:879–84. doi: 10.1002/1521-4141(200203)32:3<879::AID-IMMU879>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger AT, Caligiuri MA. The biology of human natural killer cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells. a unique immunoregulatory role for the CD56 bright subset. Blood. 2001;31:46–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 14.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626–33. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Moretta L, Biassoni R, Bottino C, et al. Human NK cells and their receptors. Microbes Infect. 2002;4:1539–44. doi: 10.1016/s1286-4579(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 16.Borrego F, Masilamani M, Kabat J, Sanni TB, Coligan JE. The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol Immunol. 2005;42:485–8. doi: 10.1016/j.molimm.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 18.Blanca IR, Bere EW, Young HA, Ortaldo JR. Human B cell activation by autologous NK cells is regulated by CD40–CD40 ligand interaction role of memory B cells and CD5+ B cells. J Immunol. 2001;167:6132–9. doi: 10.4049/jimmunol.167.11.6132. [DOI] [PubMed] [Google Scholar]
- 19.Satoskar RA, Stamm LM, Zhang X, et al. NK cell deficient mice develop a Th1 like response but fail to mount and efficient antigen-specific IgG2a antibody response. J Immunol. 1999;163:5298–302. [PubMed] [Google Scholar]
- 20.Lane P, Brocker T, Hubele S, Padovan E, Lanzaveccia A, McConell FF. Soluble CD40 ligand can replace the normal T cell derived CD40 ligand signal to B cell in T cell dependent activation. J Exp Med. 1993;177:1209–13. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akdis CA, Blesken T, Akdis M, et al. Induction and differential regulation of bee venom phospholipase A2-specific human IgE and IgG4 antibodies in vitro requires allergen specific and non-specific activation of T and B cells. J Allergy Clin Immunol. 1997;99:345–52. doi: 10.1016/s0091-6749(97)70052-6. [DOI] [PubMed] [Google Scholar]
- 22.Deniz G, Christmas SE, Brew R, Johnson PM. Phenotypic and functional cellular differences between human CD3– decidual and peripheral blood leukocytes. J Immunol. 1994;152:4255–61. [PubMed] [Google Scholar]
- 23.Akdis CA, Akdis M, Blesken T, et al. Epitope specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98:1676–83. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akdis CA, Blesken T, Wymann D, Akdis M, Wüthrich W, Blaser K. The role of IL-10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akdis CA, Blesken T, Akdis M, Alkan SS, Heusser CH, Blaser K. Glucocorticoids inhibit human antigen-specific and enhance total IgE and IgG4 production due to differential effects on T and B cells in vitro. Eur J Immunol. 1997;27:2351–7. doi: 10.1002/eji.1830270933. [DOI] [PubMed] [Google Scholar]
- 26.Herberman RR, Reynolds CW, Ortaldo JR. Mechanisms of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol. 1986;4:651–80. doi: 10.1146/annurev.iy.04.040186.003251. [DOI] [PubMed] [Google Scholar]
- 27.Whermann W, Reinhold U, Kunkel S, Franke N, Uerlich M, Kreysel HW. Selective alterations in natural killer cell subsets in patients with atopic dermatitis. Int Arch Allergy Immunol. 1990;92:318–22. doi: 10.1159/000235196. [DOI] [PubMed] [Google Scholar]
- 28.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4 independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, Miyake S, Kondo T, et al. Natural killer type 2 bias in remission of multiple sclerosis. J Clin Invest. 2001;107:23–9. doi: 10.1172/JCI11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akdis M, Trautmann A, Klunker S, et al. T helper (Th)2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J. 2003;17:1026–35. doi: 10.1096/fj.02-1070com. [DOI] [PubMed] [Google Scholar]
- 31.Moretta L, Ciccone E, Moretta A, Hoglund P, Ohlen C, Karre K. Allorecognition by NK cells: nonself or no self ? Immunol Today. 1992;13:300–6. doi: 10.1016/0167-5699(92)90042-6. [DOI] [PubMed] [Google Scholar]
- 32.Hofmeister V, Weiss HE. HLA-G modulates immune responses by diverse receptor interactions. Semin Cancer Biol. 2003;13:317–23. doi: 10.1016/s1044-579x(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 33.Vitale M, Carlomagno S, Falco M, et al. Isolation of a novel KIR2DL3-specific mAb: comparative analysis of the surface distribution and function of KIR2DL2, KIR2DL3 and KIR2DS2. Int Immunol. 2004;16:1459–66. doi: 10.1093/intimm/dxh147. [DOI] [PubMed] [Google Scholar]
- 34.Akdis CA, Akdis M, Trautmann A, Blaser K. Immune regulation in atopic dermatitis. Current Opin Immunol. 2000;12:641–6. doi: 10.1016/s0952-7915(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 35.Santamaria Babi LF, Picker LJ, Perez-Soler MT, et al. Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med. 1995;181:1935–40. doi: 10.1084/jem.181.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arai S, Yamammoto H, Itoh K, Kumagai K. Suppressive effect of human NK cells on pokeweed mitogen-induced B cell differentiation. J Immunol. 1983;131:651–7. [PubMed] [Google Scholar]
- 37.Satoskar RA, Stamm LM, Zhang X, et al. NK cell deficient mice develop a Th1 like response but fail to mount and efficient antigen-specific IgG2a antibody response. J Immunol. 1999;163:5298–302. [PubMed] [Google Scholar]
- 38.Dixon Gray J, Horwitz DA. Activated human NK cells can stimulate resting B cells to secrete Ig. J Immunol. 1995;154:5656–64. [PubMed] [Google Scholar]
- 39.Snapper CM, Yamaguchi H, Moorman MA, Mond JJ. An in vitro model for T cell independent induction of humoral immunity: a requirement for NK cells. J Immunol. 1994;152:4884–92. [PubMed] [Google Scholar]
- 40.Bacharier LB, Geha RS. Molecular mechanism of IgE regulation. J Allergy Clin Immunol. 2000;105:547–58. doi: 10.1016/s0091-6749(00)90059-9. [DOI] [PubMed] [Google Scholar]
- 41.Del Prete G, Maggi E, Paola P, Ghretien I, Tiri A, Macchia D, Banchereau J, De Vries J, Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988;140:4193–8. [PubMed] [Google Scholar]
- 42.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell FF. Soluble CD40 ligand can replace the normal T cell derived CD40 ligand signal to B cell in T cell dependent activation. J Exp Med. 1993;177:1209–13. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, De Vries JE. Anti CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified B cells via different signalling pathways. J Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 44.Akdis CA, Akdis M, Simon D, et al. T cells and T cell-derived cytokines as pathogenic factors in the nonallergic form of atopic dermatitis. J Invest Dermatol. 1999;113:628–34. doi: 10.1046/j.1523-1747.1999.00720.x. [DOI] [PubMed] [Google Scholar]
- 45.King CL, Gallin JI, Malech HL, Abramson SL, Nutman TB. Regulation of immunoglobulin production in hyper immunoglobulin E recurrent-infection syndrome by interferon gamma. Proc Natl Acad Sci USA. 1989;86:10085–9. doi: 10.1073/pnas.86.24.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pene J, Rousset F, Briere F, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferon gamma and prostaglandin E2. Proc Natl Acad Sci USA. 1988;85:6880–4. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carballido JM, Carballido-Perrig N, Oberli-Schraemmli A, Heusser CH, Blaser K. Regulation of IgE and IgG4 responses by allergen-specific T-cell clones to bee venom phospholipase A2in vitro. J Allergy Clin Immunol. 1994;93:758–67. doi: 10.1016/0091-6749(94)90256-9. [DOI] [PubMed] [Google Scholar]