Abstract
YKL-40 is secreted by macrophages and neutrophils and patients with bacterial infections have elevated circulating YKL-40. The aim was to evaluate changes in plasma YKL-40 (determined by enzyme-linked immunosorbent assay (ELISA) at 0, 2, 4, 8, 24 and 32 h) in eight healthy volunteers after injection with Esherichia coli endotoxin or saline. Plasma YKL-40 increased after endotoxin injection from 31 µg/l (range 19–39 µg/l) to a maximum of 159 µg/l (61–552 µg/l, P < 0·01) at 24 h. The finding that plasma YKL-40 increased after endotoxin injection compared with saline (P < 0·001) suggests that YKL-40 has a functional role in infections.
Keywords: CHI3L1, endotoxin, HC-gp39, TNF-α, YKL-40
Introduction
YKL-40, a phylogenetically highly conserved heparin- and chitin-binding lectin without chitinase activity [1–5], is a member of ‘mammalian chitinase-like proteins’. The gene for YKL-40 [6,7] and its crystal structure has been described previously [8,9], but the site and mode of binding of YKL-40 to cell surface receptors is unknown. YKL-40 is expressed by different inflammatory cells. Thus, neutrophil precursors start synthesizing YKL-40 at the myelocyte–metamyelocyte stage; it is stored in the specific granules of neutrophils and released from fully activated cells [10]. YKL-40 expression in vitro is absent in normal human monocytes but induced strongly during late stages of macrophage differentiation [6]. In the latter, secretion of YKL-40 appears restricted to distinct subtypes. Serial analysis of gene expression has demonstrated 288-fold increased YKL-40 transcripts in monocytes stimulated with granulocyte-macrophage colony-stimulating factor (GM-CSF), 182-fold in M-CSF-stimulated monocytes and 31-fold increased YKL-40 transcripts in lipopolysaccharide (LPS)-stimulated monocytes [11,12]. No YKL-40 expression is found in human monocytes or dendritic cells [13]. In vivo, YKL-40 mRNA and protein are expressed by a subpopulation of macrophages in different tissues, such as inflamed synovial membranes from patients with rheumatoid arthritis, osteoarthritis or spondylarthropathy [14–16], atherosclerotic plaques [17], arteritic vessels from patients with giant cell arteritis [18] and sarcoid lesions from patients with pulmonary sarcoidosis (personal observation). In patients with rheumatoid arthritis, YKL-40 protein is expressed in CD16+ monocytes with a dim expression of CD14 [15]. This CD14+, CD16+ phenotype can differentiate from classic CD142+ monocytes by maturation in vitro and is believed to be a more mature version of monocytes with properties of tissue macrophages, probably of proinflammatory type [19]. The blood count of CD14+, CD16+ monocytes is increased in numbers in patients with sepsis, rheumatoid arthritis, tuberculosis and solid tumours [19].
The regulation and definitive biological function of YKL-40 have not been fully clarified. YKL-40 probably has a function in both acute and chronic inflammatory processes. The finding that YKL-40 initiates a signalling cascade in fibroblasts which leads to increased cell proliferation suggests a role in conditions leading to tissue fibrosis [20]. Moreover, YKL-40 acts synergistically with insulin-like growth factor 1 in stimulating the growth of fibroblasts [20]. YKL-40 also acts as a chemo-attractant for vascular endothelial cells, stimulates their migration and modulates their morphology by promoting the formation of branching tubules, indicating a role in angiogenesis [21].
More than 75% of patients with Streptococcus pneumoniae pneumonia [22] and S. pneumoniae bacteraemia [23] have elevated serum concentrations of YKL-40 compared with healthy subjects, and an association exists between levels of circulating YKL-40 and the severity and fatal outcome of the disease. YKL-40 is found locally within the compartment of an infection as illustrated by high levels in cerebrospinal fluid of patients with purulent meningitis and encephalitis [24]. Finally, elevated serum YKL-40 levels are found in patients with other diseases characterized by inflammation and tissue remodelling, such as rheumatoid arthritis [2,16,25,26], giant cell arteritis [18], inflammatory bowel disease [27], liver fibrosis [28–30] and advanced cancer [31–35]. The study of these patients, however, does not allow for assessment of the dynamics of YKL release.
The human endotoxaemia model, featuring intravenous endotoxin administration to human volunteers, has been characterized previously in our laboratory [36–38]. Advantages of the model include a well-defined starting point and a reproducible, highly dynamic course displaying the features of a systemic inflammatory response that is fully reversible within hours. The systemic characteristics and safety of the human endotoxaemia model have been documented amply. The initial symptoms, consisting of headaches, chills and malaise, occur at 60 min and peak at 90 min after endotoxin injection, followed by a gradual resolution over the next 2–3 h. The temperature peaks at 4 h after endotoxin and decreases gradually thereafter. The peak in symptoms at 90 min coincide with a peak in the arterial concentration of tumour necrosis factor (TNF)-α, whereas the concentrations of interleukin (IL)-6 and IL-1β peak at 2–3 h and at 4 h hours after endotoxin, respectively [38]. The human endotoxaemia model was used in the present study to test the hypothesis that endotoxin injection in healthy subjects elevates plasma YKL-40, and to define the time–course of this elevation in the early phase after endotoxin injection.
Materials and methods
Volunteers
Eight healthy young volunteers [21–25 years, 77 ± 3 (mean ± s.e.) kg body weight] were studied. None of the subjects had a history of medical problems. A physical examination and blood analysis [haemoglobin, white blood cell and differential count, C-reactive protein (CRP), blood glucose, as well as biomarkers of kidney function, liver function and the coagulation system] prior to inclusion revealed no abnormalities. The volunteers did not use any medication and had no febrile illness in the 2 weeks preceding the study.
Study design
The design of the study has been described previously [36,37]. The study was performed in an intensive care unit setting under continuous supervision of an anaesthesiologist, with emergency and resuscitation equipment immediately available. The volunteers were studied after an overnight fast and an intravenous bolus injection of Esherichia coli endotoxin (lot EC-6, United States Pharmacopia Convention, Rockville, MD, USA; 2 ng/kg body weight) or of saline, respectively. The sequence of injections was random, and studies were spaced at least 14 days apart. Because of the obvious symptoms and clinical changes after endotoxin, neither volunteers nor staff members were blinded to the study protocol. Subjects rested in the supine position, with continuous monitoring of cardiovascular functions for 24 h. After endotoxin injection, subjects were infused with isotonic saline for 8 h (15 ml/kg/h for 1 h, followed by 7 ml/kg/h for 7 h), and were allowed to eat after 8 h. Blood samples were obtained at baseline and 2, 4, 8, 24 and 32 h after injection of endotoxin or saline. The study was approved by the regional scientific ethics committee; written informed consent was obtained from each volunteer.
Biochemical analysis
Blood samples were drawn into tubes containing ethylinediamine tetra-acetic acid (EDTA) and trasylol and spun immediately at 3500 g for 15 min at 4°C. Plasma was stored at − 70°C until analysis. Plasma concentration of YKL-40 was determined by a sandwich enzyme-linked immunosorbent assay (ELISA) (Quidel, Santa Clara, CA, USA) [26]. The intra- and interassay coefficients of variation are 3·6% and 5·3%, respectively; the detection limit is 10 µg/l. Plasma IL-6 and TNF-α were measured by ELISA (R&D Systems, Abingdon, Oxon, UK HS600 and HSTA00C). Plasma IL-6, TNF-α, neutrophils, serum CRP, orosomucoid and fibrinogen have been reported elsewhere [36,37].
Statistical analyses
Statistical analysis was performed with sigmastat, version 2·0 (SPSS Inc., Chicago IL, USA). Statistical significance between measurements obtained at the different time-points after endotoxin and after placebo injection (paired design) was ascertained by two-way repeated-measures anova. The two factors were treatment (saline versus endotoxin) and time. After a statistically significant treatment effect (or treatment versus time interaction) was detected, a multiple-comparison procedure (Tukey's test) was used to identify significant differences at each time-point. The results are given as medians (range) or box-plots as indicated. Results with P-values ≤ 0·05 were considered to be statistically significant.
Results
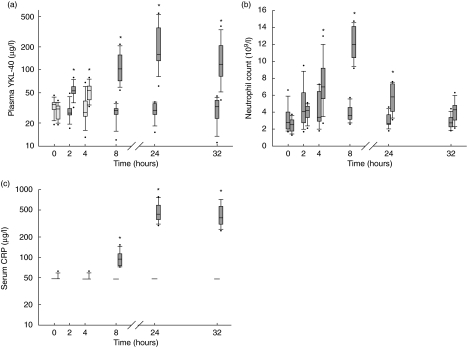
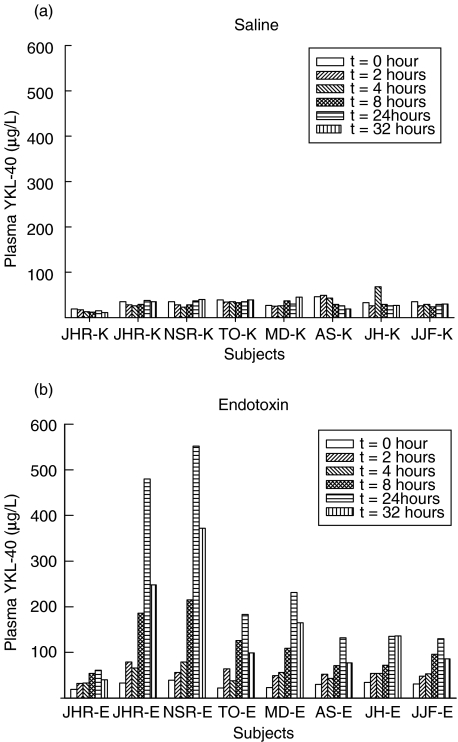
Figure 1a illustrates the changes in plasma concentrations of YKL-40 after injection of endotoxin or saline in the healthy volunteers. Plasma YKL-40 increased after administration of endotoxin compared with saline (P < 0·001, two-way repeated-measures anova) from a median level of 31 µg/l (range 19–39 µg/l) before endotoxin to a maximum of 159 µg/l (61–552 µg/l) (P < 0·01) at 24 h. The increase in plasma YKL-40 was significant (P < 0·01) at 2 h and remained significant at all later time-points after endotoxin injection. Plasma YKL-40 did not change after saline injection and had a median level of 30 µg/l (15–38 µg/l) at 24 h. Figure 2 shows the individual changes in plasma YKL-40 in the healthy subjects after endotoxin or saline injection.
Fig. 1.
Changes in plasma concentrations of YKL-40 (a), neutrophil counts (b) and serum C-reactive protein (CRP) (c) in eight healthy subjects after injection with saline (first box-plot at each time-point) or endotoxin (second box-plot at each time). Values are box and whiskers plots of plasma YKL-40, neutrophil counts and serum CRP. Boxes show 25th, 50th and 75th centiles. Whiskers illustrate 5th and 95th centiles. Circles represent outlier values. *Significant changes (P < 0·01) from baseline levels.
Fig. 2.
Individual changes in plasma concentrations of YKL-40 in eight healthy subjects after injection with saline (a) or endotoxin (b).
Plasma concentrations of TNF-α peaked at 1·5 h and plasma IL-6 at 2 h after endotoxin injection. Rectal temperature increased by approximately 2°C, reaching maximal levels at 4 h after endotoxin injection [36,37].
Changes in neutrophil counts and serum CRP over time are shown in Fig. 1b and c, respectively. Maximum increases in neutrophil count after endotoxin versus placebo injection were found at 8 h [median (range) 12·0 × 109/l (9.2–14.7) versus 3·6 × 109/l (2.6–5.7), P < 0·01] and in serum CRP at 24 h [438 mg/l (297–779) versus 48 mg/l, P < 0·01].
Discussion
The present study is the first to demonstrate that intravenous injection of healthy subjects with E. coli endotoxin results in an increase in plasma concentration of YKL-40 within 2 h. The highest value was found at the 24 h time-point after infusion. The peak value was located between 8 and 32 h after endotoxin, although its exact location was not further specified in this study.
Circulating TNF-α and IL-6 increase markedly within the first 2 h after endotoxin injection [37,38] and it is possible that they induced YKL-40. However, the present study was not designed to explore this relationship in detail.
The function of YKL-40 in infectious diseases is unknown, but it may play a role in inflammatory and tissue remodelling processes. YKL-40 expression is absent in normal human monocytes but induced strongly during the late stages of human macrophage differentiation [6,7] and after stimulation with GM-CSF, M-CSF or LPS [11,12]. Recently, the transcriptional regulation of YKL-40 during human macrophage differentiation has been described [6,7]. There are probably two independent transcription start sites; the promoter sequence contains binding sites for several known factors and specific binding of nuclear PU.1, Sp1, Sp3, USF, AML-1 and C/EBP proteins. The Sp1-family transcription factors appear to have a major role in controlling YKL-40 promoter activity. It has been suggested that the CHI3L1 gene in monocytes is in an inactive or unstable, yet primed state, full activation of which may require additional events (e.g. nucleosome remodelling) that may be initiated by additional elements upstream or downstream of the promoter [7]. Neutrophil granulocytes share a common progenitor cell with monocytes and neutrophil precursors begin to synthesize YKL-40 at the myelocyte–metamyelocyte stage [10]. YKL-40 is stored in the specific granules of neutrophils and released after full activation of the neutrophils [10]. Because YKL-40 is produced and released by activated macrophages and neutrophils, it is most likely that these cells are the major source of the observed increase in plasma YKL-40 after injection with endotoxin. It has been suggested that YKL-40 act as an opsonin with a role in the immune response or as a chitin sensor, switching on innate defences, helping to direct macrophages to the site of invasion and to regulate the inflammatory response as a consequence of infection [8]. Another of the ‘mammalian chitinase-like proteins’, Ym1/ECF-L, has been proposed to have a role in directing components of the immune system to the site of nematode infections [39]. YKL-40 is a heparin-binding protein [4], and it has been found recently that heparin-binding peptides derived from laminin isoforms, von Willebrand factor, vitronectin, protein C inhibitor, fibronectin and complement C exerted antimicrobial activities against Gram-positive and Gram-negative bacteria and the fungus Candida albicans [40]. Future studies should evaluate if heparin-binding peptides from YKL-40 have similar effects.
In the present study, we found that plasma YKL-40 increased by fivefold at 24 h after injection with endotoxin in healthy subjects. It has been shown earlier that patients with S. pneumoniae pneumonia or bacteraemia had eight to 10 times as high serum YKL-40 at the time of hospitalization as did healthy subjects [22,23]. YKL-40 can therefore be regarded as an acute phase protein, as its concentration increases by more than 25% following an inflammatory stimulus.
In patients with S. pneumoniae bacteraemia, high serum YKL-40, but not CRP, at time of diagnosis of S. pneumoniae bacteraemia was an independent prognostic marker of mortality [23]. In patients with S. pneumoniae pneumonia, who were followed during antibiotic treatment, serum CRP reached normal range a few days later than serum YKL-40 [22]. We found in the present study that the level of plasma YKL-40 was already increased after 2 h, whereas serum CRP did not increase until 8 h after endotoxin injection. Thus, plasma YKL-40 increases earlier than serum CRP, suggesting that it may add to the information provided by serum CRP and may be of diagnostic value in patients with severe acute bacterial systemic infections.
Endotoxin is a major stimulator of cytokines. Plasma TNF and IL-6 are already significantly enhanced 30 and 60 min after the administration of a bolus of endotoxin [38]. It is not known how YKL 40 is regulated on the molecular level, but according to the present study, its production, release, or both may be stimulated by cytokines such as TNF-α or IL-6, which are up-regulated early after an inflammatory stimulus. The present study suggests that YKL-40 is a true acute phase reactant, the level of which may add to the information provided by previous well-characterized proteins such as CRP during inflammation and infection.
Acknowledgments
The Copenhagen Muscle Research Center was supported by grant from the University of Copenhagen, the Faculties of Science and of Health Sciences at this University, the Copenhagen Hospital Corporation and the Danish National Research Foundation (grant 504–14). The study was also supported by grants from the Novo Nordisk Foundation, the Lundbeck Foundation, the Danish Medical Research Council, the Danish Rheumatism Association and Direktør Jens Aage Sørensen og Hustru Edith Ingeborg Sørensens Mindefond. The expert technical assistance of Tonni Løve Hansen, Herlev University Hospital is gratefully acknowledged.
References
- 1.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10. [PubMed] [Google Scholar]
- 2.Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32:949–55. doi: 10.1093/rheumatology/32.11.949. [DOI] [PubMed] [Google Scholar]
- 3.Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996;271:19415–20. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- 4.Shackelton LM, Mann DM, Millis AJT. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodelling. J Biol Chem. 1995;270:13076–83. doi: 10.1074/jbc.270.22.13076. [DOI] [PubMed] [Google Scholar]
- 5.Renkema GH, Boot RG, Au FL, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–9. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 6.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 7.Rehli M, Niller H-H, Ammon C, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003;278:44058–67. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 8.Houston DR, Recklies AD, Krupa JC, van Aalten DMF. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J Biol Chem. 2003;278:30206–12. doi: 10.1074/jbc.M303371200. [DOI] [PubMed] [Google Scholar]
- 9.Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–60. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 10.Volck B, Price PA, Johansen JS, et al. YKL-40, a mammalian member of the bacterial chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians. 1998;110:351–60. [PubMed] [Google Scholar]
- 11.Hashimoto S, Suzuki T, Dong H-Y, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999;94:837–44. [PubMed] [Google Scholar]
- 12.Suzuki T, Hashimoto S, Toyoda N, et al. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–91. [PubMed] [Google Scholar]
- 13.Hashimoto S, Suzuki T, Dong H-Y, Nagai S, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocyte-derived dendritic cells. Blood. 1999;94:845–52. [PubMed] [Google Scholar]
- 14.Kirkpatrick RB, Matico RE, McNulty DE, Strickler JE, Rosenberg M. An abundantly secreted glycoprotein from Drosophila melanogaster is related to mammalian secretory proteins produced in rheumatoid tissues and by activated macrophages. Gene. 1995;153:147–54. doi: 10.1016/0378-1119(94)00756-i. [DOI] [PubMed] [Google Scholar]
- 15.Baeten D, Boots AMH, Steenbakkers PGA, et al. Human cartilage gp-39+, CD16+ monocytes in peripheral blood and synovium. Correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43:1233–43. doi: 10.1002/1529-0131(200006)43:6<1233::AID-ANR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Volck B, Johansen JS, Stoltenberg M, et al. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthritis Cartilage. 2001;9:203–14. doi: 10.1053/joca.2000.0377. [DOI] [PubMed] [Google Scholar]
- 17.Boot RG, van Achterberg TAE, van Aken BE, et al. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–94. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 18.Johansen JS, Baslund B, Garbarsch Hansen M, Stoltenberg M, Lorenzen I, Price PA. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 1999;42:2624–30. doi: 10.1002/1529-0131(199912)42:12<2624::AID-ANR17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock HWL. Heterogeneity of human blood monocytes: the CD14+CD16+ subpopulation. Immunol Today. 1996;17:424–8. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 20.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–26. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJT. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–73. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 22.Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sørensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis. 1999;180:1722–6. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- 23.Kronborg G, Østergaard C, Weis N, et al. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated to the outcome of the disease. Scand J Infect Dis. 2002;34:323–6. doi: 10.1080/00365540110080233. [DOI] [PubMed] [Google Scholar]
- 24.Østergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin Diagn Lab Immunol. 2002;9:598–604. doi: 10.1128/CDLI.9.3.598-604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen JS, Stoltenberg M, Hansen M, et al. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology. 1999;38:618–26. doi: 10.1093/rheumatology/38.7.618. [DOI] [PubMed] [Google Scholar]
- 26.Harvey S, Weisman M, O'Dell J, et al. Chondrex: new marker of joint disease. Clin Chem. 1998;44:509–16. [PubMed] [Google Scholar]
- 27.Koutroubakis IC, Petinaki E, Dimoulios P, et al. Increased serum levels of YKL-40 in patients with inflammatory bowel disease. Int J Colorectal Dis. 2003;18:254–9. doi: 10.1007/s00384-002-0446-z. [DOI] [PubMed] [Google Scholar]
- 28.Johansen JS, Christoffersen P, Møller S, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–20. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 29.Tran A, Benzaken S, Saint-Paul M-C, et al. Chondrex (YKL-40), a potential new serum fibrosis maker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol. 2000;12:989–93. doi: 10.1097/00042737-200012090-00004. [DOI] [PubMed] [Google Scholar]
- 30.Nøjgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U EMALD Group. Serum YKL-40 and PIIINP levels as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–86. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 31.Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79:1494–9. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002;62:4364–8. [PubMed] [Google Scholar]
- 33.Dehn H, Høgdall EVS, Johansen JS, et al. Plasma YKL-40, as a prognostic tumor marker in recurrent ovarian cancer. Acta Obstet Gynecol Scand. 2003;82:287–93. doi: 10.1034/j.1600-0412.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 34.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–34. [PubMed] [Google Scholar]
- 35.Dupont J, Tanwar MK, Thaler HT, et al. Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol. 2004;22:3330–9. doi: 10.1200/JCO.2004.09.112. [DOI] [PubMed] [Google Scholar]
- 36.Keller P, Møller K, Krabbe KS, Pedersen BK. Circulating adiponectin levels during human endotoxaemia. Clin Exp Immunol. 2003;134:107–10. doi: 10.1046/j.1365-2249.2003.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bundgaard H, Kjeldsen K, Krabbe KS, et al. Endotoxemia stimulates skeletal muscle Na+-K+-ATPase and raises blood lactate under aerobic conditions in humans. Am J Physiol Heart Circ Physiol. 2003;284:H1028–34. doi: 10.1152/ajpheart.00639.2002. [DOI] [PubMed] [Google Scholar]
- 38.Krabbe KS, Bruunsgaard H, Hansen CM, et al. Ageing is associated with a prolonged fever response in human endotoxaemia. Clin Diagn Lab Immunol. 2001;8:333–8. doi: 10.1128/CDLI.8.2.333-338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owhashi M, Arita H, Hayai N. Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. J Biol Chem. 2000;275:1279–86. doi: 10.1074/jbc.275.2.1279. [DOI] [PubMed] [Google Scholar]
- 40.Andersson E, Rydengård V, Sonesson A, Mörgelin M, Björck L, Schmidtchen A. Antimicrobial activities of heparin-binding peptides. Eur J Biochem. 2004;271:1219–26. doi: 10.1111/j.1432-1033.2004.04035.x. [DOI] [PubMed] [Google Scholar]