Abstract
Chemokines, a group of cytokines that attracts and activates leucocyte subpopulations in inflamed tissue, have been associated with the pathogenesis of a number of inflammatory diseases, and some recent reports have suggested their involvement in Wegener's granulomatosis (WG). To elucidate further the possible role of chemokines in WG we examined serum levels of several CC- and CXC-chemokines in WG patients and assessed the ability of corticosteroids to modulate the expression of these mediators in vitro. Our main findings were: (i) WG patients (n = 14) had elevated serum levels of several inflammatory chemokines [i.e. regulated upon activation normal T cell expressed and secreted (RANTES), monocyte chemoattractant protein (MCP)-1 and interleukin (IL)-8] compared to healthy controls (n = 9), as assessed by enzyme immunoassays (EIAs); (ii) by using EIAs and real-time reverse transcription-polymerase chain reaction (RT-PCR), we demonstrated the ability of methylprednisolone (MP) to down-regulate both the spontaneous and the staphylococcal enterotoxin B (SEB)-induced release of chemokines from peripheral blood mononuclear cells (PBMC) in vitro in both WG patients and controls, possibly involving both transcriptional and post-transcriptional mechanisms; and (iii) the ability of MP to attenuate chemokine secretion was less pronounced in WG patients than in controls, particularly with regard to inhibition of spontaneous release. Our findings suggest a role for chemokines in the pathogenesis of WG. The diminished MP-mediated suppression of chemokines in PBMC from WG patients suggests that more specific modulators of chemokine levels should be investigated in this disorder.
Keywords: chemokines, corticosteroid, Wegener's granulomatosis
Introduction
Wegener's granulomatosis (WG) is a necrotizing, granulomatous vasculitis belonging to the small- to medium-sized vessel systemic vasculitides [1,2]. Although having a clinical predilection of affecting the upper airways, lungs and kidneys, WG is a multi-system disease, and mortality is high without specific therapy [1,2]. However, the introduction of effective treatment regimens, primarily combinations of corticosteroids and cyclophosphamide, has been found to induce remission in a large proportion of patients [3]. Yet, a significant rate of relapse and treatment-related morbidity, in particular infections secondary to drug-induced immunosuppression, still occur, underscoring the need for alternative treatment regimens [3]. To enable such developments, an improved understanding of the pathological processes underlying the development of WG, as well as knowledge on how current treatment modalities modulate these processes, is necessary.
Chemokines, a group of cytokines that attracts and activates leucocyte subpopulations in inflamed tissue, have been associated with the pathogenesis of a number of inflammatory diseases including systemic vascultitis [4–6], and there are also some recent reports suggesting their involvement in WG. For instance, Coulomb-L’Hermine et al. found elevated levels of the CC-chemokine regulated on activation normal T cell expressed and secreted (RANTES) in pulmonary WG lesions [7]. Moreover, it has been reported that antineutrophil cytoplasmic antibodies (ANCAs), which are believed to influence the development and exacerbation of WG, induce the expression of interleukin (IL)-8 in monocytes [8]. Although these and some other findings [9,10] may indicate the involvement of chemokines in WG, the specific role of these chemotactic cytokines in the pathogenesis of this disorder, and in particular the ability of immunosuppressive drugs to modulate them, is far from clear.
As an approach to these issues, we examined the levels of several CC- and CXC-chemokines in WG, and in particular tried to assess the ability of corticosteroids to modulate the expression of these mediators in peripheral blood mononuclear cells (PBMC) in vitro.
Methods
Study population
Fourteen patients with WG were recruited consecutively into the study (Table 1). All patients fulfilled the American College of Rheumatology 1990 classification criteria and the Chapel Hill Consensus Conference on the Nomenclature of Systemic Vasculitis 1992 definition of WG [11,12]. WG patients were classified as having active disease or being in remission based on clinical judgement¸ the Birmingham Vasculitis Activity Score (BVAS) [11,12] as well as levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). For comparison, blood samples were also collected in nine sex- and age-matched healthy controls. While blood samples for cell isolation were available from all controls, such samples were available only from nine of the WG patients. Informed consent for blood sampling was obtained from all subjects. The study was conducted according to the ethical guidelines at our hospital, which comply with the Helsinki declaration, and was approved by the hospital's authorized representative.
Table 1.
Characteristics of the study group.
| Patient | Sex | Age (years) | ESR (mm/h) | CRP (mg/l) | Medication | Disease activity | Organ involvement | cANCA titre |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 21 | 10 | <5 | Prednisolone | Remission | ENT | Neg |
| 2 | Male | 33 | 18 | ″5 | Prednisolone | Remission | ENT | Neg |
| 3 | Female | 29 | 10 | <5 | Trim-Sulpha | Remission | ENT, lung | 1 : 32 |
| 4 | Male | 54 | 47 | 26 | CyclophosphamidePrednisolone | Active | kidney, ENT | 1 : 64 |
| 5 | Male | 65 | 11 | <5 | Prednisolone | Remission | ENT, kidney | 1 : 16 |
| 6 | Male | 59 | 17 | <5 | Trim-Sulpha | Remission | ENT, lung | Neg |
| 7 | Male | 60 | 34 | 11 | Prednisolone | Remission | ENT, kidney | 1 : 16 |
| 8 | Male | 23 | 61 | 29 | Cyclophosphamide Prednisolone | Active | ENT, kidney | 1 : 128 |
| 9 | Male | 36 | >100 | 161 | PrednisoloneTrim-Sulpha | Active | lung, kidney | 1 : 512 |
| 10 | Female | 78 | 21 | 13 | Prednisolone | Remission | ENT | Neg |
| 11 | Female | 35 | 10 | 14 | None | Remission | ENT, kidney | 1 : 16 |
| 12 | Female | 54 | 96 | 62 | CyclophosphamidePrednisolone | Active | ENT, kidney, lung | 1 : 512 |
| 13 | Female | 52 | ″8 | ″7 | None | Remission | ENT, lung | 1 : 16 |
| 14 | Female | 25 | 28 | 62 | CyclophosphamidePrednisolone | Active | Lung, kidney | 1 : 128 |
WG patients were classified as having active disease or being in remission based on clinical judgement¸ the Birmingham Vasculitis Activity Score as well as levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Trim-Sulpha, trimethoprim-sulphametoxazol; ENT, ear, nose and throat; cANCA, cytoplasmic antineutrophil cytoplasmic antibodies. Organ involvement in those in remission represents previous involvement during active disease.
Blood sampling protocol
Peripheral venous blood was drawn between 8 and 10 a.m. into pyrogen-free vacuum blood collection tubes without additives. The tubes were immersed immediately in melting ice and allowed to clot before centrifugation (1500 g for 10 min). All samples were stored at − 80°C and thawed <3 times.
Isolation and stimulation of cells
PBMC were obtained from heparinized blood by Isopaque-Ficoll (Lymphoprep; Nycomed, Oslo, Norway) density gradient centrifugation within 45 min. Mononuclear cells were resuspended in RPMI-1640 (Gibco, Paisley, UK) with 2 mmol/l l-glutamine and 25 mmol/l HEPES buffer and 5% fetal calf serum (TCS BioSciences, Buckingham, UK) and seeded in 24-well plates (Costar, Cambridge, MA, USA; 2 × 106 cells/ml, 1 ml/well) with or without stimulants [staphylococcal enterotoxin B (SEB), Sigma, St Louis, MO, USA or 6α-methylprednisolone-21-hemisuccinate sodium (MP; Sigma) or a combination thereof]. Cell pellets and cell-free supernatants were harvested after 6 and 24 h, respectively, and stored in liquid nitrogen (pellets) or − 80°C (supernatants) until further analysis. The use of SEB and MP concentrations were based on preliminary dose–response experiments examining three different SEB concentrations (0·01 ng/ml, 1 ng/ml and 100 ng/ml) and three different MP concentrations (10−8 m, 10−7 m and 10−6m) and a combination thereof. While the SEB-stimulated chemokine response (see below) seemed to reach an optimum at 1 ng/ml, the suppressive effect of MP on chemokines showed a dose-dependent pattern with the most prominent effect at 10−6 m (data not shown). This MP concentration, in combination with 1 ng/ml SEB, was therefore used in the subsequent experiments, representing pharmacological doses of this medication with relevance to immunosuppressive therapy in WG patients.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from PBMC using the RNeasy mini kit (Qiagen, Hilden, Germany) and stored in RNA storage solution (Ambion, Austin, TX, USA) at − 80°C while awaiting further analysis. Primers and probes for real-time RT-PCR were designed using the Primer Expression version 2·0 software package (Applied Biosystems, Foster City, CA, USA; see Table 2 for details). Quantification of mRNA was performed using the ABI Prism 7000 (Applied Biosystems) [13]. Taqman and SyBr green assays (Table 2) were performed using 300 nmol/l sense and antisense primers as described previously [14]. Gene expression of the housekeeping genes β-actin and GADPH (Applied Biosystems) were used for normalization.
Table 2.
Characteristics of the real-time reverse transcription-polymerase chain reaction (RT-PCR) assays used in the study.
| Target | Sequence (5′→3′) | Acc. no. |
|---|---|---|
| β-actin* | (+)-AGGCACCAGGGCGTGAT | NM_001101 |
| (–)-TCGTCCCAGTTGGTGACGAT | ||
| ENA-78 | (+)-AAGTGGTAGCCTCCCTGAAGAAC | X78686 |
| (–)-CCTTGTTTCCACCGTCCAA | ||
| FAM-AGGAAATTTGTCTTGATCCAGAAGCCCCT-TAMRA | ||
| IL-8 | (+)-GCCAACACAGAAATTATTGTAAAGCTT | Y00787 |
| (–)-CCTCTGCACCCAGTTTTCCTT | ||
| FAM-CTGATGGAAGAGAGCTCTGTCTGGACCC-TAMRA | ||
| MCP-1 | (+)-AAGCTGTGATCTTCAAGACCATTGT | S69738 |
| (–)-TGGAATCCTGAACCCACTTCTG | ||
| FAM-CCAAGGAGATCTGTGCTGACCCCAA-TAMRA | ||
| MIP-1α | (+)-CTGCATCACTTGCTGCTGACA | M23452 |
| (–)-CACTGGCTGCTCGTCTCAAAG | ||
| FAM-TTCAGCTACACCTCCCGGCAGATTCC –TAMRA | ||
| RANTES* | (+)-CCCAGCAGTCGTCTTTGTCA | M21121 |
| (–)-TCCCGAACCCATTTCTTCTCT |
SyBr Green assays; (+), forward primers; (−), reverse primers; Acc. no., GenBank accession number. ENA, epithelial cell-derived neutrophil-activating factor; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation normal T cell expressed and secreted.
Enzyme immunoassays
Concentrations of RANTES, IL-8, macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein (MCP)-1 and epithelial cell-derived neutrophil-activating factor (ENA)-78 were analysed by enzyme immunoassay (EIA) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). The intra- and interassay coefficient of variation was <10% for all assays.
Statistical analysis
Statistical comparisons between WG patients and healthy controls were performed using the Mann–Whitney rank sum test. Responses within the same individuals were compared by the Wilcoxon's signed-rank test for paired data. P-values were two-sided and considered significant when <0·05.
Results
Serum chemokine levels in WG patients and healthy controls
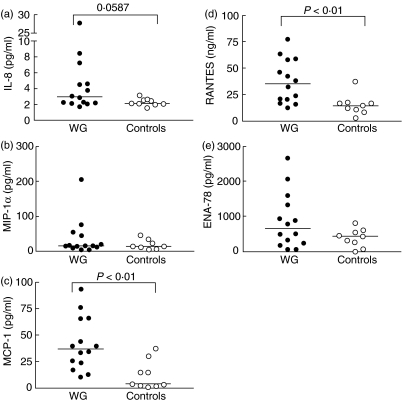
In the present study we examined serum levels and the suppressive effect of MP in vitro on several CC- and CXC-chemokines in WG patients and healthy controls (Table 3). As shown in Fig. 1, WG patients (n = 14) had raised serum levels of RANTES, MCP-1 and IL-8 compared to healthy controls (n = 9), although the difference in IL-8 levels did not reach statistical significance (P = 0·06). In contrast, serum levels of MIP-1α and ENA-78 in WG patients were comparable to those in healthy controls (Fig. 1). Although there were some trends, somewhat surprisingly we found no significant correlations between chemokine levels and the clinical parameters in the patient group as outlined in Table 1. However, the primary aim of this study was to examine the in vitro effect of steroids on chemokine levels in PBMC from WG patients and healthy controls and the study was not powered to analyse differences within the WG group. The lack of correlations within the WG patients should therefore be interpreted with caution.
Table 3.
Some selected aspects of the chemokines examined in the present study.
| Chemokines | Important cellular source | Receptors | Important target cells |
|---|---|---|---|
| C-C chemokines | |||
| ″MCP-1/CCL2 | Monocytes, macrophages | CCR2 | Monocytes, macrophages, T cells |
| ″MIP-1α/CCL3 | T cells, monocytes | CCR1, CCR5 | Monocytes, macrophages, T cells |
| ″RANTES/CCL5 | Platelets, T cells, monocytes | CCR1, CCR3, CCR5 | Monocytes, macrophages, T cells |
| C-X-C chemokines | |||
| ″GROα/CXCL1 | Platelets, monocytes | CXCR2 | Neutrophils |
| ″ENA-78/CXCL5 | Platelets, monocytes | CXCR2 | Neutrophils |
| ″IL-8/CXCL8 | Monocytes, granulocytes | CXCR1, CXCR2 | Neutrophils |
MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normally T cell expressed and secreted; GRO, growth related oncogene; ENA, epithelial neutrophil-activating peptide; IL, interleukin. In the CXC-chemokines, one amino acid separate the first two cysteine residues, whereas in the CC-chemokines, the first two cysteine residues are adjacent to each other.
Fig. 1.
Serum levels of the chemokines interleukin (IL)-8 (a), macrophage inflammatory protein (MIP)-1α (b), monocyte chemoattractant protein (MCP)-1 (c), regulated upon activation normal T cell expressed and secreted (RANTES) (d) and epithelial cell-derived neutrophil-activating factor (ENA)-78 (e) in 14 Wegener's granulomatosis (WG) patients and nine healthy controls. The concentrations of chemokines were measured by enzyme immunoassays (EIAs).
Effects of MP on unstimulated and SEB-induced gene expression of chemokines in PBMC
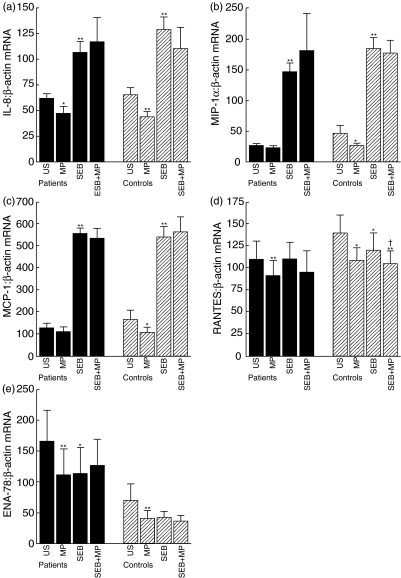
We next examined the ability of MP and SEB and a combination thereof to modulate gene expression of chemokines in PBMC from nine of the WG patients and all healthy controls (n = 9) after culturing for 6 h. Several significant findings were revealed (Fig. 2). First, while MP significantly down-regulated the expression of all examined chemokines (i.e. IL-8, RANTES, ENA-78, MCP-1 and MIP-1α) in unstimulated PBMC from healthy controls, only IL-8, RANTES and ENA-78 were significantly down-regulated in unstimulated PBMC from WG patients. However, the basal mRNA levels of MIP-1α and MCP-1 were low and any apparently suppressive effects should be interpreted with caution. Secondly, while SEB markedly enhanced the gene expression of IL-8, MCP-1 and MIP-1α, SEB had no significant effect on mRNA levels of RANTES and ENA-78, with similar patterns in WG patients and healthy controls. Finally, MP had no significant effect on the SEB-induced gene expression of IL-8, MCP-1 and MIP-1α in either patients or controls. The lack of effect of SEB on RANTES and ENA-78 mRNA levels and lack of effect of MP on the SEB-induced expression of IL-8, MCP-1 and MIP-1α were also seen after culturing for 24 h (data not shown).
Fig. 2.
Gene expression of the chemokines interleukin (IL)-8 (a), macrophage inflammatory protein (MIP)-1α (b), monocyte chemoattractant protein (MCP)-1 (c), regulation activation normal T cell expressed and secreted (RANTES) (d) and epithelial cell-derived neutrophil-activating factor (ENA)-78 (e) in peripheral blood mononuclear cells (PBMC) from Wegener's granulomatosis (WG) patients (n = 9) and healthy controls (n = 9) in response to stimulation with 1 ng/ml staphylococcal enterotoxin B (SEB) or 10−6 m methylprednisolone (MP) or both after culturing for 6 h. The results are presented as mean ± s.e.m. mRNA levels were quantified by real-time reverse transcription-polymerase chain reaction (RT-PCR) and data are presented relative to the gene expression of the house-keeping gene β-actin. *P < 0·05 and **P < 0·005 versus unstimulated cells; †P < 0·05 versus SEB-stimulated cells.
Effects of MP on unstimulated and SEB-induced release of chemokines in PBMC
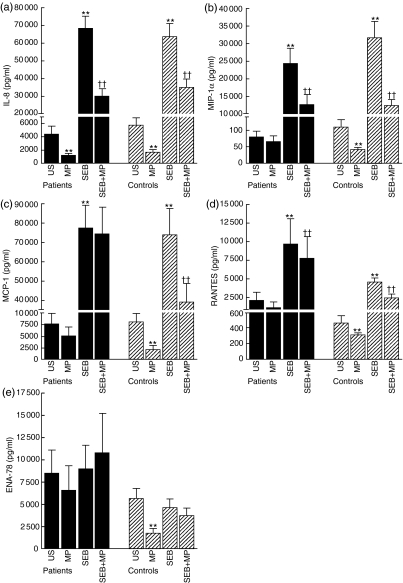
Although we found the similar pattern as outlined in Fig. 2 when using GADPH as a control gene (data not shown), quantitative results from real-time RT-PCR assays should be interpreted with caution. Therefore, we next examined the ability of MP to modulate the spontaneous and SEB stimulated release of these chemokines at the protein levels as assessed by EIA of cell supernatants after culturing for 24 h (Fig. 3). Comparable to the effect on mRNA levels, MP significantly reduced the spontaneous release of all examined chemokines in PBMC from healthy controls. In contrast, only the spontaneous release of IL-8 was significantly down-regulated by MP in cells from WG patients. Furthermore, as for mRNA levels, SEB induced a marked increase in the release of IL-8, MCP-1 and MIP-1α. However, in contrast to the mRNA levels, such an increase was also seen for the SEB-induced release of RANTES, with similar pattern in patients and controls, suggesting post-transcriptional regulation of this SEB-mediated effect. Finally, while MP had no effect on the SEB-induced gene expression of chemokines, MP attenuated the SEB-induced release of MIP-1α, IL-8 and RANTES in both WG patients and controls. In contrast, an MP-mediated suppression of the SEB-induced release of MCP-1 was seen only in cells from healthy controls. We found no relationship between the patients’ actual prednisolone dose and the response to methylpredisolone in vitro. However, relatively few patients were examined and this issue will have to be examined further in forthcoming studies.
Fig. 3.
The release of the chemokines interleukin (IL)-8 (a), macrophage inflammatory protein (MIP)-1α (b), monocyte chemoattractant protein (MCP)-1 (c), regulated upon activation normal T cell expressed and secreted (RANTES) (d) and epithelial cell-derived neutrophil-activating factor (ENA)-78 (e) in peripheral blood mononuclear cells (PBMC) from Wegener's granulomatosis (WG) patients (n = 9) and healthy controls (n = 9) in response to stimulation with 1 ng/ml staphylococcal enterotoxin B (SEB) or 10−6 m methylprednisolone (MP) or both after culturing for 24 h. The results are presented as mean ± s.e.m. Protein levels in peripheral blood mononuclear cells (PBMC) supernatants were measured by enzyme immunoassays (EIAs). **P < 0·005 versus unstimulated cells; ††P < 0·005 versus SEB stimulated cells.
Discussion
In the present study we show that patients with WG have elevated serum levels of some inflammatory chemokines (i.e. RANTES and MCP-1) compared to healthy controls. Even more importantly, we show the ability of MP to down-regulate the release of these chemotactic cytokines from PBMC in vitro in both WG patients and controls. However, the capacity of MP to attenuate chemokine secretion was less pronounced in WG patients than in controls, in particular with regard to the inhibition of spontaneous chemokine release. Our findings further suggest a role for chemokines in the pathogenesis of WG, and underscore the limitations in the ability of MP to down-regulate these inflammatory mediators in this disorder. This in vitro finding may reflect the well-known clinical observation that single treatment with corticosteroids does usually not lead to stable clinical remission in WG.
Enhanced protein expression of RANTES, MIP-1α and MIP-1β has been found previously in WG lung lesions [7,9], and there are also some reports of chemokine expression in renal biopsies from these patients [15,16]. Moreover, proteinase-3, the major antigen of WG-associated ANCAs, has been shown to enhance endothelial-cell production of IL-8 and MCP-1 [10,17]. In the present study we extend these findings by demonstrating raised serum levels of both CC- and CXC-chemokines in WG patients, suggesting systemic chemokine activation in these patients. WG lesions generally consist of a variety of leucocytes, including neutrophils, monocyte-derived tissue macrophages, giant cells and lymphocytes [1,3]. Chemokines, in turn, have emerged as the most important regulators of leucocyte trafficking during infection and inflammation [4,18]. Although we present no functional data and even though we lack data on chemokine expression at the tissue level, it is tempting to hypothesize that chemokines could contribute to the granulomatous inflammation in WG by attracting and activating leucocyte subsets into the inflamed tissue characterizing this disorder.
Corticosteroids have been used for several decades in various immune-mediated disorders and a number of in vivo and in vitro studies have demonstrated the ability of corticosteroids to down-regulate inflammatory mediators [19,20]. In the present study we show that MP has a substantial effect on the secretion of several chemokines, including both the CC- and the CXC families, from PBMC in both WG patients and healthy controls. In view of the postulated pathogenic role of Staphylococcus aureus in WG [21], the ability of MP to inhibit the SEB-induced chemokine secretion is of particular interest. While the MP-mediated effect on the spontaneous release of chemokines showed a similar pattern at the mRNA and protein levels, the MP-mediated suppression of the SEB-induced chemokine release was seen only at the protein level, suggesting post-transcriptional mechanisms for this MP effect. None the less, the ability of corticosteroids to attenuate a wide range of chemokines could clearly contribute to the ability of these drugs to prevent migration of leucocytes into inflamed tissues.
Although MP down-regulated chemokine release in PBMC from both WG patients and healthy controls, the effect was more pronounced in the latter group. In fact, in contrast to the markedly suppressive effects in healthy controls, MP had no effect on either the spontaneous release of MIP-1α, MCP-1, RANTES and ENA-78 or the SEB-induced release of MCP-1 in PBMC from WG patients. The reasons for this diminished MP response in WG patients are at present unclear but could involve factors such as down-regulation of corticosteroid receptors [22], SEB-induced corticosteroid insensitivity [23], and ‘preactivation’ in vivo of PBMC from WG patients [24]. Nevertheless, our in vitro findings suggest that some pathogenic mechanisms (i.e. chemokines) in WG may remain at least partly unmodified by corticosteroids. However, few patients were studied and we have no in vivo data on MP effects on chemokines; the comparison between WG patients and controls should therefore be interpreted with caution.
Despite some limitations (i.e. lack longitudinal data, lack of tissue samples and a relatively low number of patients), our findings in the present suggest a role for chemokines in the pathogenesis of WG. However, while we demonstrate the ability of MP to down-regulate chemokines in PBMC, the diminished MP-mediated suppression of chemokine release in PBMC from WG patients suggests that more specific modulators of chemokine levels may be desirable in this disorder.
References
- 1.Fauci A, Wolff S. Wegner's granulomatosis. Medicine. 1973;73:535–61. doi: 10.1097/00005792-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Andrassy K. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 3.Yi ES, Colby TV. Wegener's granulomatosis. Semin Diagn Pathol. 2001;18:34–46. [PubMed] [Google Scholar]
- 4.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 5.Cockwell P, Chakravorty SJ, Girdlestone J, Savage CO. Fractalkine expression in human renal inflammation. J Pathol. 2002;196:85–90. doi: 10.1002/path.1010. [DOI] [PubMed] [Google Scholar]
- 6.Tam FW, Sanders JS, George A, et al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant. 2004;19:2761–8. doi: 10.1093/ndt/gfh487. [DOI] [PubMed] [Google Scholar]
- 7.Coulomb-L’Hermine A, Capron F, Zou W, et al. Expression of the chemokine RANTES in pulmonary Wegener's granulomatosis. Hum Pathol. 2001;32:320–6. doi: 10.1053/hupa.2001.22757. [DOI] [PubMed] [Google Scholar]
- 8.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest. 1997;100:1416–24. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Huang D, Farver C, Hoffman GS. Relative importance of CCR5 and antineutrophil cytoplasmic antibodies in patients with Wegener's granulomatosis. J Rheumatol. 2003;30:1541–7. [PubMed] [Google Scholar]
- 10.Taekema-Roelvink ME, Kooten C, Kooij SV, Heemskerk E, Daha MR. Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J Am Soc Nephrol. 2001;12:932–40. doi: 10.1681/ASN.V125932. [DOI] [PubMed] [Google Scholar]
- 11.Leavitt RY, Fauci AS, Bloch DA. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 12.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 13.Yndestad A, Damås JK, Eiken HG, et al. ncreased gene expression of tumor necrosis factor superfamily ligands in peripheral blood mononuclear cells during chronic heart failure. Cardiovasc Res. 2002;54:175–82. doi: 10.1016/s0008-6363(02)00238-9. [DOI] [PubMed] [Google Scholar]
- 14.Yndestad A, Holm AM, Müller F, et al. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res. 2003;60:141–6. doi: 10.1016/s0008-6363(03)00362-6. [DOI] [PubMed] [Google Scholar]
- 15.Cockwell P, Brooks CJ, Adu D, Savage CO. Interleukin-8: a pathogenetic role in antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. Kidney Int. 1999;55:852–63. doi: 10.1046/j.1523-1755.1999.055003852.x. [DOI] [PubMed] [Google Scholar]
- 16.Cockwell P, Howie AJ, Adu D, Savage CO. In situ analysis of C-C chemokine mRNA in human glomerulonephritis. Kidney Int. 1998;54:827–36. doi: 10.1046/j.1523-1755.1998.00053.x. [DOI] [PubMed] [Google Scholar]
- 17.Mayet W, Schwarting A, Barreiros AP, Schlaak J, Neurath M. Anti-PR-3 antibodies induce endothelial IL-8 release. Eur J Clin Invest. 1999;29:973–9. doi: 10.1046/j.1365-2362.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 18.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–66. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 19.Chadda K, Annane D. The use of corticosteroids in severe sepsis and acute respiratory distress syndrome. The use of corticosteroids in severe sepsis and acute respiratory distress syndrome. Ann Med. 2002;34:582–9. doi: 10.1080/078538902321117805. [DOI] [PubMed] [Google Scholar]
- 20.Frieri M. Corticosteroid effects on cytokines and chemokines. Allergy Asthma Proc. 1999;20:147–59. doi: 10.2500/108854199778553082. [DOI] [PubMed] [Google Scholar]
- 21.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Staphylococcal superantigens and T cell expansions in Wegener's granulomatosis. Clin Exp Immunol. 2003;132:496–504. doi: 10.1046/j.1365-2249.2003.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 23.Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000;105:782–7. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]
- 24.Aukrust P, Lien E, Kristoffersen AK, et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency − possible immunologic and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]