Abstract
Complement receptor 1 (CR1) on the surface of human erythrocytes facilitates intravascular clearance of complement-opsonized pathogens. The need for complement activation can be circumvented by directly coupling the organism to CR1 using a bispecific monoclonal antibody heteropolymer (HP). Lack of a functional homologue to CR1 on mouse erythrocytes has made it difficult to study HP-dependent clearance of pathogens in small animals. We have developed a transgenic mouse that expresses human CR1 on erythrocytes. CR1 antigen is of appropriate size and in a clustered distribution as confirmed by immunoblotting and fluorescence microscopy, respectively. HP that immobilized bacteriophage ΦX174 prototype pathogen to erythrocyte CR1 of the transgenic mice increased the rate of clearance of the virus compared with HP that bound bacteriophage, but not CR1. This transgenic mouse model will allow evaluation of different HPs for their in vivo efficacy and potential as human therapeutics.
Keywords: blood-borne pathogens, complement receptor, CR1, immune clearance, transgenic mouse
Introduction
Evolution of a closed circulatory system necessitated a means to clear the intravascular space of infectious particles, apoptotic debris and, with the advent of adaptive immunity, immune complexes. It has been known for many years that the intravascular clearance process depends on complement opsonins and platelets in most experimental animals and complement opsonins and erythrocytes (E) in humans (reviewed in [1]). Nelson demonstrated in the human system that the role of specific antibody was to activate complement and thereby generate opsonins at the surface of the particle marked for clearance. He termed the binding of complement-opsonized particles to human E ‘immune adherence’ [2]. Complement receptor 1 (CR1 or CD35) is the immune adherence receptor on E [3].
Human CR1 is a type 1 transmembrane protein of about 200 kDa, which in addition to its presence on E, is found on neutrophils, monocytes, eosinophils, B cells [3], follicular dendritic cells [4] and a subpopulation of T lymphocytes [5], as well as in glomerular podocytes [6] and epidermis [7]. CR1 has 30 short consensus repeats (SCR) in its most common allele. The 28 amino terminal SCR are organized into four long homologous repeats (LHR) with distinct binding sites for the following preferred opsonic complement ligands: LHR-A, C4b; LHR-B, C4b or C3b; LHR-C, C3b; and LHR-D, C1q or mannan binding lectin [8–12]. Reconstitution of immune adherence ex vivo demonstrates that complement-opsonized immune particles adherent to E are ingested more efficiently than opsonized particles that are free in suspension [13]. The transfer process of the immune adherent particles from the surface of the E to the phagocyte is not fully understood, but in the cases of HP and the immune complexes associated with systemic lupus erythematosus transfer may involve proteolysis of CR1 [14,15]. In vivo, clearance of immune-adherent particles or immune complexes occurs when the transporting E travels to the liver or spleen where resident phagocytic cells remove the immune-adherent particle and allow the erythrocyte to return to the circulation [16–19]. Clearance of immune complexes, which efficiently activate complement, occurs rapidly in humans with a half-life (t1/2) of about 5 min [16,17]. However, the directed clearance to the liver and spleen of pathogens that do not activate complement directly is limited by the availability of specific complement activating antibody in the host.
Taylor et al. [20] have devised a means of circumventing the need for the activation of complement. They have chemically linked anti-CR1 MoAb with specific antipathogen MoAb, thereby producing a bispecific ‘heteropolymer’ (HP). Use of the HP allows the direct binding of any antigen or pathogen to CR1. When HP is infused in primates, the numerical excess of circulating E over leucocytes (about 1000 : 1) favours HP binding to CR1 on E, as opposed to leucocyte CR1. In a series of studies, Taylor and colleagues demonstrated in vitro HP-mediated binding of soluble protein antigens to primate E. This binding was found to be saturable and specific for the target antigen [21].
Testing of HP reagents in vivo has been limited because only higher primates share with humans a CR1-E-dependent immune adherence clearance mechanism. Most vertebrates, including mice, use adherent platelets to mark complement-opsonized particles for clearance, and the receptor is thought to be platelet-associated factor H [22]. Attempts in the past to use the mouse as a model for intravascular clearance relied on the infusion of human erythrocytes (hE). This heterologous model provided evidence that HP attached to hE was cleared in the liver of the recipient mouse [23]. Nardin et al. demonstrated that HP and CR1 are removed from the surface of E and a nominal pathogen (bacteriophage ΦX174) is concentrated in the liver in the mouse model [24]. Thus, the fixed macrophages of the mouse liver were capable of removing HP-complexes in a manner similar to that of humans. However, the heterologous method is cumbersome for a number of reasons: (1) infusion of hE adds an additional insult while studying natural infections; (2) HP dose is limited by the volume of hE that can be infused; (3) it is difficult to control for mouse anti-hE antibodies; and (4) it is necessary to use a recipient mouse deficient in the terminal complement pathway to avoid lysing the infused hE. To circumvent these difficulties we report the development of transgenic mice expressing human CR1 under a GATA1 promoter that drives expression in erythroid cells [25]. The mice are healthy and have been used in HP clearance studies. This practical animal model will allow testing of HPs that have been designed for therapy of humans.
Materials and methods
Monoclonal antibodies (MoAb), HPs and phage
The following murine MoAb were used: anti-CR1 MoAb YZ-1 (IgG1), which binds LHR-A, -B and -C [26]; anti-CR1 MoAb 543 (American Type Culture Collection, Manassas, VA, USA), which binds LHR-C and -D (L. B. Klickstein, unpublished observations); and anti-CR1 MoAb 7G9 (IgG2a), which binds LHR-A, -B and -C [26], was purified from ascites [27] made from the hybridoma (provided by Drs Ronald Taylor and William Sutherland, University of Virginia, Charlottesville, VA, USA); anti-ΦX174 MoAb 7B7 (IgG2a) [18] (provided by Drs Taylor and Sutherland) and anti-anthrax protective antigen MoAb 14B7 (IgG2a) [28] (provided by Stephen Little, US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, USA). Bacteriophage ΦX174 (designated ΦXcs70am-3 ATCC no. 49696-B1) was obtained from American Type Culture Collection and phage stocks were produced by PanVera Invitrogen (Carlsbad, CA, USA).
Anti-CR1 MoAb 7G9 was linked to anti-ΦX174 MoAb 7B7 (ΦX-CR1 HP) by a thioether linkage using SMCC [sulphosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate] and [SATA-(N-succinimidyl-S-acetylthioacetate] (Pierce Biotechnology, Rockford, IL, USA) activated antibodies as described previously [29]. We have developed a modification of the above method to make polyethylene glycol ∼ 5000 MW (PEG) HP, where instead of activating one MoAb with SMCC we used a biologically inactive linker/spacer molecule (modified PEG ∼5000 MW) containing a maleimide group via an NHS ester and reacted this modified MoAb with a SATA activated MoAb. A non-CR1-binding HP (ΦX-PA HP) was produced by SMCC/SATA linkage of MoAb 7B7 to MoAb 14B7.
Generation of the transgenic mouse
An expression plasmid for the human CR1 gene was constructed by obtaining a 9·9 kb Not I DNA fragment containing the complete cDNA encoding the common allele of human CR1 (hCR1) [9]. This fragment was inserted into the unique Not I site of plasmid pGATA-1, placing hCR1 under the transcriptional control of the erythroid specific promoter GATA1 [30]. Orientation of the CR1 gene was confirmed by sequencing. The transgene was purified and microinjected into fertilized oocytes harvested from F1 intercrosses of SJL × C57Bl/6 mice. Following microinjection, the oocytes were implanted into pseudopregnant SW female mice. The resulting progeny were screened for integration of the transgene by polymerase chain reaction (PCR) using mouse-tail DNA and maintained by mating with C57BL/6 mice. Positive heterozygotes were detected by PCR analysis of DNA and by expression analysis on erythrocytes by FACS analysis as described below.
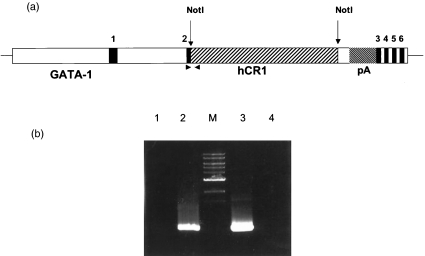
Isolation of high molecular weight DNA from mouse tails was carried out with Easy-DNA kit (Invitrogen) according to the manufacturer's protocol. Approximately 1 µg of DNA was subjected to 35 cycles of amplification on a thermal cycler. The two transgenic specific primers used for PCR analysis of transgenic mice GATA-1, forward primer (5′-ACCCTTTCTGTCC-TCACA-3′) and CR1 gene reverse primer (5′-TTTCTCCCTCCGCTTCCAGGTTG-3′), produced a 653-base pairs (bp) DNA fragment (Fig. 1a). PCR products were resolved by electrophoresis in 1% agarose gels.
Fig. 1.
Schematic map of the GATA1/hCR1 construct and identification of transgenic founders. (a) Schematic map of the GATA1/hCR1 transgenic construct: 7 kb of GATA1 upstream sequence and ∼1·5 kb of sequence downstream of exon 3 flank the hCR1 cDNA. The numbered regions indicate exons of GATA-1. The endogenous GATA-1 initiation of translation codon in exon 2 was replaced with a Not I site. The cDNA for hCR1 was inserted at this Not I site. A polyadenylation signal from simian virus 40 (pA) lies 3′ of the cDNA insert. The polymerase chain reaction (PCR) primers used to detect the transgene consisted of a forward primer specific for GATA-1 exon 2 and a reverse primer specific for hCR1 sequence (arrowheads). (b) PCR analysis on DNA isolated from tail biopsy. Transgenic founders and their transgenic offspring were identified by a diagnostic 653-bp PCR product. Lane 1, distilled water instead of DNA; lane 2, genomic DNA of the transgene mouse; M, 1-kb ladder marker; lane 3, plasmid DNA containing the transgene; lane 4, genomic DNA of a known non-transgenic mouse.
Baboons
Baboons were used under contract with IACUC approval from the colony maintained at Southwest Foundation for Biomedical Research (San Antonio, TX, USA). Prior to the study blood was obtained from 40 animals and analysed for the level of CR1 expression by FACS as described below. The results were used to select animals with 1800–3600 CR1/E for inclusion into the study.
Erythrocytes
Citrate anticoagulated mouse blood was obtained from the tail vein, and following centrifugation at 500 g the buffy coat layer was removed, and the E were washed three times in Hanks's balanced salt solution, without calcium and magnesium (HBSS) (Gibco/Invitrogen, Grand Island, NY, USA). Human E from normal donors were obtained from acid-citrate anticoagulated venous blood, which was diluted with equal volume of HBSS with 0·001 M ethylenediamine tetra-acetic acid (EDTA), 0·01% gelatin. Following centrifugation and removal buffy coat layer, the cells were washed several times in the above buffer. Finally, the cells were resuspended in HBSS containing calcium and magnesium and 0·01% gelatin (HBSS++/gelatin). All E for immune complex binding assays were used on the day of collection, and were standardized on the basis of haemoglobulin concentration, such that a 1/30 dilution of cells in water gave a haemoglobin absorbancy of 0·64 at 414 nm.
Human serum
Two ml of serum (from the donor of human E) were made 5 mM EDTA chilled to 4°C, and mixed with the washed membranes from approximately 106 wild-type mE for 60 min to absorb out human antimouse E immunoglobulins. Subsequently the absorbed human serum was centrifuged at 15 000 g to remove the mouse membranes, and the supernatant was aliquoted for storage at −80°C until use for opsonizing immune complexes.
CR1 expression
The relative CR1 expression of mE + CR1 was compared with that of E from a normal human donor using Alexa 488-conjugated 7G9 MoAb and analysis by flow cytometry. For immunoblotting, E from 100 µl blood were washed twice in HBSS= and then lysed overnight in ghosting buffer (5 mm sodium phosphate (pH 8·0), 1 mm EDTA and 1/100 dilution of ‘protease inhibitor cocktail’ (Sigma, St Louis, MO, USA). The membranes were solubilized in SDS-sample buffer (Boston Bioproducts, Boston, MA, USA), boiled for 5 min and resolved unreduced on a 4–12% NuPage gradient gel (Invitrogen, Carlsbad, CA, USA). Proteins were transferred onto Hybond nitrocellulose membrane (Amersham BioSciences, Arlington Heights, IL, USA). The membranes were immunoblotted with anti-CR1 MoAb YZ-1, followed by incubation with peroxidase-conjugated goat antimouse secondary antibody (Vector Laboratories, Burlingame, CA, USA). Blots were visualized with SuperSignal West Pico Chemiluminescence substrate (Pierce Biotechnology).
FACS analysis of CR1 expression on the surface of E
Anti-CR1 MoAb 7G9 was labelled with Alexa 488™ (Molecular Probes, Eugene, OR, USA) following the manufacturer's directions. Wild-type mE, mE + CR1, or hE were suspended to a concentration of (v/v) 1% in phosphate-buffered saline/1% bovine serum albumin buffer (PBS/BSA). Alexa 488–7G9 (100 µl of a 2 µg/ml stock) was added to 100 µl of the E and incubated for 30 min at room temperature. Unbound Alex-7G9 was removed by washing twice with PBS/BSA. E were selectively gated by flow cytometry based on their forward-scatter versus side-scatter profile and 20 000 events were analysed within that gate using a FACSCalibur™ instrument and Cellquest Pro software, version 3·3 (BD Biosciences, San Jose, CA, USA).
Distribution of CR1 on mouse and human E
Human or mouse blood was diluted in PBS/BSA, and the E were resuspended in the same buffer at a concentration of 10% (V/V). Alexa 488-labelled anti-CR1 MoAb 7G9 was added to E such that the final concentrations of antibody and E were 5·0 µg/ml and 5%, respectively. After incubation for 30 min at 37°C, the unbound MoAb was removed by centrifugation and washing. Light and fluorescence microscopy was performed using an Olympus Provis AX70 microscope (Olympus America Inc., Melville, NY, USA) using a UPlanApo 100 × 1·35 objective.
Immune complex and HP binding to E in vitro
BSA, Fraction V (Sigma) (1 mg/ml) was labelled with fluoroscein isothiocyanate (FITC) (Aldrich Chemical, Milwaukee, WI, USA) and dialysed to remove unbound FITC. The assumed concentration of BSA after labelling was 0·8 mg/ml. Mixing 30 µg FITC–BSA and 100 µl of rabbit-anti-BSA IgG (Molecular Probes) in a final volume of 250 µl for 60 min at 37°C allowed the formation of immune complexes. Thirty µl of the immune complexes were aliquoted into microfuge tubes, followed by the sequential addition of 40 µl of absorbed human serum, 4 µl stock cations (30 mm CaCl2. 100 mm MgCl2) and 150 µl E suspension. After incubation for 15 min at 37°C to allow both the opsonization of the immune complexes and the binding of the opsonized immune complex to E, the mixtures were transferred to 12 × 75 mm tubes containing 3 ml of cold HBSS-0·01% gelatin, centrifuged at 400 g, and resuspended in 3 ml of the same buffer. The cells were analysed on a FACSscan™ and 10 000 events were recorded using Cellquest Pro software version 4·01.
Binding of SMCC-HP to E was determined in the following manner. Parental mE, mE + CR1, or hE was suspended at a concentration of 10% in PBS/BSA buffer. HP (20 ng) was added to 100 µl of the E for 15 min at 37°C with shaking. Unbound HP was removed by washing twice with PBS/BSA buffer. Goat antimouse IgG-Alexa 488 (Molecular Probes) at 10 µg/ml was added to the tubes and incubated for 30 min at room temperature followed by two washes with PBS/BSA buffer. Flow cytometry was then performed (FACSCalibur) on the samples and the E were selectively gated based on their forward-scatter versus side-scatter profile; 20 000 events were analysed within that gate.
In vivo phage clearance
Baboons (8–10 kg) were injected with 1·5 × 1011 plaque-forming units (PFU) intravenously (i.v.) and injected i.v. 20 min later with 0·3 mg of ΦX-CR1 HP (PEG and SMCC), ΦX-PA HP or an equivalent volume of saline. Blood samples were obtained by femoral venipuncture into tubes containing EDTA (0·01 M final concentration) as anticoagulant, centrifuged, and the plasma frozen at −70°C. Plasma phage were determined by plaque assay as previously described [18].
Mice were injected i.v. in a 0·1 ml volume at a dose of 3 × 107 PFU/animal. Saline control, ΦX-CR1 HP or ΦX-PA HP (1, 6 or 12 µg) were injected i.v. in a 0·1 ml volume 45 min later. Blood samples (50 µl) were collected from the tails of mice into 0·5 ml Alsever's solution containing 0·04 M EDTA at various time-points, pre- or post-HP administration. Plasma was separated from cells and stored at 4°C. Plasma phage were determined by plaque assay as described previously [18].
Results
Plasmid construction and generation of transgenic mouse lines
To develop a transgenic mouse expressing human CR1 on mE we produced a transgene containing the gene for CR1 under control of the haematopoietic-specific promoter GATA1. This promoter drives transcription in some haematopoietic cells, especially erythrocytes [25]. The GATA1-CR1 transgene (Fig. 1a) was microinjected into fertilized eggs and these were implanted into surrogate mothers. Resulting progeny were screened by PCR analysis of DNA isolated from tail biopsy to identify mice that carried the transgene. A specific PCR product was generated when DNA from transgenic mice or the transgene-containing plasmid was used as template, but was absent when DNA from a wild-type mouse was used (Fig. 1b).
Evidence of expression of human complement receptor CR1 on transgenic mouse E
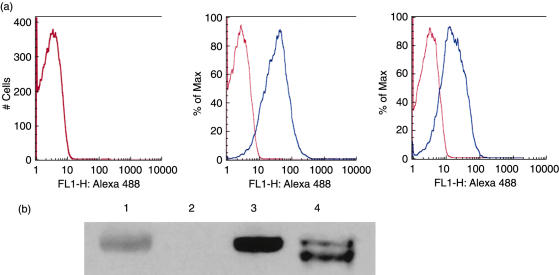
To confirm that CR1 antigen was expressed on the surface of the E from transgenic mice, flow cytometry was performed after staining cells with an Alexa-488 conjugated anti-CR1 MoAb (Fig. 2a). Analysis confirmed that CR1 antigen was expressed on the surface of hE (right panel) and on mE from the transgenic mouse (mE + CR1, centre panel), but not on mE from wild-type mice (left panel).
Fig. 2.
Confirmation that erythrocytes (E) from the transgenic mouse expressed human complement receptor 1 (CR1) antigen of appropriate molecular size. (a) human E (hE), transgenic mouse E (mE) and mE + CR1 were stained with Alexa 488 labelled anti-CR1 MoAb 7G9 and analysed by flow cytometry. CR1 antigen was expressed on the surface of the E from the transgenic mouse (centre panel), but not on E from a wild-type mouse (left panel). hE (right panel), which were used as a positive control, expressed about 50% as much CR1 on the mE + CR1. Blue histogram, anti-CR1 mAb; red histogram, isotype control mAb. (b) Immunoblotting for human CR1. Confirmation that transgenic mouse E (mE + CR1) express CR1 protein of appropriate size. Non-reduced lysates of wild-type mE, mE + CR1 and hE were resolved on a 4–12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and the proteins transferred to nitrocellulose for development with anti-CR1 MoAb YZ-1 as described in Methods. Lane 1, prestained myosin 207 kDa MW marker; lane 2, wild-type mE; lane 3, mE + CR1; lane 4, hE from a donor with CR1*1 and CR1*3 alleles of CR1.
To confirm that the CR1 of mE + CR1 was of an appropriate size, immunoblotting was performed (Fig. 2b). E from wild-type mice, CR1 transgenic mice and a normal human were lysed and the solubilized, non-reduced membrane proteins resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Following transfer the blot was reacted with anti-CR1 MoAb YZ-1, as described in Methods. The human donor was presumed to be heterozygous for the common allele CR1*1, producing a 200 kDa band and the CR1*3 allele, producing a 190 kDa band, under non-reducing conditions (Fig. 2b, lane 4) (reviewed in [31]). CR1 on E from the transgenic mouse (Fig. 2b, lane 3) was similar in molecular weight to the hE CR1 CR1*1 allele, which was consistent with the transgene. No CR1 antigen was detected by YZ-1 MoAb in the lysate of wild-type mE (Fig. 2b, lane 2).
To define the distribution of the GATA-1 promoter-driver CR-1 in the transgenic mice, tissues from the transgenic mouse were fixed by Paragon Biotech (Baltimore, MD, USA) by standard procedures, stained with a humanized anti-CR1 MoAb with the same binding specificity as MoAb 543 (see Methods), and examined for the expression of CR1. No tissues tested were found to be CR1 immunoreactive. Staining of circulating blood cells revealed no expression on lymphocytes, polymorphonuclear leucocytes or macrophages. As expected, flow cytometric analysis of blood cells and splenocytes revealed that CR1 was expressed on erythrocytes and platelets, but not splenocytes. In samples taken from GATA1-CR1 mice, the fluorescence modes, indicating the intensity of fluorescence, were 25·2 for E, 12·0 for platelets and 0·4 for splenocytes. The comparative values for wild-type were −0·2, 0·4 and 1·8, respectively. These values were calculated by subtraction of the isotype-control MoAb FL-1 fluorescence modes from those of anti-CR1 MoAb.
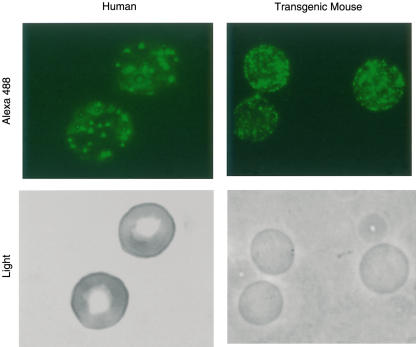
Confirmation of a cluster distribution of CR1 on hE and mE + CR1 by fluorescence microscopic analyses
As the average level of CR1 per hE is quite low (100–1000/E), and the affinity of monovalent complement ligands for CR1 is low, it is hypothesized that clusters of CR1 are required to ligate effectively a complement-opsonized particle to a hE [11,32–34]. Thus, the utility of the transgenic mouse as a model of complement-mediated immune adherence clearance might depend on the CR1 being in clusters on the mE + CR1. hE were stained as a positive control and visualized by both light and fluorescence microscopy. CR1 appeared in clusters on hE, as expected (Fig. 3, left panel). The same procedure was repeated for mE + CR1 and the CR1 also appeared in clusters (Fig. 3, right panel) that were consistently smaller but more abundant than the CR1 clusters on hE. The relative amount of CR1 per mE + CR1 was compared with the CR1 expression on E from a normal human donor with high CR1 expression, a phenotype which is linked genetically to the common or ‘F’ allele that was used in the transgene [1]. The two types of E were reacted with directly labelled anti-CR1 MoAb 7G9, and analysed by flow cytometry. The resultant geometric means of fluorescence obtained by flow cytometry indicated that the mE + CR1 had approximately twice the CR1 expression as the human E (MFC 35·5 ± 5·1 and HFC 18·3 ± 2·2, respectively).
Fig. 3.
Distribution of complement receptor 1 (CR1) on mouse and human E (hE). hE (left panels) and transgenic mouse E (mE) + CR1 (right panels) were reacted with Alexa 488 labelled anti-CR1 MoAb 7G9 and viewed by light (lower panels) and fluorescent microscopy (top panels). Clustering of CR1 is seen on both cell types, although the clusters were consistently smaller and more numerous on the mE + CR1.
Ability of mE + CR1 to bind opsonized FITC-labelled immune complexes and HP
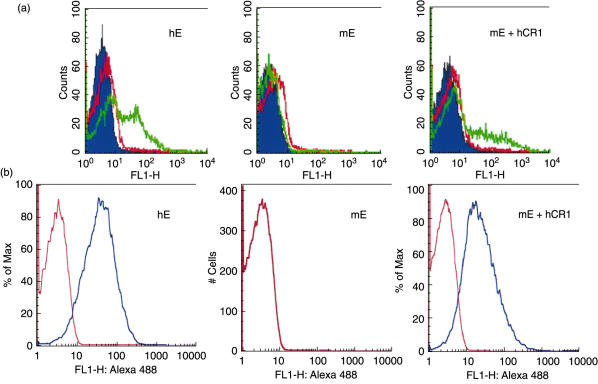
To test the functionality of the CR1 protein on mE + CR1, we examined the ability of these E to bind opsonized immune complexes. FITC-labelled immune complexes consisting of FITC–BSA and rabbit anti-BSA IgG were opsonized with complement by incubation with human serum and allowed to react with E. The samples were then analysed by flow cytometry. Binding of immune complexes to cells was demonstrated by a positive shift in fluorescence and this was seen only when the immune complexes were opsonized and the E expressed CR1, i.e. either hE or transgenic mE + CR1 (Fig. 4a).
Fig. 4.
Ability of transgenic mouse erythrocytes (mE) + complement receptor 1 (CR1) to bind human complement opsonized immune complexes or heteropolymers (HP). (a) fluoroscein isothiocyanate–bovine serum albumin (FITC–BSA)-rabbit anti-BSA immune complexes were opsonized with fresh human serum, reacted with E, and analysed by flow cytometry, as described in Methods. Left panel, normal human E (hE); middle panel, wild-type mouse E (mE); right panel, from transgenic mouse which express human CR1 (mE + CR1). The blue histogram represents E washed in buffer. FITC-labelled immune complexes were made and reacted subsequently with buffer (red histogram) or normal human serum (green histogram) in the presence of E. A shift in fluorescence indicates the binding of immune complexes to E, and this is seen only when the immune complexes were opsonized with serum (green histogram) and expressed CR1 (hE or mE + CR1, but not wild-type mE). (b) Binding of ΦX-CR1 SMCC-HP to hE and mE + CR1, but not to parental mE. The CR1 binding HP can be detected on hE and mE + CR1, but not wild-type mE. Similar results were obtained with ΦX-CR1 PEG HP. Differences in levels of binding may relate to the smaller size of mE. Blue histogram, anti-HP mAb; red histogram, isotype control mAb.
We also examined the ability of E to bind ΦΧ-CR1 HP, consisting of anti-ΦΧ174 MoAb 7B7 chemically linked by SMCC to anti-CR1 MoAb 7G9. Binding of HP to E was assessed by reactivity with Alexa 488-labelled goat-antimouse antibody and analysis by flow cytometry (Fig. 4b). It is of note that while the hE and the mE + CR1 did not bind the native immune complexes uniformly (Fig. 4a), the HP bound to the total populations of hE and mE + CR1 (Fig. 4b).
Effect of dose and type of HP on phage clearance in vivo
HP can potentially be used for the clearance of pathogens and toxins from the bloodstream. The CR1 transgenic mouse was designed as a model for testing the efficacy of HP that couples a potential pathogen to erythrocyte CR1 for clearance. Bacteriophage ΦX174 was used as a model organism for these experiments because, due in part to a lack of its receptor on mammalian cells, it is unable to replicate in mice, it is not pathogenic for mammals [35] and it is easily quantified by plaque assay. HP ΦX-CR1 was made using two different chemistries, SMCC or PEG (see Methods). Fluorescence analysis confirmed that both types of ΦX-CR1 HP bound to hE and mE + hCR1, but not to wild-type mE (data not shown). Control HP, designated ΦX-PA, was made by coupling the anti-ΦX174 MoAb to a MoAb against anthrax protective antigen (PA, 14B7 MoAb). ΦX-PA HP, which did not bind to hE or mE + CR1, was used as a control to determine the rate of soluble HP-mediated clearance. This rate would represent the sum of fluid phase viral neutralization, comprising the ability of anti-ΦX174 MoAb to neutralize the phage (data not shown) plus the clearance of the large HP complex induced by the intrinsic platelet-dependent immune adherence pathway of the mouse.
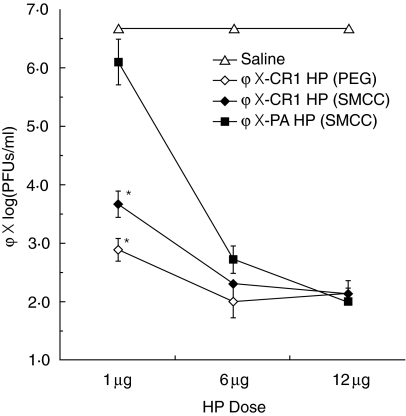
Transgenic mice were infused i.v. with bacteriophage ΦX174, followed 45 min later by either ΦX-CR1 HP (PEG), ΦX-CR1 (SMCC) or ΦX-PA HP (SMCC) at three different doses, or saline as a negative control. The transgenic mice were bled 24 h later to determine PFU of phage per ml of plasma. At 1 µg HP/mouse there was a clear difference in phage reduction in sera with both PEG and SMCC linked forms of ΦX-CR1 HP (3·8 and 3·0 log, respectively), compared with the ΦX-PA HP (0·6 log). At the higher doses of HP (6 and 12 µg/mouse), the difference between the HP that linked antigen to erythrocyte CR1 (ΦX-CR1 HP) and one that did not (ΦX-PA HP) disappeared, probably because ΦX-PA HP became more effective at phage neutralization at those doses (Fig. 5).
Fig. 5.
Effect of heteropolymer (HP) dose on phage clearance in transgenic mice. ΦX-complement receptor 1 (CR1) HPs constructed through two different conjugation methods [polyethylene glycol (PEG) or sulphosuccynimidyl 4-(N-maleimidomethyl (SMCC), see Methods] were compared with a non-E binding ΦX-PA HP (SMCC) for their ability to clear injected bacteriophage ΦX174 from the bloodstream in CR1 transgenic mice. Phage ΦX174 was injected intravenously (i.v.) at a dose of 3 × 107 plaque-forming units (PFU)/mouse. The HPs or saline control were injected intravenously (i.v.) 45 min later at doses of either 1·0 µg, 6 µg or 12 µg/mouse. The graph represents the log10 of the phage PFU/ml present in blood sampled from the tails of the mice 24 h after HP injection. Error bars represent standard errors of the means, n = 3. Asterisks indicate significant difference from the ΦX-PA HP at a P < 0·01 by Student's t-test.
Kinetics of HP-mediated clearance of bacteriophage ΦX174 from the plasma of baboons and CR1 transgenic mice
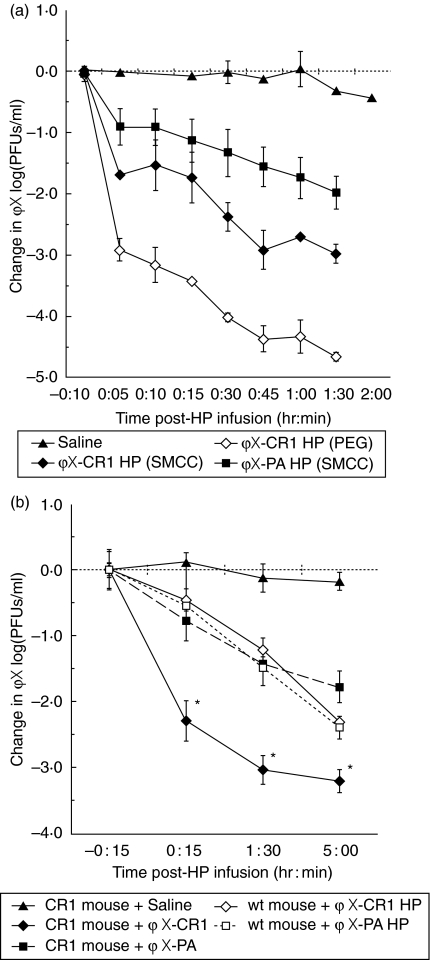
Because higher primates have CR1-expressing erythrocytes (reviewed in [36]), these animals have been used in small studies of the efficacy of HP-mediated clearance of various types of particles [21,24,29,37], including ΦX174 phage [18]. Before measuring the kinetics of clearance in the transgenic mice, we wanted to assess how efficiently our HP functioned in baboons to allow us to gauge the predictability of the transgenic mouse model. ΦX174 (1·5 × 1011 PFU) was infused at t = − 20 min. At t = 0 min, we infused SMCC- or PEG-linked ΦX-CR1 HP, non-E binding SMCC-linked ΦX-PA HP, or saline as a negative control. HP were used at 0·3 mg/animal (8–10 kg each), a dose which is roughly equivalent to the 1 µg/mouse dose on a per weight basis. Sampling of venous blood over the following 100 min revealed two phases of clearance (Fig. 6a). The first was a rapid initial phase resulting in clearance of 3·0 logs by the ΦX-CR1 PEG HP, 1·6 log by ΦX-CR1 SMCC HP and 0·8 log by the control ΦX-PA HP by 5 min post-HP infusion. The slower second phase from 5 to 90 min post-HP infusion resulted in clearance of ∼1·5 log/85 min for ΦX-CR1 HP (PEG), ∼1·0 log/85 min for ΦX-CR1 HP (SMCC), and a ∼1·0 log/85 min for ΦX-PA HP.
Fig. 6.
Clearance of phage by heteropolymers (HPs) in baboons, complement receptor 1 (CR1)-transgenic and wild-type mice. (a) HP-mediated clearance of phage in baboons. Healthy baboons were injected intravenously (i.v.) with 1·5 × 1011 plaque-forming units (PFU) of phage at time − 20 min. After 20 min, at time 0, the animals were injected intravenously (i.v.) with saline, 0·3 mg ΦX-CR1 HP [sulphosuccynimidyl 4-(N-maleimidomethyl (SMCC) or polyethylene glycol (PEG)] or ΦX-PA HP (SMCC). Blood was sampled 15 and 10 min pre-HP infusion and at multiple time-points (5–90 min) post-HP infusion. The graph represents the reduction in phage titre seen in blood at various times post-treatment. Due to limitations in the number of bleeds allowed, animals were not bled at time 0 immediately before HP administration. The values were normalized by subtracting the mean log10 (PFU/ml) found to be present in each group at time −10 min. Because the saline group shows no change in titre over the 120 min study, we would not expect a change in titre during the 15-min interval between this point and the time of HP administration. These values were 8·27 ± 0·78, 8·75 ± 0·1, 8·63 ± 0·13 and 8·54 ± 0·23 for saline, ΦX-CR1 HP (PEG), ΦX-CR1 HP (SMCC) and ΦX-PA HP (SMCC), respectively. The plots represent the means, and the bars the range, n = 2. (b) HP-mediated clearance of phage in wild-type versus CR-1 transgenic mice. ΦX-CR1 HP (SMCC) was compared against ΦX-PA (SMCC) for its ability to clear injected bacteriophage ΦX174 from the bloodstream in CR1 transgenic and C57BL/6 wild-type mice. Phage was injected i.v. at a dose of 3 × 107 PFU/mouse at time −45 min. The HPs or saline control were injected i.v. at time 0 at a dose of 1 µg/mouse. Blood was sampled at −15 min, 15 min, 90 min and 5 h post-HP injection by tail bleed. The graph represents the reduction in phage titre seen in blood at time post-treatment. The values were normalized by subtracting the mean log10 (PFU/ml) found to be present in each group at time −15 min. Because the saline group shows no change in titre over the 5-h study, we would not expect a change in titre from −15 min to time 0. These −15-min values were 6·93 ± 0·30, 7·45 ± 0·08, 6·82 ± 0·32, 7·35 ± 0·11 and 6·96 ± 0·40 for saline, CR1 mouse +ΦX-CR1 HP, CR1 mouse +ΦX-PA HP, wild-type mouse +ΦX-CR1 HP and wild-type mouse +ΦX-PA HP, respectively. Error bars represent standard errors of the means, n = 3. Asterisks indicate significant difference from the ΦX-PA HP at a P < 0·05 by Student's t-test.
Having defined a useful dose of ΦX-CR1 HP (1 µg) to use in the transgenic mouse (Fig. 4), we proceeded to define the kinetics of phage clearance in this animal. Both CR1 transgenic and wild-type parental C57BL/6 mice were injected with 3 × 107 PFU of phage per mouse. ΦX-CR1 HP (SMCC) or control ΦX-PA HP (SMCC) were injected 45 min later. Blood was drawn from the mice 15 min prior to HP injection, and 15 min, 90 min and 5 h post-HP injection. In control transgenic or wild-type mice that received a saline injection instead of HP, the phage titre in the circulation remained constant over 5 h post-injection. In CR1 transgenic mice the superiority of ΦX-CR1 HP over control ΦX-PA HP in reducing circulating phage was apparent within 15 min post-infusion and continued to 5 h post-infusion (Fig. 6b). At all time-points ΦX-CR1 HP yielded approximately a 1·5 log greater reduction in circulating phage than control ΦX-PA HP. In wild-type mice lacking CR1 on E, ΦX-CR1 HP and control ΦX-PA HP reduced the titre of circulating phage by similar levels at all time-points and this reduction was similar to the level of reduction achieved by control ΦX-PA HP in the transgenic mouse.
These results demonstrate that an antibody HP that couples phage to erythrocyte CR1 is more efficient than soluble phage-binding HP for removing phage from the circulation in the transgenic mouse. The absence of this advantage in viral clearance in wild-type mice confirms the validity of the transgenic model.
Discussion
The potential of HP technology to clear pathogens including bacterial and viral targets, toxins and autoantibodies has been demonstrated in a number of studies in non-human primates (reviewed in [38]). Recently an HP developed to remove autoantibodies in patients with systemic lupus erythematosus was found to be safe and efficacious [39]. The need to advance this technology is hampered by humane concerns as well as the high cost for purchase and housing of monkeys. Mice, which are a convenient experimental animal, have a platelet-based clearance system. Although mice have E membrane proteins that share structural homology with CR1, in that they contain SCR, they do not mediate immune adherence. In the past, use of the mouse to test HP has involved passively infusing hE, a method with limitations [23].
The goal of the present work was to develop a transgenic mouse model expressing CR1 on E for development of an animal model to examine the ability of HP to protect against blood-borne lethal pathogens or toxins. A transgenic mouse expressing human CR1 under control of the murine haematopoetic-specific promoter GATA1 was developed. E from the transgenic mouse expressed CR1 antigen (Fig. 2a) of an appropriate size for the common human allele, CR1*1 (Fig. 2b). The distribution of CR1 on hE and mE + CR1 was compared and the pattern of fluorescence is similar, although the expression of CR1 on the mE + CR1 is at least double that of hE and in smaller clusters than the CR1 on hE (Fig. 3). Clusters of CR1 are thought to be important for binding of complement-opsonized particles because of the necessity of multivalent CR1 binding. This is not an issue with anti-CR1 HP binding, but it does suggest that CR1 on both hE and mE + CR1 is attached directly or indirectly to the erythrocyte cytoskeleton.
Functionally, mE + CR1 bound antigen–antibody immune complexes that had been opsonized with human serum (Fig. 4a), but not non-opsonized or heat-inactivated serum opsonized immune complexes (data not shown). Only a fraction of the hE and mE + CR1 bound the opsonized immune complexes, although the heteropolymer bound all hE and mE + CR1 uniformly (Fig. 4b). It was reported previously that serum-opsonized Pseudomonas aeruginosa bound to only 60% of monkey E, whereas the same bacteria, when HP-ligated, bound to 100% of available E. One explanation for the binding of complement-opsonized immune complexes to a fraction of hE or mE + CR1, is that factor I produced by human leucocytes and/or contributed by contaminating serum led to processing of C3b and release of the IC from the hE and mE + CR1. We tried to minimize the presence of factor I by removing the buffy coat leucocytes from hE, terminating the E-IC-serum incubation after 15 min, and pelleting the hE-IC in cold buffer such that there was a 70-fold dilution of serum. Thus, the advantages of binding pathogens to E through HP are not only in the time saved by avoiding the three-step process of antibody binding, complement activation and finally binding CR1, but also the fact that the total population of E can be potentially involved in clearance.
The competency of either PEG or SMCC coupled ΦX-CR1 HP to clear a surrogate pathogen (ΦX174) from the circulation was tested in vivo in baboons, which have erythrocyte-based immune adherence and use a CR1-like molecule that is GPI-anchored [36]. Baboons have been used previously for studies of immune complex clearance and have been proven to be a competent experimental animal [40–42]. Phage titres in the plasma of the baboon receiving saline instead of HP were essentially unchanged over the study period, confirming that there was no specific receptor for the phage in the baboon and also suggesting that the phage was not recognized by the baboon innate immune system. PEG HP was more efficient in phage clearance than SMCC HP, resulting in an initial and final difference in clearance of 1·5 log (Fig. 6a). We postulate that the spacer afforded by PEG separates the CR1 and phage binding sites, allowing better binding to both active domains of the HP than that which occurs when the domains lack such a separation as through joining by SMCC. PEG HP at 1 µg/mouse was also more efficient at clearing phage than SMCC HP (Fig. 5), although the difference in magnitude (0·75 log) was not as large.
The SMCC coupled ΦX-CR1 HP was chosen for additional testing in the transgenic mice because this chemistry has been used in previous studies by the Taylor laboratory, and it was compared with the non-E binding ΦX-PA HP. A clear advantage was shown for HP binding of phage to mE + CR1 (Fig. 6b). Infusion of the ΦX-PA HP induced a lesser clearance equivalent to the use of ΦX-CR1 HP in the wild-type mouse, which lacked CR1 on its E. In addition to direct viral neutralization, it is possible that these HPs would act like immune aggregates and thereby activate mouse complement and be subjected to the murine platelet-dependent immune adherence clearance mechanism.
The difference in activity of PEG and SMCC HPs in both baboons and transgenic mice make a compelling case for evaluating multiple HPs in experimental animals, and monkeys, although ideal, are in short supply [43]. Our CR1 transgenic mouse model will allow the evaluation of HPs both for their ability to induce rapid clearance and eliminate the lethality of pathogenic agents, including toxins, bacteria and viruses. This mouse will also facilitate testing of HPs for their immediate and long-term toxicity. These studies will be crucial for the continued development of HP technology and are expected to lead to new novel therapeutic agents.
Acknowledgments
We thank Drs Ron Taylor and William Sutherland for their kind gift of antibodies and for many helpful discussions. We thank Dr Jean Patterson (Virology and Immunology), Southwest Foundation for Biomedical Research for help with the planning and execution of the baboon studies, and Dr Stuart Orkin (Hematology, Children's Hospital, Boston) for advice and the gift of the GATA1 plasmid. We thank Kathryn E. Bowenkamp DVM, PhD for the immunohistochemistry studies, Juan Li, Tom Bradley and Xun Chen for their assistance and Junko Kato for all her contributions in putting this manuscript together. These studies were supported by NIH grants RO116274 (to R.W.F.), R21 AI57983 (to I.G.), R01 42987 (to A.N.W.) and by a grant from Elusys.
References
- 1.Nelson DS. Immune adherence. Adv Immunol. 1963;3:131–80. [Google Scholar]
- 2.Nelson RAJ. The immune adherence phenomenon: an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953;118:733–7. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 3.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynes M, Aubert JP, Cohen JH, et al. Human follicular dendritic cells express CR1, CR2, and CR3 complement receptor antigens. J Immunol. 1985;135:2687–94. [PubMed] [Google Scholar]
- 5.Wilson JG, Tedder TF, Fearon DT. Characterization of human T lymphocytes that express the C3b receptor. J Immunol. 1983;131:684–9. [PubMed] [Google Scholar]
- 6.Gelfand MC, Shin ML, Nagle RB, Green I, Frank MM. The glomerular complement receptor in immunologically mediated renal glomerular injury. N Engl J Med. 1976;295:10–14. doi: 10.1056/NEJM197607012950103. [DOI] [PubMed] [Google Scholar]
- 7.Dovezenski N, Billetta R, Gigli I. Expression and localization of proteins of the complement system in human skin. J Clin Invest. 1992;90:2000–12. doi: 10.1172/JCI116080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klickstein LB, Wong WW, Smith JA, Weis JH, Wilson JG, Fearon DT. Human C3b/C4b receptor (CR1). Demonstration of long homologous repeating domains that are composed of the short consensus repeats characteristic of C3/C4 binding proteins. J Exp Med. 1987;165:1095–112. doi: 10.1084/jem.165.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klickstein LB, Bartow TJ, Miletic B, Rabson LD, Smith JA, Fearon DT. Identification of distinct C3b and C4b recognition sites in the human C3b/C4b receptor (CR1, CD35) by deletion mutagenesis. J Exp Med. 1988;168:1699–717. doi: 10.1084/jem.168.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krych M, Hourcade D, Atkinson JP. Sites within the complement C3b/C4b receptor important for the specificity of ligand binding. Proc Natl Acad Sci USA. 1991;88:4353–7. doi: 10.1073/pnas.88.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klickstein LB, Barbashov S, Liu T, Jack RM, Nicholson-Weller A. Complement receptor type 1 (CR1, CD35) is a receptor for C1q. Immunity. 1997;7:345–55. doi: 10.1016/s1074-7613(00)80356-8. [DOI] [PubMed] [Google Scholar]
- 12.Ghiran I, Barbashov SF, Klickstein LB, Tas SW, Jensenius JC, Nicholson-Weller A. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J Exp Med. 2000;192:1797–808. doi: 10.1084/jem.192.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson RAJ. The Immune-adherence phenomenon. A hypothetical role of erythrocytes in defense against bacteria and viruses. Proc Roy Soc Med. 1956;49:55–8. doi: 10.1177/003591575604900122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinagel ML, Taylor RP. Transfer of immune complexes from erythrocyte CR1 to mouse macrophages. J Immunol. 2000;164:1977–85. doi: 10.4049/jimmunol.164.4.1977. [DOI] [PubMed] [Google Scholar]
- 15.Ross GD, Yount WJ, Walport MJ, et al. Disease-associated loss of erythrocyte complement receptors (CR1, C3b receptors) in patients with systemic lupus erythematosus and other diseases involving autoantibodies and/or complement activation. J Immunol. 1985;135:2005–14. [PubMed] [Google Scholar]
- 16.Schifferli JA, Ng YC, Estreicher J, Walport MJ. The clearance of tetanus toxoid-anti-tetanus toxoid immune complexes from the circulation of humans. Complement- and erythrocyte complement receptor 1-dependent mechanisms. J Immunol. 1988;140:899–904. [PubMed] [Google Scholar]
- 17.Davies KA, Peters AM, Beynon HLC, Walport MJ. Immune complex processing in patients with systemic lupus erythematosus. J Clin Invest. 1992;90:2075–83. doi: 10.1172/JCI116090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor RP, Sutherland WM, Martin EN, et al. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J Immunol. 1997;158:842–50. [PubMed] [Google Scholar]
- 19.Taylor RP, Ferguson PJ, Martin EN, et al. Immune complexes bound to the primate erythrocyte complement receptor (CR1) via anti-CR1 MoAbs are cleared simultaneously with loss of CR1 in a concerted reaction in a rhesus monkey model. Clin Immunol Immunopathol. 1997;82:49–59. doi: 10.1006/clin.1996.4286. [DOI] [PubMed] [Google Scholar]
- 20.Reist DJ, Liang HY, Denny D, Martin EN, Scheld WM, Taylor RP. Cross-linked bispecific monoclonal antibody heteropolymers facilitate the clearance of human IgM from the circulation of squirrel monkeys. Eur J Immunol. 1994;24:2018–25. doi: 10.1002/eji.1830240913. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RP, Reist CJ, Sutherland WM, Otto A, Labuguen RH, Wright EL. In vivo binding and clearance of circulating antigen by bispecific heteropolymer-mediated binding to primate erythrocyte complement receptor. J Immunol. 1992;148:2462–8. [PubMed] [Google Scholar]
- 22.Alexander JJ, Hack BK, Cunningham PN, Quigg RJ. A protein with characteristics of factor H is present on rodent platelets and functions as the immune adherence receptor. J Biol Chem. 2001;276:32129–35. doi: 10.1074/jbc.M101299200. [DOI] [PubMed] [Google Scholar]
- 23.Henderson AL, Lindorfer MA, Kennedy AD, Foley PL, Taylor RP. Concerted clearance of immune complexes bound to the human erythrocyte complement receptor: development of a heterologous mouse model. J Immunol Meth. 2002;270:183–97. doi: 10.1016/s0022-1759(02)00296-x. [DOI] [PubMed] [Google Scholar]
- 24.Nardin A, Lindorfer MA, Taylor RP. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol Immunol. 1999;36:827–35. doi: 10.1016/s0161-5890(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 25.McDevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc Natl Acad Sci USA. 1997;94:7976–81. doi: 10.1073/pnas.94.15.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickells M, Hauhart R, Krych M, et al. Mapping epitopes for 20 monoclonal antibodies to CR1. Clin Exp Immunol. 1998;112:27–33. doi: 10.1046/j.1365-2249.1998.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus SE, Lukacher N, Mohamed N, et al. Evaluation of antigen-based heteropolymer for treatment of systemic lupus erythematosus in a nonhuman primate model. Clin Immunol. 2002;105:141–54. doi: 10.1006/clim.2002.5274. [DOI] [PubMed] [Google Scholar]
- 28.Little SF, Leppla SH, Cora E. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect Immun. 1988;56:1807–13. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindorfer MA, Nardin A, Foley PL, et al. Targeting of Pseudomonas aeruginosa in the bloodstream with bispecific monoclonal antibodies. J Immunol. 2001;167:2240–9. doi: 10.4049/jimmunol.167.4.2240. [DOI] [PubMed] [Google Scholar]
- 30.Visvader JE, Fujiwara Y, Orkin SH. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 1998;12:473–9. doi: 10.1101/gad.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klickstein LB, Moulds JM. In: The complement facts book. Morley BJ, Walport MJ, editors. New York: Academic Press; 2000. pp. 136–45. CR1. [Google Scholar]
- 32.Edberg JC, Wright E, Taylor RP. Quantitative analyses of the binding of soluble complement-fixing antibody/dsDNA immune complexes to CR1 on human red blood cells. J Immunol. 1987;139:3739. [PubMed] [Google Scholar]
- 33.Paccaud JP, Carpentier JL, Schifferli JA. Direct evidence of the clustered nature of complement receptors type 1 on the erythrocyte membrane. J Immunol. 1988;141:3889–94. [PubMed] [Google Scholar]
- 34.Chevalier J, Kazatchkine MD. Distribution in clusters of complement receptor type one (CR1) on human erythrocytes. J Immunol. 1989;142:2031–6. [PubMed] [Google Scholar]
- 35.Och HD, Davis SD, Wedgwood RJ. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J Clin Invest. 1971;50:2559–68. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birmingham DJ, Hebert LA. CR1 and CR1-like: the primate immune adherence receptors. Immunol Rev. 2001;180:100–11. doi: 10.1034/j.1600-065x.2001.1800109.x. [DOI] [PubMed] [Google Scholar]
- 37.Craig ML, Reinagel ML, Martin EN, Schlimgen R, Nardin A, Taylor RP. Infusion of bispecific monoclonal antibody complexes into monkeys provides immunologic protection against later challenge with a model pathogen. Clin Immunol. 1999;92:170–80. doi: 10.1006/clim.1999.4739. [DOI] [PubMed] [Google Scholar]
- 37.Lindorfer MA, Hahn CS, Foley PL, Taylor RP. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol Rev. 2001;183:10–24. doi: 10.1034/j.1600-065x.2001.1830102.x. [DOI] [PubMed] [Google Scholar]
- 38.Iking-Konert C, Stocks S, Weinsberg F, et al. First clinical trials of a new heteropolymer technology agent in normal healthy volunteers and patients with systemic lupus erythrematosus: safety and proof-of-principle of the antigen-heteropolymer ETI-104. Ann Rheum Dis. 2004;63:1104–12. doi: 10.1136/ard.2003.016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornacoff JB, Hebert LA, Smead WL, Van Aman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–47. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waxman FJ, Hebert LA, Cornacoff JB, et al. Complement depletion accelerates the clearance of immune complexes from the circulation of primates. J Clin Invest. 1984;74:1329–40. doi: 10.1172/JCI111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waxman FJ, Hebert LA, Cosio FG, et al. Differential binding of immunoglobulin A and immunoglobulin G1 immune complexes to primate erythrocytes in vivo. Immunoglobulin A immune complexes bind less well to erythrocytes and are preferentially deposited in glomeruli. J Clin Invest. 1986;77:82–9. doi: 10.1172/JCI112306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall C. Monkeys for research: much coveted, and hard to come by. New York Times. 2004. p. D1. April 6.