Abstract
We report for the first time a significant increased lymphoproliferative response to alpha tropomyosin as well as observing autoantibodies to tropomyosin observed in Behçet's disease (BD) patients with posterior uveitis. Peripheral blood mononuclear cells (PBMCs) from 18 BD patients with posterior uveitis, 18 patients with other forms of noninfectious uveitis, 9 patients with retinal damage due to photocoagulation as well as 18 healthy donors were evaluated for antigen-specific lymphoproliferative responses to alpha tropomyosin and its derivative peptides. The proliferative responses of PBMCs to these antigens were studied using 3H thymidine incorporation assay. Serum samples were also screened by ELISA for autoantibodies against tropomyosin. Six of the 18 (33%) BD patients with posterior uveitis showed increased proliferative response to alpha tropomyosin or its derivative peptides, while none of the healthy, disease controls were positive. The mean lymphoproliferative responses to tropomyosin were significantly higher (P < 0·02) in the BD patients compared to healthy or disease controls. Higher titres of anti-tropomyosin antibodies were also seen in four of the 18 BD patients but none in the healthy or disease control groups (P < 0·002). The occurrence of these abnormalities supports a possible role for alpha tropomyosin as a self-antigen in a subset of patients with Behçet's disease.
Keywords: Alpha tropomyosin, Autoantigen, Autoantibody, Behçet's disease
Introduction
Behçet's disease (BD) is an inflammatory disorder involving multiple systems including the eyes, skin, oral cavity, joints, genital system, central nervous system and blood vessels [1]. In the eye, BD is a major cause of posterior uveitis and visual loss [2]. Ocular inflammation constitutes one of the major diagnostic criteria for Behçet's disease and the principal manifestations include posterior uveitis and retinal vasculitis, predominantly affecting the retinal veins [3]. Even though numerous aetiologies have been put forward, BD is believed to be autoimmune in origin [4]. Laboratory studies show the presence of autoantibodies in Behçet's patients to oral as well as ocular antigens [5,6]. Other groups have shown immune responses to retinal S antigen and interphotoreceptor retinoid binding protein in BD patients with ocular complications [6,7].
Tropomyosins are a family of proteins expressed in muscle as well as in nonmuscle cells including epithelial cells, fibroblasts, and platelets. It is the simplest form of alpha helical fibrous proteins consisting of two identical polypeptide chains wound around each other [8]. In nonmuscle cells, tropomyosin forms an integral part of the cytoskeleton and is involved in the regulation of cellular contraction. Invertebrate tropomyosins are a major food allergen, and it is believed that cross-reactivities to the crustaceans are due to this protein [9]. Autoimmune responses to tropomyosin and related antigenic epitopes have been reported in other chronic diseases including ulcerative colitis [10] Alzheimer's disease [11] and autoimmune hepatitis [12].
Recent work showed the presence of autoantibodies to alpha tropomyosin in serum samples from patients with Behçet's disease, and immunization with alpha tropomyosin induced uveitis in Lewis rats [13]. Experimental animal model of uveitis as well as evidence from clinical disease in humans demonstrates that T cells of Th1 subtype play an important role in etiopathogenesis of posterior uveitis [14,15]. Moreover, evidence from literature points to the role of T cells in the underlying mechanism in the pathogenesis of Behçet's disease [2]. Hence this study was initiated to learn whether peripheral blood cells from BD patients with posterior uveitis might manifest antigen-specific immune responses to alpha tropomyosin. We here report for the first time, that peripheral blood cells from a subset of BD patients displayed a specific response to alpha tropomyosin and its derivative peptides when compared to healthy or disease controls. These findings indicate that an autoimmune response to tropomyosin might characterize a subset of patients with Behçet's disease.
Materials and methods
Patients
Eighteen BD patients with posterior uveitis (BD group) who fulfilled the diagnostic criteria of the International study group for Behçet's disease [2,3] and were followed in the outpatient clinic at the National Eye Institute were studied. Eighteen patients with other forms of noninfectious uveitis including anterior uveitis, retinal vasculitis, sarcoidosis, panuveitis, Birdshot retinochoroidopathy (non BD group), 9 patients with end stage diabetic retinopathy who had undergone pan retinal photocoagulation and 18 healthy individuals served as controls. Blood samples from all subjects were obtained after IRB approval and informed consent prior to enrollment. While BD, diabetic as well as other noninfectious uveitis patients were enrolled from the outpatient clinic of National Eye Institute, blood samples from healthy donors were obtained from NIH blood bank.
PBMCs and serum samples
Blood samples were collected either in heparinized tubes for isolation of PBMCs or in sterile glass tubes without anticoagulants to collect serum. PBMCs were isolated immediately by density gradient centrifugation using Ficoll-Paque plus® (Amersham Biosciences Corporation, NJ, USA) as previously described [16]. PBMC's from both patients and control blood samples from NIH blood bank were isolated within 2 h of collection and were used for proliferation studies on the same day to avoid sample variations. Collected serum samples were frozen at −20°C for later ELISA assay.
Antigen-specific lymphoproliferative assay
To avoid any artifact effect during freeze-thaw procedure, the isolated PBMCs were used immediately for the antigen-specific lymphoproliferative assay. Briefly, the isolated PBMCs were cultured in 96-well flat bottomed plates (Nalge Nunc International, Rochester, NY, USA) at 2 × 106/ml in 0·2 ml/well of RPMI-1640 medium (Gibco-BRL, MD, USA) containing 10% human AB serum (Sigma, St. Louis, MO, USA) supplemented with 2 m m glutamine, 1× antibiotics and antifungal cocktail (Gibco-BRL). All experiments were done in triplicates. Bovine tropomyosin was purchased from Sigma, and the recombinant human muscle tropomyosin was a generous gift from Dr Hitchkock-DeGregori (Department of Neuroscience and Cell Biology, Robert Wood Johnson Medical School, NJ, USA). The sequences for the tropomyosin derivative peptides were P1 (5–21: KKKMQML KDKENALDR), P2 (73–89: LAEKKATDAEADVASLN) and P3 (201–217: TNNLKSLEAQAEKYSQK). For cell stimulation, 100 µl of cell suspension were plated with 100 µl of culturing medium in the presence or absence of the following stimulants in final concentrations: bovine tropomyosin (BT, 10 µg/ml or 20 µg/ml), human tropomyosin (HT, 10 µg/ml or 20 µg/ml), synthetic tropomyosin peptides (P1, P2 or P3 at 10 µg/ml or 20 µg/ml), or the mitogen ConA (2 µg/ml, CalBiochem, San Diego, CA, USA). After 5 days culturing at 37°C in 5% CO2, the cultures were pulsed with 3H thymidine (2·5 µCi/ml) (Amersham, Buckinghamshire, UK) and continuously cultured for 8–10 h. The cells were then harvested and uptake of 3H thymidine was measured by a beta counter (Perkin Elmer life sciences). The effect of antigen stimulation was expressed as stimulation index (SI), e.g. the ratio of mean counts per minute with stimulant to mean counts per minute without stimulant. A stimulation index of above Mean + 3 SD of the normal healthy control was considered as significant proliferative response.
ELISA for anti-tropomyosin antibodies
To screen sera for anti-tropomyosin antibodies, a direct tropomyosin ELISA assay was established in the laboratory. The pilot experiments determined the optimum conditions for the ELISA assay, including coating, blocking and assaying buffer, optimum concentrations for coating antigen, dilution of HRP conjugate as well as other assaying conditions. The sensitivity of the ELISA assay was determined to be around 200 pg/ml. For screening, briefly, 96-well flat-bottomed microplates (Immulon 4Hbx, Thermo Labsystems, MA, USA) were coated with 1 µg/ml of tropomyosin in 100 µl of PBS at 4°C overnight. The plates were washed 3 times with washing buffer (PBS/0·1% Tween-20) and blocked with blocking buffer (PBS/0·1%Tween-20 containing 0·5% non fat milk) at room temperature for 2 h. The plates were then washed with washing buffer and patient serum samples diluted in PBS (1 : 10, 1 : 50, 1 : 250 and 1 : 1250) were added and incubated at 37°C for 2 h. The plates were again washed with washing buffer and 100 µl/well of detecting antibody in 1 : 10 000 dilution (HRP conjugated anti-human IgG antibody, Pierce laboratories, PA, USA) was added. At the end of a 20-minute incubation period at room temperature, the plates were washed, and TMB substrate solution (Pierce laboratories) added at 100 µl/well. The reaction was stopped by addition of 50 µl/well 0·2 m H2SO4 and the plates read at 450 nm by an ELISA reader (Labtech instruments, MO, USA). The optical density (OD) background values were determined as the mean of the OD values from wells with no coating antigen. The background values were subtracted from the test values. Standard serum, described earlier [13] included in the plate served as positive controls. A value of Mean + 3 SD of the normal serum samples was taken as the cut-off value. All samples were measured in duplicate.
To demonstrate that the autoantibodies present in patients’ sera were specific against tropomyosin, sera were preincubated with 10 µg/ml and 20 µg/ml of tropomyosin antigen at 37°C for 30 min. The tropomyosin blocked serum samples were then tested using the same ELISA assay for titres of anti-tropomyosin antibodies.
Statistical analysis
Analysis of lymphocyte proliferation as well as serum tropomyosin antibodies was done using independent Student's t-test. P-values ≤ 0·05 were considered statistically significant.
Results
Clinical information
Eighteen patients with BD were included in this study. All patients had a history of posterior uveitis, as well as recurrent oral ulcers (9 males, 9 females, mean age 40·3 ± 15·4 years). Fourteen (77·78%) patients had genital ulcerations; 11 (61·11%) had arthritis and 8 (44·44%) had skin involvement (Table 1). 17/18 patients were clinically quiescent and all the patients had a long duration of disease ranging from 2 to 20 years. At the time of sampling, 14/18 patients were on treatment. Six patients were on more than one drug. Six were on treatment with systemic steroids while two patients were on cyclosporin. Four patients were on Steroid/Cyclosporine. Six patients who had very severe disease with ocular ischemia had reached end stage and were on no treatment. Eighteen (9 males and 9 females, age 45·1 ± 13·1 years) uveitis patients (anterior uveitis = 2, primary retinal vasculitis = 4, sarcoidosis = 3, intermediate uveitis = 2, panuveitis = 5, Birdshot retinochoroidopathy = 2) (Table 2) as well as 9 patients with severe diabetic retinopathy (5 males and 4 females, age 55·4 ± 12·1 years) who had undergone photocoagulation treatment served as disease controls. Control blood samples obtained from NIH blood bank were (9 males, 9 females; age 40 ± 12·4 years) age and gender matched to the study group.
Table 1.
Clinical characteristics of patients with Behçet's disease.
| Patient | Age/ sex | VA | Duration (year) | HLA | Systemic involvement | Ocular diagnosis | Ocular ischaemia | Severity | Stimulation indices | Anti-tropomyosin antibodies | Disease state & activity | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/F | 20/16020/160 | 7 | N/A | OU, A, S | (OU) Chronic panuveitis | + | Severe | 1·1 | < 1 : 10 | Burnt out | Steroid |
| 2 | 17/M | 20/40020/32 | 2 | B-38,44 | OU | (OU) Chronic panuveitis(OD) Retinal detachment | _ | Severe | 2·3 | < 1 : 10 | Stable, Quiet | Steroid/mycophenolate |
| 3 | 34/M | 20/1220/32 | 9 | B-7,15 | OU | (OU) Chronic panuveitis | _ | Moderate | 14·3 | < 1 : 10 | Stable, Quiet | Cyclosporine |
| 4 | 33/M | 20/16HMCF | 12 | N/A | OU, GU, A, S | (OS) chronic panuveitis | + | Severe | 19·3 | < 1 : 10 | Burnt out | Steroid/colchicine |
| 5 | 36/M | 20/4020/100 | 12 | B-8,27 | OU, GU | (OU) Chronic panuveitis | _ | Moderate | 11·3 | < 1 : 10 | Stable, Quiet | Steroid |
| 6 | 72/F | 20/16020/25 | 2 | B-35 | OU, GU, A, S | (OU) Chronic recurrent posterior uveitis | + | Severe | 6 | 1 : 250 | OD Burnt outOS Stable, Quiet | Steroid |
| 7 | 53/M | HMCF20/60 | 20 | B-35,39 | OU, GU, A | (OU) Chronic panuveitis | + | Severe | 20·3 | < 1 : 10 | OD Burnt outOS Stable, Quiet | No treatment |
| 8 | 30/F | 20/2520/20 | 3 | B-07,40 | OU, GU | (OU) Chronic panuveitis | _ | Severe | 5·3 | < 1 : 10 | Stable, Quiet | No treatment |
| 9 | 28/F | 20/2020/60 | 2 | B-13,44 | OU, GU, A | (OU) Chronic panuveitis(OS) Macular hole | _ | Severe | 12·9 | < 1 : 10 | Stable, Quiet | Steroid/cyclopsorine |
| 10 | 19/F | HMCF20/20 | 5 | B-7,14 | OU, GU, S | (OU) Chronic panuveitis | + | Severe | 2·4 | 1 : 1250 | OD Burnt outOS Stable, Quiet | Steroid/cyclosporine |
| 11 | 58/F | 20/2020/20 | 3 | B-08,57 | OU, GU | (OU) Chronic panuveitis | _ | Moderate | 5·6 | < 1 : 10 | Stable, Quiet | Cyclosporine |
| 12 | 38/F | LP20/80 | 10 | B-7 | OU, GU, A | (OD) Phthisis(OS) Chronic recurrent posterior uveitis | + | Severe | 1·4 | 1 : 250 | Burnt out | No treatment |
| 13 | 48/M | 20/3220/20 | 8 | B-14,33 | OU, A | (OU) Chronic panuveitis | _ | Moderate | 20·1 | < 1 : 10 | Stable, Quiet | No treatment |
| 14 | 45/M | 20/2020/25 | 12 | B-08 | OU, GU, A | (OU) Chronic recurrent posterior uveitis | _ | Moderate | 2·4 | Active | Steroid | |
| 15 | 65/M | 20/8020/40 | 14 | B-34 | OU, GU, A, S | (OU) Chronic panuveitis | _ | Severe | 6·8 | 1 : 250 | Stable, Quiet | Steroid |
| 16 | 32/M | 20/4020/30 | 4 | B-35/50 | OU, GU, S | (OU) Chronic panuveitis | _ | Severe | 2·7 | < 1 : 50 | Stable, Quiet | Steroid |
| 17 | 45/F | 20/1220/32 | 5 | B-51/57 | OU, GU, A, S | (OU) Chronic panuveitis | _ | Severe | 1·8 | < 1 : 10 | Stable, Quiet | Steroid/cyclosporine |
| 18 | 23/F | 20/2020/20 | 6 | B-7, 57 | OU, GU, A | (OU) Chronic panuveitis | _ | Severe | 2·8 | < 1 : 10 | Stable, Quiet | Steroid/cyclosporine |
VA, visual acuity; N/A, not available; OU, oral ulcers; GU, genital ulcers; A, arthritis; S, skin involvement.
Table 2.
Clinical characteristics of control uveitis patients.
| Patient | Age/ sex | Duration (year) | Diagnosis | Ocular clinical findings |
|---|---|---|---|---|
| 1 | 44/M | 8 | Primary retinal vasculitis | Vascular sheathing, Retinal haemorrhages, Sclerotic vessels and capillary dropout. |
| 2 | 34/M | 3 | Anterior uveitis | Anterior chamber cells and flare |
| 3 | 45/F | 4 | Primary retinal vasculitis | Vascular sheathing, Retinal haemorrhages, Neovascularization and capillary dropout. |
| 4 | 22/F | 5 | Intermediate uveitis | Vitreous cells and opacities, CME |
| 5 | 24/M | 2 | Anterior uveitis | Anterior chamber cells and flare |
| 6 | 56/F | 12 | Primary retinal vasculitis | Vascular sheathing, Retinal haemorrhages, Scleroticvessels and capillary dropout. |
| 7 | 62/M | 8 | Primary retinal vasculitis | Vascular sheathing, Retinal haemorrhages, Sclerotic vessels and capillary dropout. |
| 8 | 38/F | 10 | Intermediate uveitis | Vitreous cells and opacities, Vascular sheathing, CME |
| 9 | 62/M | 5 | Sarcoidosis | Anterior chamber cells, Vitreous cells and opacities |
| 10 | 49/F | 11 | sarcoidosis | Anterior chamber cells, Vitreous cells and opacities |
| 11 | 38/F | 3 | Panuveitis | Anterior chamber cells and flare, Vitreous cells, Retinal vascular sheathing, CME |
| 12 | 66/M | 4 | Sarcoidosis | Vitreous cells and opacities, Retinochoroiditis |
| 13 | 29/M | 5 | Birdshot retinochoroidopathy | Vitritis, Retinal vasculitis, Chorioretinal lesions |
| 14 | 50/M | 14 | Chronic panuveitis | Retinochoroidal scarring, sclerosed vessels, cystoid macular oedema, Multifocal choroiditis |
| 15 | 35/M | 8 | Chronic granulomatous panuveitis | |
| 16 | 57/F | 7 | Chronic granulomatous panuveitis | Retinal vasculitis, sclerosed vessels, chorioretinal scars |
| 17 | 45/F | 4 | Birdshot retinochoroidopathy | Retinal vasculitis, chorioretinal lesions |
| 18 | 56/F | 5 | Chronic panuveitis | Multifocal chorioretinal lesions |
CME, cystoid macular oedema.
Lymphoproliferative responses to tropomyosin antigen and its derivative peptides
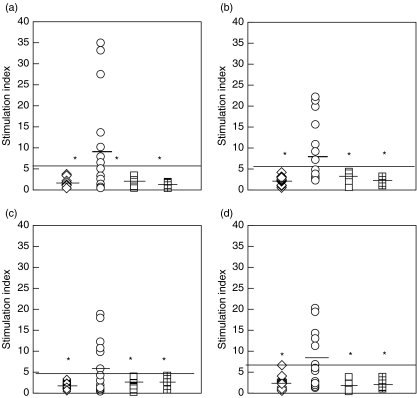
Using 3H thymidine assay we evaluated the lymphoproliferative responses of the patient as well as controls. The range of proliferation values for the normal healthy controls was in the range of 200–500 CPM while that of the BD patients who responded ranged from 2000 to 15000 CPM. We were interested in knowing whether the lymphocytes from BD patients with posterior uveitis would respond to the stimulation of tropomyosin and its derivative peptides and whether there would be differences comparing with patients in the non-BD group or healthy donors. Figure 1(a–d) shows the proliferative responses among BD patients, non-BD groups and healthy controls to tropomyosin. In the BD group 7/18 (38%) of patients had significantly higher proliferative response (> Mean ± 3 SD) to bovine tropomyosin, while 6/18 (33%) responded to human tropomyosin compared with the non BD group or healthy controls. Little differences were found between healthy donors, non-BD group and diabetic controls to the stimulation at either 10 or 20 µg/ml of bovine tropomyosin (1·9 ± 0·9 versus 2·3 ± 0·8 versus 1·2 ± 0·3, or 2·1 ± 1·0 versus 2·9 ± 1·2 versus 1·8 ± 0·7). But significantly higher mean lymphoproliferative response was seen in BD patients (P < 0·02) to bovine tropomyosin (Fig. 1a,b) compared to either disease or healthy controls (8·6 ± 11·4–10 µg/ml of antigen, or 7·8 ± 7·0–20 µg/ml of antigen). This increased lymphoproliferative responses from BD patients were reproduced when using a recombinant human tropomyosin as antigen (Fig. 1c,d). While the mean stimulation indices to recombinant human tropomyosin in the healthy controls, non-BD uveitis group and diabetic controls was 1·7 ± 0·8 or 2·4 ± 0·9 or 1·7 ± 1·1–10 µg/ml of antigen and 2·5 ± 1·3 or 2·2 ± 0·9 or 1·7 ± 0·9 for 20 µg/ml of antigen, respectively, the indices were significantly higher in the Behçet's subjects (5·8 ± 5·7 for 10 µg/ml of antigen and 7·7 ± 6·8 for 20 µg/ml of antigen, respectively). All the patients that responded to the bovine tropomyosin also responded to the recombinant tropomyosin except patient 10.
Fig. 1.
Scatter diagram showing stimulation indices (SI) of PBMCs from normal subjects (open diamonds; n = 18), BD patients (open circles; n = 18) non-BD group (open squares; n = 18) and patients with retinal damage due to photocoagulation (checked squares; n = 9) stimulated with tropomyosin. (a,b) shows responses against Bovine tropomyosin (BT) 10 µg/ml and 20 µg/ml while (c,d) indicates responses against the human tropomyosin (HT) 10 µg/ml & 20 µg/ml, respectively. Black bars indicate the mean proliferative responses of each group. Solid black line indicates the upper limit of normal proliferative responses (Mean + 3SD). *indicate significant proliferative responses in BD patients compared with normal or disease controls P-value < 0·02.
As shown in Table 3, the stimulation indices to tropomyosin derivative peptides (20 µg/ml) for the healthy controls, BD group, non-BD group and diabetic controls were (1·4 ± 0·7, 2·8 ± 3·2, 1·2 ± 0·5 and 0·8 ± 0·3) against P1 (1·4 ± 0·8, 3·1 ± 3·1, 1·8 ± 0·9 and 0·9 ± 0·4) against P2, or (1·3 ± 0·8, 3·0 ± 2·8, 1·3 ± 0·4 and 0·9 ± 0·5) against P3, respectively. Among the three peptides tested P2 and P3 showed significantly increased proliferative responses in the BD group compared to only healthy controls (P < 0·05). The three patients who responded to the peptides P2 and P3 also responded to the whole protein while patient 10 who responded to P1 only, showed response to bovine tropomyosin. However, we did not see correlation between proliferative responses and specific clinical manifestation or without treatment.
Table 3.
Stimulation indices of PBMCs from normal donors, BD patients, non BD group and control diabetic patients who had retinal damage due to photocoagulation.
| P1 = 20 µg/ml | P2 = 20 µg/ml | P3 = 20 µg/ml | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sl no. | NL | BD | UV | DM/PHC | NL | BD | UV | DM/PHC | NL | BD | UV | DM/PHC |
| 1 | 1·2 | 1·6 | 1·3 | 1·1 | 1·2 | 1·8 | 1·2 | 0·7 | 0·9 | 1·2 | 1·3 | 0·6 |
| 2 | 1·1 | 1·3 | 1·9 | 0·9 | 1 | 1 | 1·3 | 0·9 | 0·8 | 1 | 1·8 | 0·9 |
| 3 | 1·8 | 1·1 | 2·1 | 1·0 | 1·8 | 1·3 | 2·9 | 1·1 | 2·4 | 1·1 | 2·1 | 1·1 |
| 4 | 0·9 | 3 | 2 | 1·1 | 1 | 0·9 | 2·3 | 1·1 | 0·2 | 1·2 | 2 | 1·0 |
| 5 | 0·8 | 3·5 | 1·7 | 1·1 | 0·5 | 1·9 | 1·2 | 0·6 | 1 | 1·4 | 1·7 | 2·0 |
| 6 | 1·5 | 2·9 | 1·2 | 0·3 | 1·1 | 8·7* | 2·3 | 0·5 | 1 | 9·8* | 1·2 | 0·3 |
| 7 | 0·3 | 0·7 | 0·6 | 0·3 | 0·2 | 1·9 | 1·9 | 0·2 | 1·7 | 0·7 | 0·9 | 0·2 |
| 8 | 1 | 0·7 | 0·7 | 0·7 | 2·9 | 13·2* | 2·3 | 1·1 | 0·6 | 1·2 | 0·7 | 1·0 |
| 9 | 1·9 | 0·6 | 0·9 | 1·1 | 2·3 | 0·4 | 3 | 1·3 | 1·7 | 0·6 | 0·9 | 1·4 |
| 10 | 0·9 | 7·5* | 1·4 | 1·5 | 3·3 | 2·8 | 0·8 | 5·7* | 1·4 | |||
| 11 | 1·5 | 2·9 | 1·9 | 1·5 | 1·8 | 3 | 2·3 | 2·2 | 1·9 | |||
| 12 | 2 | 14·1* | 1·6 | 1·9 | 5·7* | 3·5 | 2·9 | 9* | 1·6 | |||
| 13 | 1·7 | 2·1 | 0·5 | 2·2 | 2 | 0·5 | 1·65 | 2·1 | 0·7 | |||
| 14 | 1·3 | 3·9 | 0·4 | 1·1 | 2·7 | 0·8 | 1·4 | 4·6 | 1·0 | |||
| 15 | 3·4 | 1·5 | 1·2 | 3·1 | 3 | 1·2 | 2·7 | 3·1 | 1·2 | |||
| 16 | 2·1 | 1·3 | 0·4 | 1·7 | 2·4 | 0·8 | 1·3 | 3·1 | 1·2 | |||
| 17 | 1 | 0·9 | 1·2 | 0·7 | 1 | 1·9 | 0·6 | 1·2 | 2·1 | |||
| 18 | 1·3 | 1 | 0·7 | 1·1 | 2·5 | 1·0 | 1·01 | 4·1 | 0·9 | |||
NL, healthy controls; BD, Behcets disease group; UV, other uveitis (non BD group); DM/PHC, control diabetic patients who had retinal damage due to photocoagulation. P1, P2 and P3 are the three Tropomyosin derivative peptides.
indicates significant proliferative responses in BD patients compared with disease well as healthy controls (P < 0·02).
Serum levels of anti-tropomyosin antibodies
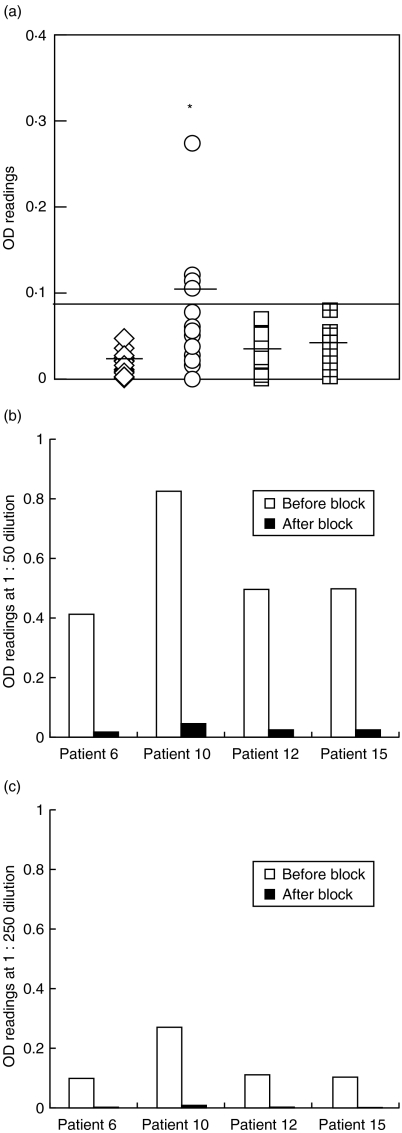
In addition to the cellular immune response of BD patients to tropomyosin antigen, we also examined whether there is a humoral immune responses in BD patients to tropomyosin. We screened all serum samples using a highly sensitive and specific tropomyosin ELISA assay. Although there were no anti-tropomyosin antibodies detectable from either healthy donors, diabetic controls or from non-BD group, 4/18 patients in the BD group demonstrated significantly higher titres (P < 0·002) of anti-tropomyosin antibodies (> 1 : 10 dilution) (Fig. 2a). Moreover, when the positive serum samples were blocked by tropomyosin antigen and then tested for anti-tropomyosin antibody titres, there was a dramatic decrease of autoantibodies at lower concentration (10 µg/ml) of the blocking antigen. At higher concentration (20 µg/ml) of blocking antigen there were no detectable autoantibodies (Fig. 2b,c). But there was no correlation between the levels of antitropomyosin antibodies and the T cell proliferative responses.
Fig. 2.
ELISA for serum anti-tropomyosin antibodies in BD patients as well as healthy and disease controls. (a) shows optical density (OD) values for serum anti-tropomyosin antibody (1 : 250 dilution) from BD patients (open circles); healthy controls (open squares); disease controls (open diamonds) and patients with retinal damage due to photocoagulation (checked squares). Black bars indicate the mean OD value for each group. OD values above the solid black line indicate positive sera (OD > mean + 3SD). *indicates significantly higher levels of anti- tropomyosin antibodies in the BD patients compared to the healthy and disease controls P < 0·002. (b,c) represents ELISA data from a blocking experiment done on four patients who were positive for anti-tropomyosin antibodies. Serum dilution (b) 1 : 50 and (c) (1 : 250). □ OD readings without blocking; ▪ OD readings after pre incubating the serum with human tropomyosin antigen at 20 µg/ml before doing the ELISA. Note the lower values for the black bars for each patient indicating the specificity of ELISA.
Discussion
Behçet's syndrome is a multisystemic inflammatory disorder with a number of immunological alterations from the normal suggesting an autoimmune pathogenesis. Autoantibodies have been reported to occur against mucosal cells [5], endothelial cells [17], lymphocytes [18] and cardiolipin [19]. Unlike SLE and rheumatoid arthritis, BD does not manifest classical features such as hyper gammaglobulinaemia or anti-nuclear antibodies.
In an earlier study, we reported high titres of anti-tropomyosin antibody in 26·6% of BD patients without posterior uveitis. However we did not see any T cell specific antigenic responses to tropomyosin in the peripheral blood of affected patients [13]. In the present study, we compared antigen-specific responses to tropomyosin in BD patients with posterior uveitis, healthy donors as well as disease controls. In this series, 33% of BD patients demonstrated lymphoproliferative response to tropomyosin antigen in vitro. This response was not observed in other forms of noninfectious uveitis, patients with retinal damage (photocoagulation) nor in healthy donors. Among these patients, 4/18 patients also showed significantly higher titres of anti-tropomyosin antibodies. Although no correlation was seen between clinical status and the antigen specific responses, all BD patients in this series had posterior uveitis. The T cell response seen in these BD patients with posterior uveitis is consistent with the proposed Th1 mechanism in the experimental model of uveitis (EAU) [15] and suggests a potential role of tropomyosin in the pathogenesis of a subgroup of BD patients.
Tropomyosins are a family of proteins found in virtually all tissues. The nonmuscle isoforms are associated with the regulation of cytoskeletal structure. Nevertheless, tropomyosin has been implicated as a target autoantigen in various inflammatory conditions, including ulcerative colitis [10]. Das et al. [20] demonstrated in ulcerative colitis patients the presence of autoantibodies to a colonic protein p40, which has structural similarity to cytoskeletal protein tropomyosin. Alpha tropomyosin has been shown to be a major food allergen present in crustaceans, insects and mollusks [9]. Direskeneli et al. [21] suggested that molecular mimicry between microbial and HSP peptides can trigger autoimmune responses in BD patients based on sequence homology between microbial and human HSP. Studies looking at the sequence homology between streptococcal cell wall M proteins as well as tropomyosin reveal shared immunological eptitopes. Fenderson et al. [22] reported a homology of 31% when comparing particular segments of mammalian tropomyosin with streptococcal M protein as well as cross reactivity's between M protein and tropomyosin using monoclonal antibodies. Cross reactivity can occur because of shared epitopes among different proteins. In rheumatic fever and myocarditis, a cross-reacting response between streptococcal cell wall antigens and tropomyosin has been implicated. Immune responses against microbial cell wall proteins can activate a cross-reactive response to self-antigens, thus resulting in an autoimmune disease. Similarities between the streptococcal surface protein and tropomyosin suggests that molecular mimicry might be important in eliciting an immune reaction to tropomyosin leading to the inflammatory manifestations in BD. Past clinical history for evidence of streptococcal infection in these patients even though ambiguous, still did not rule out the possibility of a similar mechanism.
Preliminary experimental results indicate that the autoantibodies produced in these patients react with the normal human retinal tissues as shown by the positive immunolabelling (data not shown). This raises the possibility that tropomyosin might indeed be a target self-antigen in a group of patients with BD. However it is still not clear whether tropomyosin acts as the self-antigen or is an epiphenomenon in BD patients. Alternatively these findings might be secondary phenomena, resulting from a deregulated immune system producing secondary autoantibodies against exposed antigens following any kind of tissue damage in these patients. All BD patients in this study had severe retinal damage. However it is interesting to note that we did not observe similar immune responses to tropomyosin in other causes of retinal damage such as in diabetics following pan retinal photocoagulation.
Various antigens that have been implicated in the pathogenesis of BD including sensitivities to cows milk protein, oral mucosal antigens, heat shock proteins and ocular S antigen. Different studies have shown T cell proliferation to heat shock proteins and its peptides [23]. In an earlier series of 49 patients, a proliferative response to different peptides of HSP60/65 was seen in 52% of BD patients[21]. A review of the literature suggests that BD patients may have cell-mediated responses to different antigens [5–7,23]. Indeed, the diagnosis of BD is based on an association of different clinical findings, as there is no definite diagnostic test. This may reflect a heterogeneous disease population with different immunopathogenic mechanisms, which explains that only a subpopulation, responds to a particular antigenic stimulation.
Tropomyosins from different species as well as different tissues exhibit similarities in functional as well as chemical properties. There are few differences in the peptide sequences of tropomyosin between different species even though the antigenic structure and epitopes varies from one mammalian species to another [24]. In this study, the six patients who responded to human recombinant tropomyosin showed proliferative responses to the bovine as well. Only four of these patients had shown responses to the peptide sequences that we had selected for in vitro studies. These peptides were designed from the rat sequence based on an MHC motif, which might partially explain these differences.
There are several limitations to the present study. All except one patient were in a clinically quiescent stage of the disease and all had a long duration of disease. The long duration of the disease also raises the question of epitope spread and shift in immune responses. Although many questions yet remain to be answered, it is quite possible that autoimmunity to alpha tropomyosin may not merely be an epiphenomenon; immunization of Lewis rats with alpha tropomyosin peptides led to pathogenic inflammatory lesions consistent with anterior uveitis [13]. Thus, autoimmunity to alpha tropomyosin might, in humans as in rats, trigger uveitis. Further studies looking for evidence of antigen release in the serum of these patients during the active stage of disease would shed light more in to the understanding of these autoimmune phenomena.
Acknowledgments
We would like to thank Prof Hitchkock-De Gregori for supplying us the recombinant human tropomyosin. I.R.C. is the incumbent of the Mauerberger Chair in Immunology at the Weizmann Institute, and the Director of the Center for the Study of Emerging Diseases, Jerusalem.
References
- 1.Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB, Whitcup SM, Palestine AG. Uveitis. Fundamentals and Clinical practice. 3. Philadelphia: Mosby; 2004. Behçet's disease: Concept of disease pathogenesis: pp. 334–53. [Google Scholar]
- 3.Bloch-Michel E, Nussenblatt RB. International Uveitis Study group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–5. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- 4.Stanford MR. Behçet's syndrome. Br J Ophthalmol. 2003;87:381–2. doi: 10.1136/bjo.87.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok AM, deVries J, Rothova A, Zaal MJ, Schweitzer CM, Bos JD, Kijlstra A. Antibodies against ocular and oral antigens in Behçet's disease associated with uveitis. Curr Eye Res. 1989;8:957–62. doi: 10.3109/02713688909082657. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto JH, Minami M, Inaba G, Masuda K, Mochizuki M. Cellular autoimmunity to retinal specific antigens in patients with Behçet's disease. Br J Ophthalmol. 1993;77:584–9. doi: 10.1136/bjo.77.9.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Smet MD, Dayan M. Prospective determination of T-cell responses to S-antigen in Behçet's disease patients and controls. Invest Ophthalmol Vis Sci. 2000;41:3480–4. [PubMed] [Google Scholar]
- 8.Smillie LB. Structure and functions of tropomyosins from muscle and non-muscle sources. Tren Bio Chem Sci. 1979;4:151–5. [Google Scholar]
- 9.Reese G, Ayuso R, Lehrer SB. Tropomyosin. An Invertebrate Pan-Allergen. Int Arch Allergy Immunol. 1999;119:247–58. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 10.Biancone L, Monteleone G, Marasco R, Pallone F. Autoimmunity to tropomyosin isoforms in ulcerative colitis (UC) patients and unaffected relatives. Clin Exp Immunol. 1998;113:198–205. doi: 10.1046/j.1365-2249.1998.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galloway PG, Mulvihill P, Siedlak S, Mijares M, Kawai M, Padget H, Kim R, Perry G. Immunochemical demonstration of tropomyosin in the neurofibrillary pathology of Alzheimer's disease. Am J Pathol. 1990;137:291–300. [PMC free article] [PubMed] [Google Scholar]
- 12.Dighiero G, Lymberi P, Monot C, Abuaf N. Sera with high levels of anti-smooth muscle and anti-mitochondrial antibodies frequently bind to cytoskeleton proteins. Clin Exp Immunol. 1990;82:52–6. doi: 10.1111/j.1365-2249.1990.tb05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mor F, Weinberger A, Cohen IR. Identification of alpha-tropomyosin as a target self–antigen in Behçet's syndrome. Eur J Immunol. 2002;32:356–65. doi: 10.1002/1521-4141(200202)32:2<356::AID-IMMU356>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Nussenblatt RB, Fortin E, Schiffman R, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci USA. 1999;96:7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–5. [PubMed] [Google Scholar]
- 16.McDyer JF, Li Z, John S, Yu X, Wu CY, Ragheb JA. IL-2 receptor blockade inhibits late, but not early, IFN-gamma and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and – independent pathways of IFN-gamma production. J Immunol. 2002;169:2736–46. doi: 10.4049/jimmunol.169.5.2736. [DOI] [PubMed] [Google Scholar]
- 17.Direskeneli H, Keser G, D’Cruz D, et al. Anti-endothelial cell antibodies, endothelial proliferation and von Willebrand factor antigen in Behçet's disease. Clin Rheumatol. 1995;14:55–61. doi: 10.1007/BF02208085. [DOI] [PubMed] [Google Scholar]
- 18.Matsui T, Kurokawa M, Kobata T, Oki S, Azuma M, Tohma S, Inoue T, Yamamoto K, Nishioka K, Kato T. Autoantibodies to T Cell Costimulatory Molecules in Systemic Autoimmune Diseases. J Immunol. 1999;162:4328–35. [PubMed] [Google Scholar]
- 19.Tokay S, Direskeneli H, Yurdakul S, Akoglu T. Anticardiolipin antibodies in Behçet's disease: a reassessment. Rheumatology. 2001;40:192–5. doi: 10.1093/rheumatology/40.2.192. [DOI] [PubMed] [Google Scholar]
- 20.Das KM, Dasgupta A, Mandal A, Geng X. Autoimmunity to cytoskeletal protein Tropomyosin. J Immunol. 1993;150:2478–93. [PubMed] [Google Scholar]
- 21.Direskeneli H, Eksioglu-Demiralp E, Yavuz S, Ergun T, Shinnick T, Lehner T, Akoglu T. T cell responses to 60/65-kDa-heat shock protein derived peptides in Turkish patients with Behçet's disease. J Rheumatol. 2000;27:708–13. [PubMed] [Google Scholar]
- 22.Fenderson PG, Fischetti VA, Cunningham MW. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989;142:2475–81. [PubMed] [Google Scholar]
- 23.Lehner T. The role of heat shock protein, microbial and autoimmune agents in the aetiology of Behçet's disease. Int Rev Immunol. 1997;14:21–32. doi: 10.3109/08830189709116842. [DOI] [PubMed] [Google Scholar]
- 24.Leger J, Bouveret P, Lompre AM, Scwartz K. Species-dependent immunological differences between various mammalian cardiac tropomyosins. Biochim Biophys Acta. 1979;26:314–21. doi: 10.1016/0005-2795(79)90406-9. [DOI] [PubMed] [Google Scholar]