Abstract
Enhanced production of macrophage migration inhibitory factor (MIF) is recognized in patients with inflammatory bowel disease (IBD) and mice with experimental colitis; however, the precise molecular function of MIF in colitis is not fully understood. To further investigate this matter, we examined the pathological features of MIF transgenic mice with dextran sulphate sodium (DSS)-induced colitis. We generated transgenic mice carrying a murine MIF cDNA driven by a cytomegalovirus enhancer and a β-actin/β-globin promoter. Mice were orally administered 1–4% DSS in drinking water for 7 days. Clinical disease activity, survival and histological features were evaluated. The level of myeloperoxidase (MPO) activity in the colon tissue was measured to assess neutrophil infiltration. The level of corticosterone in the serum was measured by enzyme linked-immunosorbent assay (ELISA). MIF mRNA and protein were markedly up-regulated in the colon and serum obtained from MIF transgenic mice. The severity of the colitis induced by 1% DSS treatment was markedly higher in MIF transgenic mice than in wild-type mice. We also found that MPO activity was significantly higher in MIF transgenic mice than wild-type mice in response to DSS stimulation. Interestingly, the corticosterone level remained unchanged in MIF transgenic mice. MIF enhances DSS-induced colitis, in part via neutrophil accumulation and inhibition of glucocorticoid bioactivity.
Keywords: corticosterone, dextran sulphate sodium-induced colitis, inflammatory bowel disease, macrophage migration inhibitory factor, neutrophil
Introduction
Inflammatory bowel disease (IBD) is characterized as a chronic and relapsing inflammation of the bowel, mainly consisting of ulcerative colitis and Crohn's disease. Although the aetiology of these intractable diseases remains unclear, there are several reports suggesting the involvement of proinflammatory cytokines in their pathogenesis and exacerbation [1–3]. Indeed, the neutralization of tumour necrosis factor (TNF)-α using anti-TNF-α antibody has been found remarkably effective for the treatment of IBD [4,5].
Macrophage migration inhibitory factor (MIF) was originally identified as a cytokine-derived activated T lymphocyte in vitro; however, its precise biological function has been not sufficiently elucidated until the cloning of MIF cDNA [6]. MIF is released from the anterior pituitary gland in response to endotoxic stimuli [7], and this response has been shown to counter-regulate the immunosuppressive effects of glucocorticoids [8,9]. Moreover, MIF is released from a variety of cells in response to stimuli as a proinflammatory cytokine, and induces the production of TNF-α by macrophages [10].
MIF plays an important role in the pathogenesis of inflammatory diseases. The serum and the local expression of MIF are significantly up-regulated in inflammatory diseases such as rheumatoid arthritis [11], acute pulmonary diseases [12] and glomerulonephritis [13]. It is of note that neutralization of MIF activity by the anti-MIF antibody ameliorated inflammation in these experimental models [14–16]. It has been reported that MIF is increased in the sera of patients with human IBD and experimental colitis [17–19]. In regard to immunohistochemistry, strong positive staining of MIF has been observed in epithelial cells and infiltrating immune cells in colon tissue [18]. Moreover, neutralization of MIF bioactivity by the anti-MIF antibody significantly reduced the production of TNF-α, interferon (IFN)-γ and matrix metalloproteinases in the colon of mice. Although these results suggest that MIF plays a critical role in inflammation in the colon, the molecular basis of its mechanism of action has not been fully elucidated.
Dextran sulphate sodium (DSS)-induced colitis is considered to be a useful experimental model for investigating the mechanism of IBD because its pathological features are similar to those of the chronic inflammatory colonic disorders [20]. We have reported previously that MIF expression was up-regulated in the serum and colon tissues in murine DSS colitis and that anti-MIF antibody treatment effectively reduced the severity of colitis [18]. However, the effect of overexpression of endogenous MIF in the experimental model has not been fully clarified.
In this study, we generated MIF transgenic (Tg) mice to further clarify the role of MIF in the development of colitis, and investigated the effect of overexpression of MIF on DSS-induced colitis using MIF Tg mice.
Materials and methods
Mice and generation of MIF Tg mice
Eight- to 10-week-old C57BL6 mice weighing 22–25 g were purchased from Charles River Japan (Shizuoka, Japan). In this study, MIF Tg mice were established by using C57BL6 mice, which were in turn established from 8-week-old ICR mice (Charles River Japan, Shizuoka, Japan), as foster mothers [21]. In brief, the expression of the transgene was regulated by a hybrid promoter composed of a cytomegalovirus (CMV) enhancer and a β-actin/β-globin promoter [22]. The cDNA sequence used for microinjection was obtained by polymerase chain reaction (PCR) amplification of a reverse transcript of mouse liver total RNA, which was cloned into pBluescript ΙΙ KS (+), and the insert was confirmed by sequencing analysis. The cDNA was then subcloned into pCXN2, a derivative of pCAGGS [23,24]. The 2·7-kb SalΙ-HindΙΙΙ fragment was separated from the vector sequence and used as a transgene. This fragment contained the CMV-IE enhancer, a modified chicken β-actin promoter, the MIF cDNA, and a rabbit β-globin 3′-flanking sequence (Fig. 1a). The transgene was microinjected into male pronuclei of fertilized C57BL6 mouse embryos, which were transplanted into the oviducts of pseudopregnant C57BL6 mice used as foster mothers. Founder mice, which had integrated the transgene, were bred with mice of the same strain. The resultant litters were analysed by Southern blotting of DNA extracted from their tails to distinguish between Tg mice and non-Tg mice. All mice were maintained on standard laboratory chow and given food and water ad libitum. This study adhered to the principles of the Declaration of Helsinki and was approved by the Animal Experiment Ethics Committee of the Graduate School of Medicine of Hokkaido University.
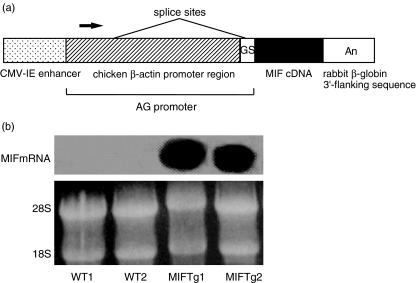
Fig. 1.
Construction of the macrophage migration inhibitory factor (MIF) transgene and expression of MIF mRNA in the colon of mice. (a) The fragment contained the cytomegalovirus (CMV)-IE enhancer, a modified chicken β-actin promoter, the MIF cDNA and a rabbit β-globin 3′-flanking sequence. GS, globin splice acceptor; An, poly A signal. (b) Northern blot analysis of MIF Tg and WT mouse colons in two independent lines. The levels of MIF mRNA expression were higher in the colon of MIF Tg mice than in that of WT mice. Similar results were obtained in three independent experiments.
Northern blot analysis
Colon tissues were obtained from mice under anaesthesia. Northern blot analysis was carried out as described previously [25]. In brief, RNA was isolated from these colon tissues using an Isogen RNA extraction kit (Wako, Osaka, Japan). These samples were separated into 10 µg units by electrophoresis on 1% agarose gel containing 0·6 m formaldehyde, and blotted onto nylon membrane filters. The probe for mouse MIF was labelled with [α-32P]deoxycytidine triphosphate using a DNA random primer labelling kit (Takara, Kyoto, Japan). Hybridization was carried out at 42°C for 48 h. After hybridization the membranes were washed at 65°C for 15 min in × 0·2 standard saline citrate (SSC) with 0·1% sodium dodecyl sulphate (SDS). Bands were visualized by autoradiography on Kodak X-AR film. 18S and 28S of ribosomal RNA were used as a loading control.
Induction and assessment of DSS colitis
Experimental colitis was induced by administration of 1·0% (wt/vol) DSS (molecular weight, approximately 40 000; ICN Biochemicals, Aurora, OH, USA) from day 0 to day 7 in drinking water. Mice were weighed and observed visually for rectal bleeding and diarrhoea every day beginning on day 0. The percentage weight change for each mouse was calculated compared to that on day 0. Prior to colon removal, mice were euthanized by intraperitoneal injection of thiopental. In a separate series of experiments, mice were sacrificed after various time intervals for measurement of cytokines and myeloperoxidase (MPO) activity. The colon was collected and stored at − 80°C until use. The severity of colitis was evaluated by assessment of colon length and histological examination on day 7 after the first DSS administration. To examine the survival rate, the 1% DSS was switched to 4% DSS in drinking water, and the mice were inspected every day.
Disease activity index based on clinical features
The disease activity index (DAI) was used to assess the grade of colitis based on a previously published scoring system [26] (Table 1). The score ranges from 0 to 4 (total score), and represents the sum of the scores for body weight loss, stool consistency and rectal bleeding divided by three. The DAI has been well correlated with tissue damage scores and with specific measurements of inflammation, such as myeloperoxidase (MPO) activity [26].
Table 1.
Scoring system for the disease activity index (DAI).
| Score | Weight loss (%) | Stool consistency | Occult/gross rectal bleeding |
|---|---|---|---|
| 0 | <1 | Normal | Negative |
| 1 | ″1–5 | ||
| 2 | ″5–10 | Loose stool | Haemo-occult positive |
| 3 | 10–20 | ||
| 4 | >20 | Diarrhoea | Gross bleeding |
These clinical criteria were used to evaluate the severity of colitis in mice. Scores were tailed for each category and then divided by three to obtain the DAI.
Histological evaluation of colitis
The colon tissue samples were longitudinally opened, fixed with 10% neutral buffered formalin, embedded in paraffin, sectioned on glass slides, and stained with haematoxylin and eosin (H&E). For histological evaluation of the tissue damage, areas of inflammatory lesions were microscopically evaluated and scored by the pathologist in a blind fashion. The histological score was assessed as follows. The tissue damage grades were: 0, normal mucosa; 1, infiltration of inflammatory cells; 2, shortening of the crypt by less than half; 3, shortening of the crypt by more than half; 4, crypt loss; and 5, destruction of epithelial cells. The grades of the lesion extent were 0, 0%; 1, 1–20%; 2, 21–40%; 3, 41–61%; 4, 61–80%; and 5, 81–100%.
Measurement of MPO activity in the colon
The level of tissue MPO was determined in accordance with a standard enzymatic procedure as described by Krawisz et al. [27], with minor modifications. In brief, the tissue specimen was homogenized in 50 m m potassium phosphate buffer (pH 6·0) with 0·5% hexadecyltrimethylammonium bromide using a Polytron-type homogenizer three times for 30 s each on ice. The sample was centrifuged at 20 000 g for 10 min at 4°C, and the supernatant was collected. This sample (100 µl) was added to 2·9 ml of 50 m m phosphate buffer (pH 6·0) containing 0·167 mg/ml O-dianisidine hydrocholoride and 0·0005% hydrogen peroxide. The absorbance at 460 nm in the sample was measured using a spectrometer at 25°C. The protein concentration of the supernatant was determined using a Bradford assay kit (Bio-Rad Laboratories, Hercules, CA, USA) for calibration, and the values were standardized using MPO purified from human leucocytes (Sigma, St Louis, MO, USA). One unit of change in MPO level was defined as the value that can degrade 1 µm H2O2 per minute at 25°C.
Enzyme-linked immunosorbent assay for MIF and cytokines
MIF in the samples of plasma and colon tissue were measured with an enzyme-linked immunosorbent assay (ELISA) kit specific for MIF. This assay was performed in accordance with the procedure described previously [18].
Enzyme-linked immunosorbent assay for corticosterone
Corticosterone in the serum was measured with an AngioMax Corticosterone ELISA kit (Angiopharm, O’Fallon, MO, USA). The assay was carried out according to the manufacturer's protocol.
Statistics
All data are presented as the mean ± standard error (s.e.). The results were analysed statistically using analysis of variance (anova) for ranks and post hoc tests using Student's unpaired t-test (StatView; SAS Institute, Cary, NC, USA). Survival curves of Kaplan–Meier plots were compared using a log rank test and final outcomes were calculated using Fisher's exact test. Values of P < 0·05 were considered to indicate statistical significance.
Results
Enhancement of MIF mRNA and protein expression in the serum and colon tissues of MIF Tg mice
Northern blot analyses were performed on the colon samples from MIF Tg mice and those from untreated wild-type (WT) mice. The expression of MIF mRNA was stronger in MIF Tg mice than in WT mice (Fig. 1b). We determined the level of MIF in the colon tissues by ELISA. In MIF Tg mice, MIF levels in the serum and colon were more than 10-fold higher than in WT mice (Table 2). Moreover, the increase of MIF was remarkably higher in MIF Tg mice than in WT mice at day 7 after the induction of 1·0% DSS-induced colitis (Table 2).
Table 2.
MIF contents in the serum and colon in C57B6 and macrophage migration inhibitory factor (MIF) transgenic (Tg) mice.
| Serum (ng/ml) | Colon (ng/mg protein) | |
|---|---|---|
| WT + water | ″16·4 ± 1·6 | ″37·8 ± 2·7 |
| WT + DSS | ″25·0 ± 2·8* | ″63·0 ± 8·2* |
| MIF Tg + water | 229·8 ± 20·8** | 1152·0 ± 115·6** |
| MIF Tg + DSS | 2016·4 ± 349·4*** | 2053·6 ± 313·6*** |
Values are expressed as the mean ± s.e. n = 10 mice per group.
P < 0·05 versus the values in untreated WT mice;
P < 0·01;
P < 0·001 versus the values in wild-type (WT) mice treated with 1% dextran sulphate sodium (DSS) for 7 days.
Severity of DSS-induced colitis in MIF Tg mice
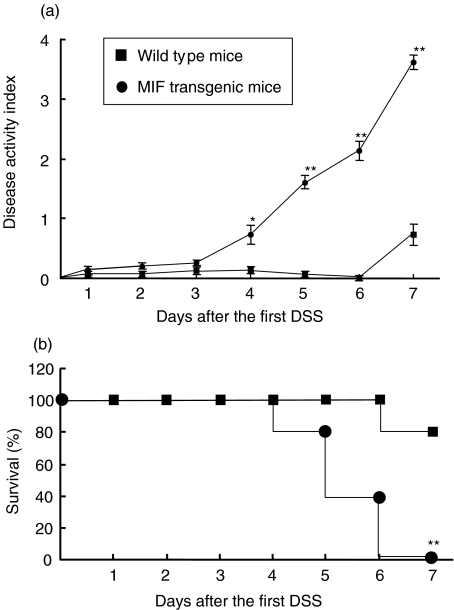
The DAI value in WT mice increased on day 7 (0·73 ± 0·17), and that in MIF Tg mice treated by 1% DSS was significantly higher than that in WT mice on days 4, 5, 6 and 7 (0·73 ± 0·15, 1·60 ± 0·11, 2·13 ± 0·16 and 3·60 ± 0·12, respectively) (Fig. 2a). To examine the survival rate in DSS-induced colitis, mice were fed 4% DSS in drinking water. Eighty per cent of WT mice given 4% DSS survived until day 7. On the other hand, 20% of MIF Tg mice given 4% DSS died on day 4, and all had died by day 7 (Fig. 2b).
Fig. 2.
(a) The disease activity index (DAI) in macrophage migration inhibitory factor (MIF) transgenic (Tg) mice was significantly higher than that in wild-type (WT) mice during the 1% dextran sulphate sodium (DSS) treatment. DAI was assessed daily during the 1% DSS treatment as described in Materials and methods. (b) Survival rate during DSS-induced colitis. Four per cent DSS in the drinking water was given to MIF Tg mice (n = 10) and WT mice (n = 10). Survival was recorded daily. All data are presented as the mean ± s.e. Statistical significance was assessed compared with WT mice on the same day. *P < 0·05, **P < 0·001.
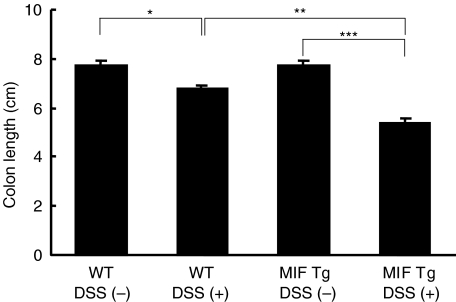
Measurement of colon length has often been used as a morphological parameter for the degree of inflammation in DSS colitis. To assess the colon length in the present study, mice in each group were sacrificed at the indicated times. At day 7 after DSS treatment, we found that the colon length in MIF Tg mice was significantly shorter than that in WT mice (5·4 ± 0·2 and 6·8 ± 0·1, respectively) (Fig. 3).
Fig. 3.
Colon length at 7 days after 1% dextran sulphate sodium (DSS) treatment was significantly shorter in macrophage migration inhibitory factor (MIF) transgenic (Tg) mice than in wild-type (WT) mice. Data are expressed as the mean ± s.e. There were 10 mice in each group. Statistical significance was assessed compared with WT mice. *P < 0·05, **P < 0·01, ***P < 0·001. WT, WT mice; Tg, Tg mice.
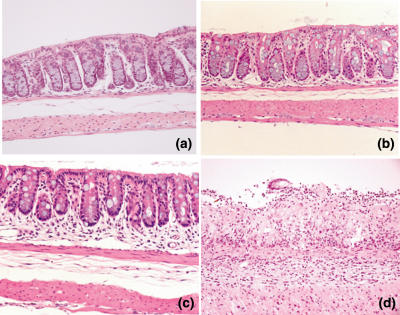
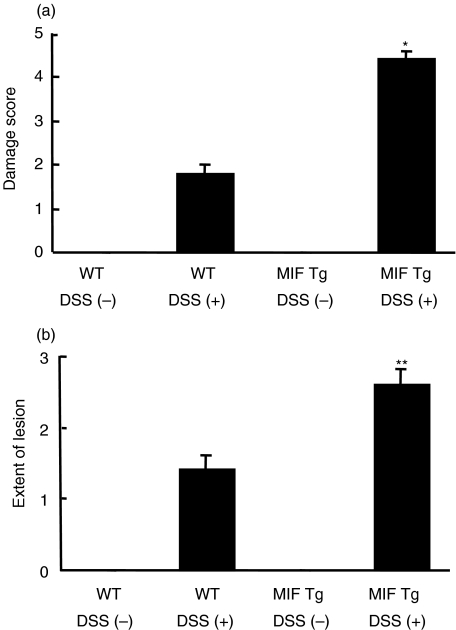
In the histological examination, both the colon samples of Tg mice and those of WT mice were normal (Fig. 4a,b). When mice were fed 1% DSS, the crypts in the mucosa of the colon in MIF Tg mice were shortened to less than half the length of those in WT mice at day 7 (Fig. 4c). The section of colon obtained from MIF Tg mice given 1% DSS for 7 days showed severe inflammatory cell infiltration and destruction of the epithelial cells in the mucosa (Fig. 4d). The scores for both tissue damage and the extent of the lesion were significantly higher in the colons of DSS-treated MIF Tg mice than in the colons of DSS-treated WT mice (Fig. 5).
Fig. 4.
Histology of colon in macrophage migration inhibitory factor (MIF) transgenic (Tg) mice. Sections of the colon from wild-type (WT) mice (a) and MIF Tg mice (b) without dextran sulphate sodium (DSS) treatment show normal crypts and no inflammatory infiltrate. (c) The section of WT mice 7 days after DSS treatment shows shortening of crypts and mild inflammatory cell infiltrate. (d) Section of a MIF Tg mouse at 7 days after DSS treatment shows complete crypt loss, destruction of the epithelial cells and severe inflammatory infiltrate. Magnification × 100.
Fig. 5.
The histological scores for the colons of macrophage migration inhibitory factor (MIF) transgenic (Tg) mice (n = 7) at day 7 after administration of 1·0% dextran sulphate sodium (DSS) were significantly higher than those of wild-type (WT) mice (n = 7). Histological evaluation of the tissue damage (a) and the extent of inflammatory lesions (b) was made using a microscope and quantified by the method described in Materials and methods. Statistical significance was assessed compared with WT mice. *P < 0·05, **P < 0·001.
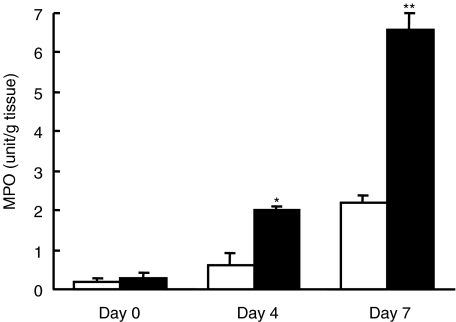
Increase of MPO activity in MIF Tg mice
MPO is an enzyme produced mainly by polymorphonuclear leucocytes and is well associated with the degree of neutrophil infiltration in tissues. The level of MPO activity was not significantly different between MIF Tg mice and WT mice before administration of DSS (0·2 ± 0·1 and 0·3 ± 0·1, respectively). It was significantly higher in MIF Tg mice than in WT mice on days 3 and 7 after administration of DSS (2·0 ± 0·1 versus 0·6 ± 0·3 (P < 0·05) and 6·6 ± 0·4 versus 2·2 ± 0·2 (P < 0·01), respectively) (Fig. 6).
Fig. 6.
The myeloperoxidase (MPO) level in the colon of macrophage migration inhibitory factor (MIF) transgenic (Tg) mice (n = 6) increased at days 3 and 7 after treatment of 1% dextran sulphate sodium (DSS) compared with that of wild-type (WT) mice (n = 6). MPO level was determined as described in Materials and methods. Open box, WT mice; closed box, MIF Tg mice. All data are expressed as the mean ± s.e. Statistical significance was assessed compared with WT mice on the same day. *P < 0·05, **P < 0·01.
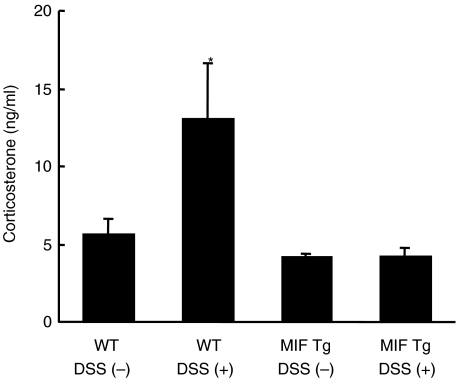
No increase of corticosterone level in the serum from MIF Tg mice with DSS-induced colitis
Corticosterone in mice is analogous to glucocorticoid in humans, and suppresses inflammation and the immune system in a similar manner. In this experiment, the level of corticosterone in the serum was measured by ELISA. The corticosterone level in serum was not significantly different between MIF Tg mice and WT mice prior to DSS treatment (4·1 ± 0·2 and 5·6 ± 1·0, respectively) (Fig. 7). On the other hand, the corticosterone level in WT mice increased on day 7 after DSS treatment (13·0 ± 3·6); however, the corticosterone level in MIF Tg mice remained unchanged (4·2 ± 0·5) (Fig. 7).
Fig. 7.
Corticosterone level in the serum from macrophage migration inhibitory factor (MIF) transgenic (Tg) mice and wild-type (WT) mice. The serum obtained before and 7 days after 1% dextran sulphate sodium (DSS) treatment was measured by enzyme-linked immunosorbent assay (ELISA). Corticosterone was significantly increased in WT mice treated with DSS compared with non-treated WT, non-treated MIF Tg and DSS-treated MIF Tg mice (*P < 0·05). The level of corticosterone in MIF Tg mice did not change between before and 7 days after DSS treatment. No significant differences were observed between non-treated WT, non-treated MIF Tg and DSS treated MIF Tg mice. All data are expressed as the mean ± s.e. There were five mice in each group. WT, WT mice; Tg, Tg mice.
Discussion
In this study, we demonstrated for the first time that colitis could be induced by a very low concentration of DSS in MIF Tg mice. The histological features of inflammation and tissue damage in the colon of MIF Tg mice were remarkably severe compared with those of WT mice. In addition, MPO activity was markedly higher in MIF Tg mice than WT mice, and this increase in MPO activity occurred at a very early stage after DSS treatment. The level of corticosterone significantly increased in the serum from WT mice given DSS, whereas this level showed only minimal change in MIF Tg mice.
It has been suggested that macrophages play a critical in the induction of DSS-induced colitis. Dieleman et al. [28] demonstrated that acute DSS-induced colitis occurred in severe combined immunodeficient (SCID) mice deficient of T and B lymphocytes. Although the histological features of colitis in SCID mice are similar to those of DSS-induced colitis in BALB/c mice, the cells that had infiltrated into the lesions of the SCID mice consisted of mainly macrophages, along with a smaller number of lymphocytes and granulocytes. Okayasu et al. [20] demonstrated that the phagocytes in the mucosa of mice with DSS-induced colitis were mainly macrophages. Similarly, we found that large numbers of phagocytes had infiltrated into the mucosa of mice with DSS-induced colitis in the present study. Onodera et al. [29] reported that MIF could modulate the function of phagocytosis in macrophages. They demonstrated that recombinant MIF enhanced the phagocytosis of foreign particles in the RAW 264·7 cells in a dose-dependent manner. In this study, we showed that DSS-induced colitis in MIF Tg mice was more severe than that in WT mice. This result suggests that MIF has the potential to enhance the phagocytosis of DSS by macrophages in the colon.
Makita et al. [15] reported that MIF modulated neutrophil accumulation in the lungs in an acute pneumonitis rat model. Further, they showed that injection of lipopolysaccharides (LPS) up-regulated MIF expression in both the serum and the lungs. In their model, neutrophil accumulation increased significantly in the lesions of alveoli. Neutralization of MIF by an anti-MIF antibody was effective therapeutically and reduced the alveolar neutrophil accumulation. Baumann et al. [30] reported that MIF played a critical role in the apoptosis of neutrophils in vitro. MIF delayed the apoptosis of cultured neutrophils obtained from the abdominal cavity in mice in a time- and dose-dependent manner, whereas the anti-MIF antibody promoted programmed cell death. This fact suggests that MIF expression contributes to the recruitment of neutrophils at inflammatory loci and promotes acute inflammation. As for MIF Tg mice, we found increased neutrophil accumulation and rapid increase of the activity of MPO, a molecular component of neutrophils, on day 3 after the first DSS treatment. On day 7 after DSS treatment, MPO activity was significantly higher in MIF Tg mice than in WT mice. Consistent with these findings, de Yong et al. [17] reported that chronic colitis induced by transfer of T lymphocytes containing high levels of CD45RB occurred in Rag-2-deficient mice and MIF was produced by innate immune cells.
Sasaki et al. [21] observed podocyte injury and progressive mesangial sclerosis in the kidneys of MIF Tg mice. In their study, MIF was expressed strongly in the mesangial cells and phagocytes of the MIF Tg mice. However, infiltration of macrophages into the kidneys of MIF Tg mice was not significantly greater than that in WT mice. We found here that there were no differences between MIF Tg and WT mice with regard to histological findings of the colon under normal conditions. However, low-dose DSS, which induced only minimal colitis in WT mice, induced severe colitis in MIF Tg mice. The levels of MIF protein in both the serum and colon tissues were significantly increased in MIF Tg mice compared with those in WT mice. Moreover, DSS treatment increased the MIF level in the serum and colon tissue of MIF Tg mice. These results indicate that the increased MIF content produced by the MIF gene transfer contributes to the inflammatory process in DSS-induced colitis.
Corticosterone in mice is analogous to glucocorticoid in humans. Glucocorticoids show critical anti-inflammatory and pro-apoptotic activities within the immune system. The anti-inflammatory action of glucocorticoids originates in the suppression of the expression of proinflammatory genes at the level of DNA transcription. Calandra et al. [8] demonstrated that MIF modulated the secretion and activity of glucocorticoids in vitro. A low concentration of glucocorticoids induced MIF production from macrophages. Moreover, MIF over-rode glucocorticoid-mediated inhibition of cytokine secretion by LPS-stimulated monocytes and overcame glucocorticoid protection against lethal endotoxaemia. Another study [31] reported that exogenous glucocorticoids modulated the expression of MIF in rat tissues after ablation of the hypothalamic-pituitary-adrenal axis. Interestingly, we demonstrated that overexpression of MIF inhibited the level of corticosterone in vivo. The level of corticosterone in the serum from WT mice given DSS for 7 days increased significantly. On the other hand, the level of corticosterone in the serum remained unchanged in MIF Tg mice given DSS. This fact suggests that overexpression of MIF suppresses the up-regulation of corticosterone and progresses the inflammatory process in DSS-induced colitis.
In conclusion, we have shown for the first time that transgene of MIF accelerates inflammation in murine experimental colitis. DSS-induced colitis in MIF Tg mice was clinically and histologically more severe than that in WT mice. MPO activity, which is one of the inflammatory parameters in acute colitis, was remarkably increased in the MIF Tg mice. Moreover, overexpression of MIF inhibited the glucocorticoid response in MIF Tg mice. Our results suggest that MIF is critical for the inflammatory process in DSS-induced colitis. Suppression of MIF bioactivity may have therapeutic implications for the treatment of IBD.
Acknowledgments
The authors thank Somako Tone and Rika Nagashima for their technical assistance. This research was supported by a Grant-in-Aid for research (No.12213008) from the Ministry of Education, Science and Culture of Japan.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:S5–11. [PubMed] [Google Scholar]
- 3.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–98. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 4.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. Crohn's Disease cA2 Study Group. [DOI] [PubMed] [Google Scholar]
- 5.van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 6.Weiser WY, Temple PA, Witec-Giannoti JS, et al. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1989;86:7522–7. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 8.Bucala R. MIF rediscovered: pituitary hormone and glucocorticoid-induced regulator of cytokine production. FASEB J. 1996;7:19–24. doi: 10.1016/1359-6101(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 9.Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bernhagen J, Mitchell RA, et al. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onodera S, Tanji H, Suzuki K, Kaneda K, et al. High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine. 1999;11:163–7. doi: 10.1006/cyto.1998.0402. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly SC, Haslett C, Reid PT, et al. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3:320–3. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 13.Lan HY, Yang N, Nikolic-Paterson DJ, et al. Expression of macrophage migration inhibitory factor in human glomerulonephritis. Kidney Int. 2000;57:499–509. doi: 10.1046/j.1523-1755.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Mikulowska A, Metz CN, Bucala R, et al. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–17. [PubMed] [Google Scholar]
- 15.Makita H, Nishimura M, Miyamoto K, et al. Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharade-induced pulmonary neutrophil accumulation. Am J Respir Crit Care Med. 1998;158:573–9. doi: 10.1164/ajrccm.158.2.9707086. [DOI] [PubMed] [Google Scholar]
- 16.Lan HY, Bacher M, Yang N, et al. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med. 1997;185:1455–65. doi: 10.1084/jem.185.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong YP, Abadia-Molina AC, Satoskar AR, et al. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–6. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawara T, Nishihira J, Takeda H, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–70. doi: 10.1053/gast.2002.34236. [DOI] [PubMed] [Google Scholar]
- 19.Murakami H, Akbar SMF, Matsui H, et al. Macrophage migration inhibitory factor in the sera and at the colonic mucosa in patients with ulcerative colitis: clinical implications and pathogenic significance. Eur J Clin Invest. 2001;31:337–43. doi: 10.1046/j.1365-2362.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- 20.Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, Nishihira J, Ishibashi T, et al. Transgene of MIF induces podocyte injury and progressive mesangial sclerosis in the mouse kidney. Kidney Int. 2004;65:469–81. doi: 10.1111/j.1523-1755.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 22.Ayagi Y, Isaka Y, Akagi A, et al. Transcriptional activation of a hybrid promoter composed of cytomegalovirus enhancer and β-actin/β-globin gene in glomerular epithelial cells in vivo. Kidney Int. 1997;51:1265–9. doi: 10.1038/ki.1997.172. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki J, Takaki S, Araki K, et al. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989;79:269–77. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- 24.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 25.Nishihira J, Koyama Y, Mizue Y. Identification of macrophage migration inhibitory factor (MIF) in human vascular endothelial cells and its induction by lipopolysaccharide. Cytokine. 1998;10:199–205. doi: 10.1006/cyto.1997.0276. [DOI] [PubMed] [Google Scholar]
- 26.Cooper HS, Murthy SNS, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–50. [PubMed] [Google Scholar]
- 27.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 28.Dieleman LA, Ridwan BU, Tennyson GS, et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 29.Onodera S, Suzuki K, Matsuno T, et al. Macrophage migration inhibitory factor induces phagocytosis of foreign particles by macrophages in autocrine and paracrine fashion. Immunology. 1997;92:131–7. doi: 10.1046/j.1365-2567.1997.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumann R, Casaulta C, Simon D, et al. Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J. 2003;17:2221–30. doi: 10.1096/fj.03-0110com. [DOI] [PubMed] [Google Scholar]
- 31.Fingerle-Rowson G, Koch P, Bikoff R, et al. Regulation of macrophage migration inhibitory factor expression by glucocorticoids in vivo. Am J Pathol. 2003;162:47–5. doi: 10.1016/S0002-9440(10)63797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]