Abstract
The interaction between stroma and blood cells in the human spleen has received little attention, despite their well-defined roles during blood cell development in bone marrow. We have reported previously that human spleen-derived fibroblasts display a differentiated myofibroblast phenotype and constitutively express a biologically active form of membrane interleukin (IL)-15 that can drive co-cultured CD34+ blood cells to differentiate into activated natural killer (NK) cells. Here, we show that, in addition to NK cells, CD34/fibroblast co-cultures also yield myeloid CD1a+CD38+CD68+CD86+ HLA-DR+CD14–CD80– dendritic cells (DCs) after 3–4 weeks in culture. We found that DC development depended on endogenously secreted stromal macrophage colony-stimulating factor (M-CSF) and CD40/CD40L interaction rather than on fibroblast- and CD34-derived membrane IL-15. CD1a+ cells were necessary for co-produced NK cells to acquire lytic functions by a mechanism involving cell-to-cell contact and DC-derived IL-12. This study highlights the importance of spleen myofibroblasts in the in vitro generation of two distinct cell types (DC and NK cells) from the innate immune system and suggests that the human spleen is involved in the generation of NK cells from circulating progenitors.
Keywords: blood progenitor, dendritic cell, immune regulation, myofibroblast, natural killer cell
Introduction
Unlike rodent spleens, the human spleen lacks a sinus lining at the extensive interface between the white pulp and red pulp. This lining appears to be replaced by a ring of a peculiar cell type, the myofibroblast. The myofibroblast shows ultrastructural similarities with smooth muscle cells and expresses alpha smooth muscle actin (ASMA) and cytokeratin 8 [1]. These specialized stromal cells are thought to attract lymphocytes and to guide them from the blood into the white pulp [2].
Our previous in vitro studies suggested that the stroma/blood cell interaction plays a more important role than expected in the innate immune system. Indeed, we showed that human spleen-derived fibroblasts, which homogeneously display a differentiated ASMA+ myofibroblast phenotype, specifically express a membrane form of interleukin (IL)-15 able to drive the differentiation of co-cultured unprimed peripheral blood CD34+ cells into mature, activated NK cells [3–5]. Besides its major role in NK cell differentiation [6], IL-15 alone or together with cytokines has been reported to induce the differentiation of cord blood-derived CD34+ cells and circulating monocytes into dendritic cells (DCs) [7–9]. Furthermore, DCs play a co-operative role in NK cell proliferation, maturation and lytic function [10–14]. We used our in vitro co-culture model to determine whether DCs could be generated from blood-derived CD34+ cells when cultured with human splenic IL-15-expressing fibroblasts and whether their presence affects NK cell maturation and activation.
Materials and methods
Reagents
The following antibodies were used: HLA-DR, phycoerythrin (PE)-conjugated CD1a, and CD56, fluorescein isothiocyanate (FITC)-conjugated CD14, and CD83 (Beckman Coulter, Rássy CDG, France); PE-conjugated CD38, CD68, CD80, and CD86 (Immunotools, Friesoythe, Germany); perforin, and FITC-conjugated CD34 (BD Biosciences, Le Pont de Clay, France); CD40L, IL-12 p70, and PE-conjugated IL-15 (R&D Systems, Lille, France); CD94, p30, p44, and p46 (kindly provided by Dr A. Moretta, Gaslini Institute, Genoa, Italy); α-smooth muscle actin (ASMA), and cytokeratin (CK) 8 (Sigma, Saint Quentin Fallavier, France). Recombinant (rh) macrophage colony-stimulating factor (M-CSF), rhIL-15 and enzyme-linked immunosorbent assay (ELISA) kits for CD40L, IL-12 and macrophage colony-stimulating factor (M-CSF) were purchased from R&D Systems. RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) was purchased from Invitrogen, Cergy Pontoise, France.
Cell culture
Fibroblasts were isolated from postmortem human spleens (French Transplant Organization, Hospital Kremlin-Bicêtre, France), as described previously [15], and subcultured at a 1 : 2 split ratio for up to three passages before experiments. The fibroblastic nature of the cultures was ascertained by the absence of endothelial (CD31) and macrophagic (CD11b) marker expression. CD34+ cells were isolated and purified from peripheral blood mononuclear cells after Ficoll-plaqueTM gradient centrifugation, overnight culture followed by immunomagnetic selection and passage through varioMACS columns (Miltenyi Biotec, Auburn, CA, USA) as described previously [4,5,15]. Fibroblasts grown to confluence in 6-well or 0·4-µm pore size transwell plates (Costar, ATGC Biotechnologies, Noisy-le-Grand, France) were incubated with purified CD34+ cells (3 × 104) in culture medium with no exogenous cytokine added. When necessary, rhIL-15 (50 ng/ml), rhM-CSF (100 ng/ml), neutralizing monoclonal antibodies to CD40L (5 µg/ml), IL-12 (10 µg/ml), IL-15 (0·1 µg/ml) or M-CSF (10 µg/ml) were added to the cultures at the start and in the half-medium changes performed every other week.
Flow cytometry analysis
For flow cytometry, cells were stained with various antibodies according to conventional direct and indirect techniques. For perforin expression, cells were first stained with a PE-conjugated anti-CD56 antibody. Cells were then fixed and permeabilized by treatment with ORTHOPermeafix (Ortho Diagnostic Systems, Roissy, France) for 45 min at room temperature. Intracellular staining was performed by adding control FITC-conjugated IgGs and antiperforin antibody. For IL-12p70 expression, cells were first stained with a PE-conjugated anti-CD1a antibody, fixed and permeabilized in 0·05% saponin/phosphate buffered saline (PBS) for 10 min at 4°C. Intracellular staining was performed by adding control FITC-conjugated IgGs and anti-IL-12 p70 antibody (clone 24910, R&D Systems), which specifically recognizes the secreted form of IL-12. After 30 min, cells were rinsed with the saponin/PBS solution and rapidly analysed by flow cytometry. All cell analyses were performed on a FACScanTM flow cytometer (BD Biosciences) using the CellQuestTM (BD Biosciences) and WinMDI programs (Scripps Research Institute, La Jolla, CA, USA).
Results
Blood CD34+ cells cultured with human spleen-derived fibroblasts yield CD1a+ DCs
Highly purified (>98%) peripheral unprimed blood-derived CD34+ cells were cultured for 3 and 4 weeks with fibroblasts positive for ASMA, CK8 and membrane IL-15 (data not shown). Immunophenotyping of the non-adherent cell population revealed the presence of a few (<5%) CD1a+ and CD14+ cells after 3 weeks in culture (not shown). A number (12·5% ± 6·4%) of CD1a+ cells were detected consistently in the various CD34+ cell populations tested. Dual flow cytometry analysis showed that a fraction of CD1a+ cells (∼30%) co-expressed CD14 or HLA-DR but not CD83 (Fig. 1a). This is consistent with an immature myeloid DC phenotype [16]. In addition, up to 40% of non-adherent CD56+ cells were also recovered from the culture supernatants. Moreover, 45–80% of the CD56+ cell population coexpressed mature NK cell markers (Fig. 2), including CD94, p46, p44 and p30 as well as the lytic mediator perforin (≥20% of the CD56+ cells), consistent with an activated, mature phenotype. Perforin expression is a reliable activation marker for this NK cell type, which has been shown not to release interferon (IFN)-γ[5].
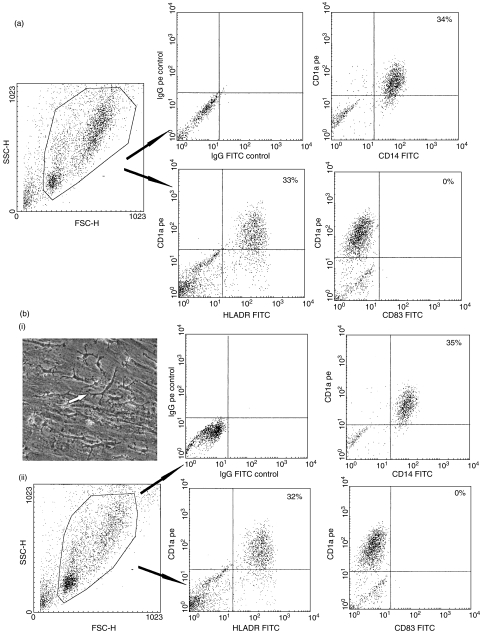
Fig. 1.
Production of myeloid CD1a+ dendritic cells (DCs) in cultures of peripheral blood CD34+ cells with human spleen-derived fibroblasts. CD34+ cells were cultured with fibroblasts without any exogenous cytokines for 3 weeks. Non-adherent and adherent cells were harvested, and analysed by flow cytometry after staining with indicated antigens. (a) Analysis of the FSC/SSC size profile and expression of DC and control antigens in non-adherent cells. (b) Morphology and phenotype of adherent monolayer-derived cells. (i) Morphology of the macrophage-like cells (arrow), developing onto the fibroblast monolayer observed under inverted phase-contrast microscope (original magnification × 100). (ii) Analysis of the forward scatter (FSC)/side scatter (SSC) size profile and expression of DC and control antigens. Results are representative of more than three independent experiments.
Fig. 2.
Phenotype of natural killer (NK) cells generated from blood CD34+ cells cultured with splenic fibroblasts. Non-adherent cells from 3-week-old cultures were harvested, and analysed by flow cytometry after staining with indicated mature NK cell markers. Results are representative of more than three independent experiments.
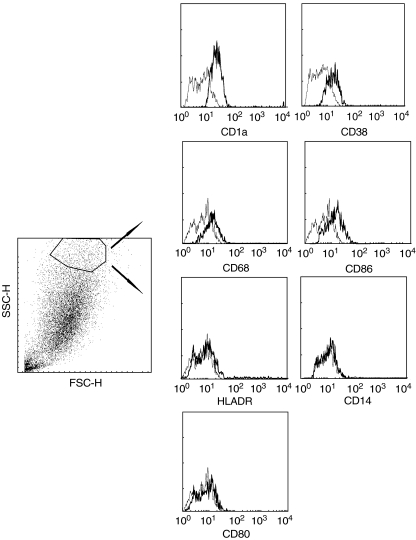
In addition to non-adherent haematopoietic cells, numerous adherent cells with macrophage-like morphology were seen to develop progressively on the fibroblast monolayer (Fig. 1b). After 3 weeks in culture, flow cytometry showed that ∼35% of the cells from the monolayer expressed CD1a. Dual flow cytometry revealed that, like the non-adherent cells, adherent CD1a+ cells coexpressed CD14, and HLA-DR but not CD83 (Fig. 1b). With longer culture periods (4 weeks), low levels of CD38, CD68, CD86, HLA-DR and lack of CD14 or CD80 expression could be detected, suggestive of a more mature DC phenotype (Fig. 3). No DC marker expression was detected in control fibroblasts under the same culture conditions but without the presence of CD34+ cells (data not shown).
Fig. 3.
Phenotype of adherent monolayer-derived CD1a+ dendritic cells (DCs) generated from blood CD34+ cells cultured with splenic fibroblasts. Adherent cells from 4-week-old cultures were harvested, and analysed by flow cytometry after staining with indicated antigens. Dark-lined histograms show antigen expression after electronic gating on CD1a-positive cells. Light-lined histograms represent staining with an isotope control antibody.
Expansion of CD1a+ cells is independent of IL-15
To determine whether the IL-15 exposed on the surface of splenic fibroblasts plays a role in DC development, we added neutralizing anti-IL-15 antibody to the cultures for 3 weeks, and monitored the cells with the myeloid DC phenotype on the basis of CD1a expression. The total (non-adherent and adherent) number of CD1a+ cells was similar in anti-IL-15-treated cultures and controls (Fig. 4a). However, when CD34+ cells were cultured in transwells to prevent direct contact with fibroblasts, CD1a+ cells were found only in the insert, but in lower numbers than in standard conditions (14·5% ± 2·1%versus 29·5% ± 6·4% CD1a+ cells, respectively). Addition of exogenous rhIL-15 (50 ng/ml) to the transwell cultures did not increase CD1a+ cell numbers. No CD56+ cells were detected in these culture conditions (data not shown).
Fig. 4.
Implication of stromal macrophage colony-stimulating factor (M-CSF) and CD40L in the production of CD1a+ cells from blood CD34+ cells cultured with splenic fibroblasts. (a) Percentage of CD1a+ cells recovered from the cell monolayer after treatment with indicated neutralizing antibodies for 3 weeks. In the case of transwell cultures, values represent the total percentage of adherent and non-adherent CD1a+ cells. (b) Photographic images of cellular aggregates (arrow, original magnification × 100) and surviving cell numbers in cultures of CD34+ cells with rhM-CSF (100 ng/ml), neutralizing anti-M-CSF (10 µg/ml) and non-reactive antibodies (10 µg/ml) for 3 weeks. (c) Constitutive expression of CD40L in living splenic fibroblasts analysed by flow cytometry. Results are representative of at least two independent experiments. The dark line histogram indicates antigen expression. The light line histogram represents staining with an isotype control antibody.
We have shown previously that peripheral blood CD34+ cells also express a membrane form of IL-15 that is necessary for the full differentiation of NK progenitors in response to stromal IL-15 [15]. Indeed, fewer CD56+ cells were produced when CD34+ cells were incubated for 4 h with neutralizing anti-IL-15 antibody before culturing with splenic fibroblasts than when cells were untreated (6%versus 13% CD56+ cells, respectively). Pretreatment with anti-IL-15 antibody did not, however, affect the number of CD1a+ cells (Fig. 4a). Thus, unlike NK cells, the expansion of CD1a+ cells does not require fibroblast- and CD34+-derived membrane IL-15 and is more efficient when CD34+ cells and fibroblasts are in direct contact.
M-CSF is required for CD1a+ cell expansion
The haematopoietic growth factor M-CSF can efficiently stimulate CD14+-derived DC precursors to differentiate into macrophage-like cells [17]. As reported previously [3], splenic fibroblasts constitutively produced abundant amounts of M-CSF with optimal values of 2·7 ± 0·2 ng/ml by week 1, a level that remained unchanged under co-culture conditions (data not shown). Addition of neutralizing anti-MCSF antibody to CD34/fibroblast cultures for 3 weeks blocked CD1a+ cell development (<3% recovered cells; Fig. 4a). Contrastingly, CD56+ cell production was not affected, and no intracellular perforin expression was detected in these cells (data not shown). When blood-derived CD34+ cells were cultured without fibroblasts in the presence of 100 ng/ml of rhM-CSF for 3 weeks, a few cells survived and formed aggregates (Fig. 4b). The number of surviving cells was six-fold lower than in standard culture conditions (3·2 × 103versus 18·0 × 103 cells, respectively). No surviving cells were recovered after treatment with rhM-CSF plus neutralizing anti-M-CSF antibody, whereas the number of surviving cells was not affected by non-reactive antibody.
Role of cell-to-cell contact in CD1a+ expansion
The fact that CD1a+ cells developed less well in transwell culture conditions and with rhM-CSF alone prompted us to investigate the effect of direct contact between CD34+ progenitors and fibroblasts. We have reported previously that human fibroblasts from various tissue sources, including the spleen, consistently expressed CD40 [18]. Although undetected in formalin-fixed cells and culture supernatants, flow cytometry detected CD40L on the surface of living fibroblasts (Fig. 4c). Addition of neutralizing anti-CD40L antibody to the culture for 3 weeks abrogated CD1a+ cell development without affecting CD56+ cell production (Fig. 4a). No perforin-expressing cells were detected in the CD56+ cell population (data not shown).
Role of adherent CD1a+ cells in the activation of co-produced NK cells
As culture conditions that prevented CD1a+ cell development consistently yielded mature NK cells lacking intracellular perforin expression, we investigated the role of CD1a+ cells in CD56+ cell activation. The DC-derived cytokine IL-12 has been shown to stimulate the lytic functions of resting NK cells via the two lytic mediators, perforin and granzyme [19]. Double cell staining revealed the presence of intracellular IL-12 p70 in CD1a+ cells, suggestive of IL-12 secretion (Fig. 5a). Addition of neutralizing anti-IL-12 to the culture for 3 weeks did not alter CD1a+ and CD56+ cell numbers (Fig. 5b). However, no perforin-expressing CD56+ cells were detected, whereas ≥ 20% of untreated cells was CD56+ (data not shown).
Fig. 5.
Lack of perforin expression in CD56+ cells generated from blood CD34+ cells cultured with splenic fibroblasts and anti-interleukin (IL)-12 antibody. CD34+ cells were cultured for 3 weeks with splenic fibroblasts in the presence or absence of neutralizing anti-IL-12 antibody. Non-adherent and adherent cells were harvested and analysed by flow cytometry after staining with CD56-PE, perforin and IgG-FITC, and CD1a-PE, IL-12p70 and IgG-FITC, respectively. (a) Histograms of intracellular expression of IL-12 p70 (dark line) in adherent monolayer-derived cells after electronic gating on PE-positive cells. The light line histogram represents staining with an antibody control. (b) Percentage of adherent CD1a-positive cells and non-adherent cells positive for CD56 and perforin in controls (blank histograms), and cultures treated with neutralizing anti-IL-12 antibody (shaded histograms). Results are representative of two independent experiments.
Discussion
DCs are found commonly as precursor populations from myeloid and lymphoid lineages in bone marrow and blood, and as more mature forms in tissues of non-lymphoid origin as well as in the T cell zones of lymphoid organs [20]. We used an in vitro model consisting of spleen-derived ASMA+ myofibroblasts to show that a population of adherent immature myeloid CD1a+ DCs could be generated progressively from co-cultured peripheral blood CD34+ progenitors. DCs in the human spleen are mainly immature CD11c+CD14–HLA-DR+ cells and localize in the T and B cell zones as well as in the marginal zone, although CD1a+ DCs have also been described in other reports [21,22]. In marginal zones, they formed concentric rings around the white pulp with the first line of red pulp CD14+ macrophages. This is similar to the distribution of the ASMA+CK8+ myofibroblasts that replace the missing sinus lining of the human spleen [1].
A peculiar feature of spleen-derived ASMA+ myofibroblasts was the highly specific expression of a bioactive membrane IL-15 that specifically stimulated the generation of mature CD56+CD3–NK cells from blood CD34+ cells [5]. Expansion of CD1a+ cells, unlike that of NK cells, depends on fibroblast-derived M-CSF rather than on IL-15, as shown by their near complete disappearance in the presence of neutralizing antibodies to M-CSF but not IL-15. However, similarly to NK cells, the expansion of CD1a+ cells required intimate contact with fibroblasts. This probably occurred through CD40–CD40L interactions, as indicated by the abrogation of CD1a+ cell expansion in the presence of neutralizing anti-CD40L antibodies.
We found that the spontaneous activation of co-produced NK cells required the presence of CD1a+ cells, consistent with DC playing a co-operative role in NK cell functions [11–13]. Indeed, all culture conditions that suppressed CD1a+ cell expansion without affecting the formation of mature CD56+ NK cells were associated with lack of intracellular perforin expression, a marker of lytic potentialities. This activation process appeared to be mediated by DC-derived signals such as IL-12 and most probably required direct cell-to-cell contacts. It is interesting that IL-12 production, similarly to CD1a+ cell expansion, has been found to depend on CD40/CD40L interactions [14].
A limited number of human fibroblasts from other tissue sources have been reported to induce myelopoiesis through the production of haematopoietic factors and cell-to-cell contact. However, each differentiation event is highly specific and strictly dependent on the type of fibroblasts involved and the repertoire of haematopoietic factors produced [3,4,23–27]. It has been recently reported that cutaneous fibroblasts can generate CD1a+ DCs from cord blood CD34+ cells through M-CSF production and cell-to-cell contact [27]. In this model, however, the cross-talk between blood progenitors and fibroblasts only generated immature CD1a+ DCs that required tumour necrosis factor (TNF)-α to mature. These data highlight the specific functions and unique properties of spleen-derived fibroblasts, which generate mature NK cells that require myeloid DCs to be activated.
Recent in vitro evidence emphasizes the importance of NK/DC interactions for NK cell activation, DC maturation and their immunoregulatory cross-talk [28]. However, important questions about the physiological site of NK/DC cross-talk in vivo (peripheral inflammatory and tumoral sites versus secondary lymphoid tissues) remain unanswered. Although our in vitro model cannot reproduce all aspects of the in vivo situation, it nevertheless underlines the potential role of spleen-derived fibroblasts in the dynamic process of NK/DC development within the human spleen. Thus in this organ, fibroblasts, with their unusual microanatomic localization and differentiated myofibroblastic phenotype, can be viewed not only as a guide for lymphocyte trafficking but also as effector cells in the regulation of innate immune cell homeostasis.
Acknowledgments
We thank Dr J.-J. Lataillade and the staff from Hôpital Kremlin-Bicêtre for supplying us with normal blood and spleen tissue samples. This work was supported by grants from the Association Nouvelle Recherche Biomédicale. D.B. was the recipient of a fellowship from the Association de Recherche contre le Cancer.
References
- 1.Steiniger B, Barth P. Microanatomy and function of the spleen. Adv Anat Embryo Cell Biol. 2000;151:1–01. doi: 10.1007/978-3-642-57088-9. [DOI] [PubMed] [Google Scholar]
- 2.Steiniger B, Barth P, Hellinger A. The perifollicular and marginal zones of the human splenic white pulp. Do fibroblasts guide lymphocyte immigration? Am J Pathol. 2001;159:501–12. doi: 10.1016/S0002-9440(10)61722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouty-Boyé D, Doucet C, Clay C, Le Bousse-Kerdilès MC, Lampidis TJ, Azzarone B. Phenotypic diversity in human fibroblasts from myelometaplasic and non-myelometaplasic hematopoietic tissues. Int J Cancer. 1998;76:767–73. doi: 10.1002/(sici)1097-0215(19980529)76:5<767::aid-ijc24>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Brouty-Boyé D, Briard D, Azzarone B, et al. Effects of human fibroblasts from myelometaplasic and non-myelometaplasic hematopoietic tissues on CD34+ stem cells. Int J Cancer. 2001;92:484–7. doi: 10.1002/ijc.1222. [DOI] [PubMed] [Google Scholar]
- 5.Briard D, Brouty-Boyé D, Azzarone B, Jasmin C. Fibroblasts from human spleen regulate NK cell differentiation from blood CD34+ progenitors via cell surface IL-15. J Immunol. 2002;168:4326–32. doi: 10.4049/jimmunol.168.9.4326. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human diseases. Blood. 2001;97:97–109. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 7.Bykovskaia SN, Buffo M, Zhang H, et al. The generation of human dendritic and NK cells from hemopoietic progenitors induced by interleukin-15. J Leuk Biol. 1999;66:659–66. doi: 10.1002/jlb.66.4.659. [DOI] [PubMed] [Google Scholar]
- 8.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;16:1179–87. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 9.Mohamadzadeh M, Berard F, Essert G, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–9. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions. Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 11.Amakata Y, Fujiyama Y, Andoh A, Hodohara K, Bamba T. Mechanism of NK cell activation induced by co-culture with dendritic cells derived from peripheral blood monocytes. Clin Exp Immunol. 2001;124:214–22. doi: 10.1046/j.1365-2249.2001.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–81. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 15.Briard D, Brouty-Boyé D, Giron-Michel J, Azzarone B, Jasmin C, Le Bousse-Kerdilès MC. Impaired NK cell differentiation of blood-derived CD34+ progenitors from patients with myeloid metaplasia with myelofibrosis. J Clin Immunol. 2003;106:201–12. doi: 10.1016/s1521-6616(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 16.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamps AW, Hendriks D, Smit JW, Vellenga E. Role of macrophage colony stimulating factor in the differentiation and expansion of monocytes and dendritic cells from CD34+ progenitor cells. Med Oncol. 1999;16:46–2. doi: 10.1007/BF02787358. [DOI] [PubMed] [Google Scholar]
- 18.Brouty-Boyé D, Pottin-Clémenceau C, Doucet C, Jasmin C, Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000;30:914–9. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Lehman C, Zeis M, Uharek L. Activation of natural killer cells with interleukin 2 (IL-2) and IL-12 increases perforin binding and subsequent lysis of tumour cells. Br J Haematol. 2001;114:660–5. doi: 10.1046/j.1365-2141.2001.02995.x. [DOI] [PubMed] [Google Scholar]
- 20.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 21.McIlroy D, Troadec C, Grassi F, et al. Investigation of human spleen dendritic cell phenotype and distribution reveals evidence of in vivo activation in a subset of organ donors. Immunobiol. 2001;97:3470–7. doi: 10.1182/blood.v97.11.3470. [DOI] [PubMed] [Google Scholar]
- 22.Buckley PJ. Phenotypic subpopulation of macrophages and dendritic cells in human spleen. Scan Microsc. 1991;5:147–57. [PubMed] [Google Scholar]
- 23.Barak Y, Karov Y, Levin S, Barash A, Ben-Hur H, Lancet M. Regulation of in vitro granulopoiesis by human fetal liver stromal cells. Prog Clin Biol Res. 1985;193:157–65. [PubMed] [Google Scholar]
- 24.Slaper-Cortenbach I, Ploemacher R, Lowenberg B. Different stimulative effects of human bone marrow and fetal liver stromal cells on erythropoiesis in long-term culture. Blood. 1987;69:135–9. [PubMed] [Google Scholar]
- 25.Yamamoto R, Lin LS, Lowe R, Warren MK, White TJ. The human lung fibroblast cell line, MRC-5, produces multiple factors involved with megakaryocytopoiesis. J Immunol. 1990;144:1808–16. [PubMed] [Google Scholar]
- 26.Eckardt KU. Erythropoietin production in liver and kidneys. Curr Opin Nephrol Hypertens. 1996;5:28–4. doi: 10.1097/00041552-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Mollah ZU, Setsuya A, Manome H, Yoshimo Y, Tagami H. Cord blood CD34+ cells differentiate into dendritic cells in co-culture with cutaneous fibroblasts or stromal cells. J Invest Dermatol. 2002;118:450–60. doi: 10.1046/j.0022-202x.2001.01692.x. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–2. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]