Abstract
This study was designed to investigate the relationship between influx of extracellular Ca2+, activation of NFκB and synthesis of interleukin-8 (IL-8) following exposure of human neutrophils to subcytolytic concentrations (8·37 and 41·75 ng/ml) of the pneumococcal toxin, pneumolysin, as well as the potential of the omega-3 polyunsaturated fatty acid, docosahexaenoic acid, to antagonize these events. Activation and translocation of NFκB were measured using a radiometric electrophoretic mobility shift assay, while influx of extracellular Ca2+ and synthesis of IL-8 were determined using a radioassay and an ELISA procedure, respectively. Exposure of neutrophils to pneumolysin was accompanied by influx of Ca2+, activation of NFκB, and synthesis of IL-8, all of which were eliminated by inclusion of the Ca2+-chelating agent, EGTA (10 m m), in the cell-suspending medium, as well as by pretreatment of the cells with docosahexaenoic acid (5 and 10 µg/ml). The antagonistic effects of docosahexaenoic acid on these pro-inflammatory interactions of pneumolysin with neutrophils were not attributable to inactivation of the toxin, and required the continuous presence of the fatty acid. These observations demonstrate that activation of NFκB and synthesis of IL-8, following exposure of neutrophils to pneumolysin are dependent on toxin-mediated influx of Ca2+ and that these potentially harmful activities of the toxin are antagonized by docosahexaenoic acid.
Keywords: calcium, docosahexaenoic acid, neutrophils, nuclear factor kappa B (NFκB), pneumolysin
Introduction
Streptococcus pneumoniae (pneumococcus) remains one of the major human pathogens and one of the most common causes of community-acquired pneumonia, otitis media, sinusitis and meningitis. Notwithstanding the threat posed by emerging antibiotic resistance and human immunodeficiency virus, the mortality rate among those patients with acute pneumococcal disease who receive appropriate antimicrobial chemotherapy remains unacceptably high. Better understanding of the immunopathogenesis of infections caused by the pneumococcus may lead to additional options for treatment and prevention.
We have previously reported that exposure of human neutrophils to pneumolysin, one of the best-characterized virulence factors produced by the pneumococcus, results in activation of synthesis of interleukin-8 (IL-8), which is secondary to toxin-mediated influx of Ca2+[1,2]. IL-8 in turn, not only amplifies neutrophil recruitment and activation, but also confers resistance to the pro-apoptotic actions of corticosteroids on these cells [3]. However, rather than contributing to eradication of the infection, pneumolysin-mediated potentiation of neutrophil influx and activation in a murine model of experimental pneumococcal infection of the airways was found to favour persistence and extrapulmonary dissemination of the pneumococcus [4], possibly as a consequence of inflammation-mediated damage to airway epithelium [5].
Pneumolysin has been proposed to represent a possible target for adjunctive therapy to antibiotics in patients with acute pneumococcal infection [6]. Notwithstanding antimicrobial agents which inhibit synthesis of the toxin, potential strategies include toxin-targeted monoclonal antibodies [7], or pharmacological agents which antagonize the interactions of the toxin with eukaryotic cells and/or suppress the inflammatory processes activated by it. One such group of agents is the omega-3 polyunsaturated fatty acids, which have been reported to possess beneficial immunomodulatory and anti-inflammatory properties in acute and chronic inflammatory disorders of both infective and noninfective origin [8–10], and to attenuate the pro-inflammatory interactions of Escherichia coli haemolysin with rabbit macrophages [11]. In agreement with these observations, we have recently reported that these agents antagonize the pro-oxidative interactions of pneumolysin with human neutrophils by interfering with Ca2+ influx [12]. Interestingly, omega-3 polyunsaturated fatty acids have also been reported to exclude proteins from the lipid rafts of eukaryotic cell membranes [13]. Lipid rafts are putative binding sites for perfringolysin O, the cholesterol-binding, pore-forming toxin of Clostridium perfringens[14], as well as for the beta toxin of this microbial pathogen [15]. Pneumolysin is also a cholesterol-binding toxin and shares a high degree of homology with perfringolysin O [16].
Nevertheless, the development of pneumolysin-directed chemotherapeutic strategies clearly requires additional insights into the mechanisms, which underpin both the pro-inflammatory activities of the toxin and the anti-inflammatory actions of omega-3 polyunsaturated fatty acids. In the current study, we have investigated the involvement of the transcription factor, nuclear factor kappa B (NFκB) in the Ca2+-dependent activation of synthesis of IL-8 by pneumolysin-exposed human neutrophils, as well as the potential of the omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA), to modulate these pro-inflammatory processes.
Materials and methods
Chemicals and reagents
Unless otherwise indicated, all chemicals and reagents were obtained from the Sigma Chemical Co. (St Louis, MO, USA).
Neutrophils
These were prepared from the heparinized venous blood of healthy adult human volunteers and were separated from mononuclear leucocytes by centrifugation on Histopaque-1077 (Sigma Diagnostics) cushions at 400 g for 25 min at room temperature as described elsewhere [1]. The neutrophils were routinely of high purity (>90%) and viability (>95%).
Recombinant pneumolysin
Recombinant pneumolysin was expressed in Escherichia coli and was purified from cell extracts as described previously [17]. Protein homogeneity was confirmed by SDS-PAGE. The stock concentration was 80 µg/ml, which corresponds to 3·2 × 105 haemolytic units/ml, and was essentially free of contaminating bacterial endotoxin (<2 pg/ml). The toxin was diluted in endotoxin-free RPMI 1640 tissue culture medium or Hanks’ balanced salt solution (HBSS; pH 7·4; 1·25 m m CaCl2; indicator-free; Highveld Biological, Johannesburg, South Africa) and was used at fixed, final concentrations of 8·37 and 41·75 ng/ml which are well within the range (0·85–180 ng/ml) of those reported to occur in the cerebrospinal fluid of patients with pneumococcal meningitis [18]. We have previously found that pneumolysin at these concentrations causes influx of Ca2+ into neutrophils and activates synthesis of IL-8 by these cells in the absence of cytolysis [1,2].
Construction of N-terminal green fluorescent protein (GFP)-pneumolysin (GFP-Ply) fusion plasmid and expression and purification of eGFP-Ply
A green fluorescent protein (GFP)/pneumolysin construct was used to measure the binding of the toxin to neutrophils as described below.
The coding sequence of pneumolysin was amplified with the introduction of appropriate restriction sites by PCR using primers PlyPetFwd (CCG GAT CCG GCA AAT AAA GCA GTA AAT GAC TTT; BamH1 site underlined) and PlyPetRev (GAC GGA GCT CGA CTA GTC ATT TTC TAC CTT ATC; Sac1 site underlined). The PCR product was ligated into BamHI/SacI digested pET33b (Novagen, Madison, WI, USA) and transformed into TOP10 E. coli. The presence of a correctly sized insert in pET33b was confirmed by BamHI/SacI digestion followed by agarose gel electrophoresis.
The GFP coding sequence was amplified from pNF320 [19] and appropriate restriction sites introduced by PCR using primers GFPpET33bFwd (GT CAG GCT AGC ATG AGT AAA GGA GAA GAA C; Nhe1 site underlined) and GFPpET33bRev (CC ACG CAG ATC TTT GTA TAG TTC ATC C; BglII site underlined). The PCR product was cut with NheI and BglII, ligated into NheI/BamHI digested pET33bPLY and transformed into TOP10 E. coli. The plasmid was recovered and mutations F64L and S65T [20] were introduced into GFP by site directed mutagenesis (Quikchange SDM Kit, Stratagene, La Jolla, CA, USA) using primers GFP-S65T-F64LmutaFWD (CAC TTG TCA CTA CTC TGA CTT ATG GTG TTC AAT GC) and GFP-S65T-F64LmutaREV (GCA TTG AAC ACC ATA AGT CAG AGT AGT GAC AAG TG). The sequence was confirmed and the plasmid was transformed into BL21 (DE3) E. coli (Stratagene, La Jolla, CA, USA).
Recombinant eGFP-Ply was expressed in terrific broth by IPTG induction. Cells were disrupted using a French Press and resuspended in PBS. Crude supernatants were purified by nickel affinity chromatography and eluted on 0–300 m m imidazole concentration gradient. Fractions containing purified eGFP-Ply were dialysed against a greater than 50-fold volume of PBS three times at 4°C.
With respect to haemolytic activity, the construct and the free protein have equivalent specific activities on a molar basis. The construct also competes with the wild type protein for binding to nucleated cells i.e GFP/fusion binding is reduced by mixing with unlabelled toxin and molar ratios suggest binding is similar for both forms of the toxin. We have not measured complement activation by the construct.
NFκB activation
For these investigations, neutrophils were suspended in RPMI 1640 tissue culture medium supplemented with 0·5% human serum albumin (HSA). Following 10 min of preincubation at 37°C, pneumolysin (8·37 and 41·75 ng/ml) or an equal volume of RPMI 1640 (control systems) was added to the cells which were then incubated for 15 or 30 min at 37°C. The final volume in each tube was 1 ml containing 5 × 106 cells. Following incubation, detection of NFκB nuclear translocation was determined as described previously [21], with slight modifications. Briefly, cells were harvested and resuspended in 0·4 ml buffer (10 m m HEPES/10 m m KCl/2 m m MgCl2/1 m m DTT/0·1 m m EDTA/0·2 m m NaF/0·2 m m Na3VO4) supplemented with the protease inhibitors 1 mg/l leupeptin and 0·4 m m PMSF. After 15 min on ice, 25 µl 10% Igepal CA-630 was added and the cells vortexed for 15 s and pelleted by centrifugation. Pellets containing the nuclear proteins were resuspended in buffer (50 m m HEPES/50 m m KCl/300 m m NaCl/0·1 m m EDTA/1 m m DTT 10% glycerol/0·2 m m NaF/0·2 m m Na3VO4) supplemented with 0·1 m m PMSF and incubated on ice on a rotating platform for 20 min. After centrifugation for 5 min at 4°C, supernatants were collected and protein determinations performed.
For the electrophoretic mobility shift assay (EMSA), 7 µg of nuclear extract protein was incubated with 32P-radiolabelled NFκB-specific oligonucleotide (Amersham Biosciences UK Ltd, Amersham, UK) for 20 min at room temperature. Binding of NFκB nuclear proteins to the oligonucleotide results in a retardation (‘shift’) of the electromobility on a 5% nondenaturating polyacrylamide gel. These shifts were visualized by phosphor-imaging using the Personal Molecular Imager® FX and software from BIO-RAD Laboratories, Inc. Specificity of NFκB DNA binding was ascertained by competition with excess unlabelled olignucleotides, resulting in disappearance of NFκB complexes, and results are shown as either the mean percentage counts/mm2 of the pneumolysin-free control system, or as the complete phosphor-images for representative experiments.
Additional experiments were performed to investigate the effects of the following on pneumolysin-mediated activation of NFκB in neutrophils: (i) inclusion of the extracellular Ca2+-chelating agent EGTA (10 m m, final) in the cell-suspending medium; (ii) the effects of pretreatment of the cells for 5 min with docosahexaenoic acid (DHA, 5 and 10 µg/ml, final), or with diphenyleneiodonium chloride (10 µm, final), an inhibitor of the activity of the phagocyte NADPH oxidase [22].
Interleukin-8
Neutrophils were preincubated for 10 min at 37°C with and without DHA (5 and 10 µg/ml) in HSA (0·5%) supplemented RPMI 1640, followed by the addition of a fixed concentration of 8·37 ng/ml, pneumolysin or an equal volume of RPMI 1640 to control systems. This is the concentration of pneumolysin which we have previously found to cause maximal synthesis of IL-8 by neutrophils [2]. The tubes, containing 2 × 106 cells in a final volume of 1 ml, were then incubated for 6 h at 37°C. Following removal of cells by centrifugation, total IL-8 was assayed in the supernatants by an antibody-capture ELISA procedure (Roche Diagnostics GmbH, Mannheim, Germany).
Calcium influx
Neutrophils which had been preincubated for 10–15 min at 37°C in Ca2+-replete HBSS, to achieve filling of intracellular stores, were washed and transferred to HBSS containing 100 µm CaCl2. After 9 min of incubation at 37°C, 2 µCi of 45Ca2+ (calcium-45 chloride, specific activity 13·27 mCi/mg; Perkin Elmer Life Sciences, Boston, USA) was added to the cells, followed by pneumolysin at a fixed, final concentration of 8·37 ng/ml. The tubes which contained 107 neutrophils in a total volume of 5 ml HBSS were incubated for a further 5 min at 37°C, after which the reactions were stopped and the cells washed twice with ice-cold PBS. The cell pellets were then lysed and the radioactivity in the lysates determined by liquid scintillation spectrometry. The effects of pretreatment of the cells with DHA (5 µg/ml) or EGTA (10 m m) on pneumolysin-mediated Ca2+ influx were also determined.
In an additional series of experiments designed to investigate possible direct, inactivating effects of DHA on pneumolysin, the toxin (4·137 µg/ml) was mixed with DHA (50 µg/ml) for 5 min at 37°C in a final volume of 100 µl HBSS. This was followed by dilution (1 : 500) and assessment of the influx of Ca2+ following treatment of neutrophils with DHA-treated or -untreated toxin. The final concentration of pneumolysin was 8·37 ng/ml, while that of DHA was 0·1 µg/ml, which was without effect in the assay system.
To assess the requirement for continuous exposure of neutrophils to DHA to achieve antagonism of Ca2+ influx, neutrophils (2 × 106/ml) in HBSS were incubated with the fatty acid (5 µg/ml) for 10 min at 37°C followed by addition of bovine serum albumin (BSA, 5 mg/ml final) or an equal volume of HBSS to control cells followed by washing of the cells (twice). The cells were resuspended in HBSS (2 × 106/ml) containing 100 µm CaCl2 and influx of 45Ca2+ measured 5 min after the addition of pneumolysin (8·37 ng/ml). The responses of cells which had been treated with DHA followed by washing with and without BSA were compared with those of similarly processed cells to which DHA was added following washing.
Pneumolysin-binding assay
Neutrophils were preincubated with DHA (5 and 10 µg/ml) for 10 min at 37°C in HBSS followed by addition of the eGFP-Ply construct (500 ng/ml) and a further incubation for 5 min at 37°C. The final volume in each tube was 3 ml, containing 2 × 106 neutrophils/ml. Following incubation, pneumolysin binding to neutrophils was determined flow cytometrically using an Altra cell sorter equipped with a water-cooled Coherent Enterprise laser (Beckman Coulter, Miami, FL, USA). In an additional series of experiments, neutrophils were pretreated with DHA (10 µg/ml) for 5 min followed by washing of the cells, addition of pneumolysin and measurement of binding of the toxin by the cells.
Haemolytic activity of pneumolysin
Human erythrocytes were used to investigate the effects of DHA (5 µg/ml) on the haemolytic activity of pneumolysin (4·19 and 8·37 ng/ml). The erythrocytes, at a final concentration of 0·5% in HBSS, were preincubated with DHA for 10 min at 37°C followed by addition of pneumolysin and a further incubation period of 35 min at room temperature. Thereafter, the residual erythrocytes were pelleted by centrifugation and haemolysis determined spectrophotometrically at a wavelength of 450 nm according to the extent of release of haemoglobin. Relative to neutrophils, erythrocytes are more sensitive to the cytolytic actions of pneumolysin [1].
Statistical analysis
Levels of statistical significance were calculated using the paired Student's t-test or by analysis of variance with a subsequent Tukey-Kramer multiple comparisons test for multiple groups. The level of statistical significance was P < 0·05. The results of each series of experiments are expressed as the mean values ± s.e.m.
Results
Activation of NFκB in pneumolysin-treated neutrophils
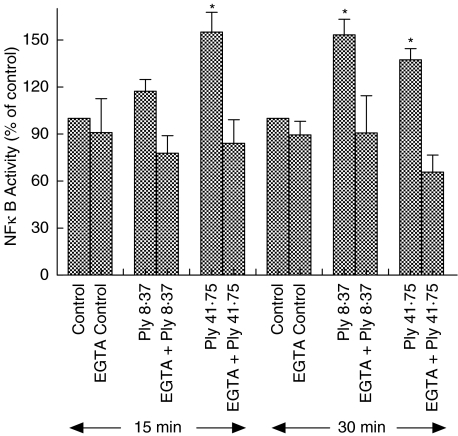
Exposure of neutrophils to pneumolysin caused a time- and dose-related activation of NFκB which was attenuated by inclusion of EGTA in the cell-suspending medium or by pretreatment of the cells with DHA. As can be seen in Fig. 1, the effects of pneumolysin at 41·75 ng/ml, were evident at 15 min (maximal) and 30 min, while those of 8·37 ng/ml pneumolysin were statistically significant only at 30 min. The effects of EGTA are also shown in Fig. 1 and demonstrate complete attenuation of activation of NFκB following exposure of neutrophils to pneumolysin at both 8·37 and 41·75 ng/ml.
Fig. 1.
Effects of exposure of neutrophils to pneumolysin (Ply, 8·37 and 41·75 ng/ml) for 15 min and 30 min in the absence and presence of EGTA (10 m m) on nuclear translocation of NFκB. The results are presented as the mean percentages ± s.e.m. of the corresponding pneumolysin-free control systems (data from 12 experiments). *P < 0·05 for comparison with the pneumolysin-free control system.
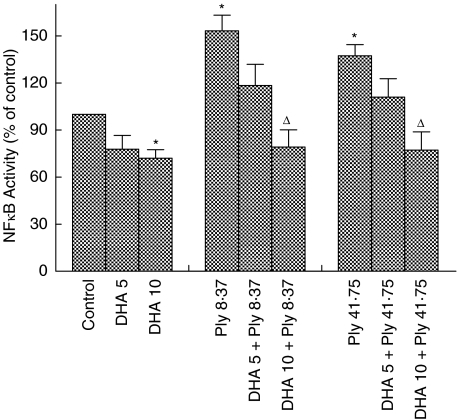
The effects of DHA on activation of NFκB by pneumolysin were measured only at 30 min after addition of the toxin to neutrophils and are shown in Fig. 2. Activation of NFκB by pneumolysin was effectively antagonized by DHA at 10 µg/ml, and to a lesser extent at 5 µg/ml.
Fig. 2.
Effects of pretreatment of neutrophils with docosahexaenoic acid (5 and 10 µg/ml) on pneumolysin (Ply, 8·37 and 41·75 ng/ml)-mediated nuclear translocation of NFκB following 30 min exposure to the toxin. The results are presented as the mean percentages ± s.e.m. of the pneumolysin-free control system (data from 6 experiments). *P < 0·05 for comparison with the pneumolysin-free control system. ΔP < 0·05 for comparison with the corresponding pneumolysin-treated, DHA-free system.
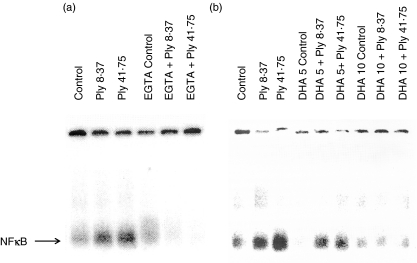
Representative phosphor-images from experiments designed to investigate the effects of EGTA and DHA on pneumolysin-mediated activation of NFκB in neutrophils are shown in Fig. 3.
Fig. 3.
Phosphor-images showing the effects of pneumolysin (Ply 8·37 and 41·75 ng/ml) on nuclear translocation of NFκB in human neutrophils in the absence and presence of (a) 10 m m EGTA or (b) 5 and 10 µg/ml docosahexaenoic acid (DHA). Neutrophils were treated with pneumolysin for 15 min at 37°C in the absence and presence of EGTA or DHA and the nuclear extracts then analysed by electrophoretic mobility shift assay.
Pneumolysin-mediated activation of NFκB was unaffected by DPI (not shown).
Effects of pneumolysin on interleukin-8 by neutrophils
As reported previously [2], exposure of neutrophils to pneumolysin was accompanied by an increase in the synthesis of IL-8, which was attenuated by treatment of the cells with DHA. In the absence of pneumolysin, the amounts of IL-8 produced by neutrophils only, or neutrophils treated with 5 or 10 µg/ml DHA were 324 ± 27·8, 361 ± 39·9 and 371 ± 60·2 pg/ml, respectively. The corresponding values for cells treated with the toxin (8·37 ng/ml) were 578 ± 66·4, 363 ± 28·9 (P < 0·05), and 365 ± 33·1 (P < 0·05) pg/ml (data from 3 experiments with 5 replicates in each). EGTA was not included in these experiments because we have previously reported that this agent completely abolished pneumolysin-induced synthesis of IL-8 by neutrophils [2].
Pneumolysin effects on calcium influx
The effects of DHA (5 µg/ml) and EGTA (10 m m) on the influx of Ca2+ into neutrophils following exposure to pneumolysin (8·37 ng/ml) are shown in Tables 1 and 2, respectively. Treatment of neutrophils with pneumolysin was accompanied by influx of Ca2+, which is in agreement with previous studies using spectrofluorimetric procedures [1,12]. Pneumolysin-mediated influx of Ca2+ was completely eliminated by inclusion of EGTA in the cell-suspending medium and substantially decreased by pretreatment of the cells with DHA.
Table 1.
Effects of docosahexaenoic acid (DHA) treatment of neutrophils on pneumolysin-mediated influx of Ca2+
| System | Amount of cell-associated 45Ca2+ (pmoles/107 cells/5 min) |
|---|---|
| Neutrophils only | 180 ± 10 |
| Neutrophils + 5 µg/ml DHA | 224 ± 19 |
| Neutrophils + 8·37 ng/ml pneumolysin | 2406 ± 50* |
| Neutrophils + DHA + pneumolysin | 332 ± 23 |
The results are expressed as the mean values ± s.e.m. of a single representative experiment (3 in the series) with 6 replicates for each system.
P < 0·05 for comparison with each of the other systems.
Table 2.
Effects of addition of EGTA to the neutrophil-suspending medium on pneumolysin-mediated influx of Ca2+
| System | Amount of cell-associated 45Ca2+ (pmoles/107 cells/5 min) |
|---|---|
| Neutrophils only | 179 ± 6 |
| Neutrophils + 10 m m EGTA | 132 ± 5 |
| Neutrophils + 8·37 ng/ml pneumolysin | 2031 ± 91* |
| Neutrophils + EGTA + pneumolysin | 160 ± 10 |
The results are expressed as the mean values ± s.e.m. of a single representative experiment (2 in the series) with 5 replicates for each system.
P < 0·05 for comparison with each of the other systems.
Exposure of pneumolysin (4·137 µg/ml) to DHA (50 µg/ml) for 5 min at 37°C followed by dilution (1 : 500) and measurement of toxin (8·37 ng/ml)-mediated influx of 45Ca2+ into neutrophils was not accompanied by detectable loss of the pore-forming actions of the toxin (data not shown).
Treatment of neutrophils with DHA (5 µg/ml) followed by washing only (twice), or especially pretreatment with BSA (5 mg/ml) followed by washing, attenuated the inhibitory effects of DHA on influx of 45Ca2+ on subsequent exposure of the cells to pneumolysin (8·37 ng/ml). Washing only, or BSA treatment + washing in particular, significantly (P < 0·05) reduced the protective effects of DHA on toxin-mediated influx, the values being 1718 ± 68, 189 ± 36, 1195 ± 59 and 1449 ± 55 pmoles 45Ca2+/107 cells/5min for the pneumolysin control system and for systems with DHA present throughout, DHA + washing only, and DHA + BSA + washing, respectively (data from 5 measurements with values for spontaneous uptake of 45Ca2+ subtracted).
Pneumolysin binding to neutrophils
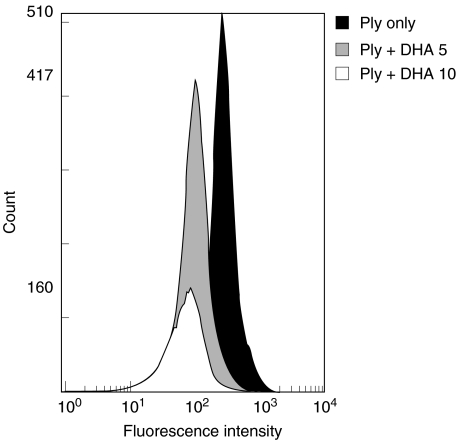
The effects of DHA (5 and 10 µg/ml) on the binding of pneumolysin to neutrophils are shown in Fig. 4. DHA antagonized the binding of the toxin to the cells. In a larger series of experiments, the mean percentages inhibition (reduction in fluorescence intensity) of uptake of pneumolysin by cells treated with 5 and 10 µg/ml DHA were 18 ± 5 and 45 ± 7 (P < 0·05), respectively (data from 3 separate experiments with 4 replicates in each). Pretreatment of neutrophils with DHA (10 µg/ml), followed by washing of the cells also resulted in decreased binding of pneumolysin to the cells, compatible with a cell-directed mechanism of action of DHA, as opposed to direct inactivation of the toxin (results not shown).
Fig. 4.
Effects of docosahexaenoic acid (DHA, 5 and 10 µg/ml) on the binding of the eGFP-Ply construct (500 ng/ml) to neutrophils. The cells were treated with DHA for 5 min at 37°C followed by addition of the construct and flow cytometric analysis of cell-associated toxin. The results shown are those of a single representative experiment with three in the series.
Pneumolysin-mediated haemolysis
The mean percentages haemolysis observed following treatment of human erythrocytes with pneumolysin at 8·37 ng/ml alone or in the presence of 5 µg/ml DHA were 56·3 ± 2 and 44 ± 1·4, respectively (data from 2 experiments with 8 replicates for each system in each experiment; P < 0·05). The corresponding values for treatment of erythrocytes with 4·19 ng/ml pneumolysin in the presence and absence of 5 µg/ml DHA were 33·3 ± 1·7% and 22·7 ± 1·3 (P < 0·05).
Discussion
In the current study, we have demonstrated that NFκB is activated following exposure of neutrophils to pneumolysin, which is accompanied by synthesis of IL-8. Compatible with these findings, IL-8 gene expression in neutrophils and other types of inflammatory cells has been reported to involve coordination of several mechanisms, including transcriptional activation by the NFκB and JUN-N terminal protein kinase pathways [23]. Activation of NFκB by pneumolysin has previously been described in murine macrophages [24], but not, to our knowledge, in human neutrophils. However, the mechanism of pneumolysin-mediated activation of the transcription factor in murine macrophages is clearly different from that described in the current study for human neutrophils, since it is dependent on recognition by Toll-like receptor 4 and requires considerably greater concentrations of the toxin [24].
Increased cytosolic Ca2+ has been reported to result in activation of transcription factors in immune and inflammatory cells, with activation of NFκB requiring a relatively large increase in the concentration of the cation [25]. Such a mechanism appears to be operative in the case of pneumolysin-activated neutrophils. This contention is based on our previous findings that pneumolysin, but not a mutant version of the toxin inactivated with respect to pore-forming activity, causes influx of Ca2+ into neutrophils [1,12], while activation of NFκB by the toxin, as reported here, as well as synthesis of IL-8, as reported previously [2], are attenuated by the Ca2+-chelating agent, EGTA. The absence of effects of DPI appears to exclude involvement of NADPH oxidase and intracellular oxidative stress in the pneumolysin-mediated activation of NFκB in neutrophils.
The proposed relationship between pneumolysin-mediated influx of Ca2+, activation of NFκB and synthesis of IL-8 by neutrophils is strengthened by the finding that all of these events were effectively antagonized by pretreatment of the cells with DHA at concentrations which have been reported to be cytoprotective for various eukaryotic cell types [26–28]. Interestingly, data derived from mixing experiments revealed that DHA does not cause direct inactivation of pneumolysin, while extensive washing of DHA-treated cells, and albumin pretreatment plus washing in particular, significantly reduced the inhibitory effects of the fatty acid on pneumolysin-mediated Ca2+ influx, demonstrating a requirement for the continuous presence of DHA. These findings, together with observations that pretreatment of erythrocytes and neutrophils with DHA was accompanied by decreased haemolysis and binding of pneumolysin, respectively, appear to be compatible with a mechanism whereby the polyunsaturated fatty acid interferes with the binding of pneumolysin to target cells.
Nevertheless, we believe that other, as yet unidentified mechanisms, are also likely to be operative. This contention is based on the moderate levels of protection afforded by DHA against pneumolysin (8·37 ng/ml)-mediated haemolysis and binding of the toxin to neutrophils (21·8% and 18%, respectively, at 5 µg/ml DHA) in comparison with the magnitude of reduction of influx of Ca2+ (86%) into neutrophils. In this respect it is noteworthy that polyunsaturated fatty acids have been reported to stimulate the plasma membrane Ca2+-ATPase (Ca2+ efflux) of eukaryotic cells [29], and to antagonize influx of Ca2+ via interference with various types of Ca2+ channels, including receptor-operated-, l-type voltage-gated-, and store-operated Ca2+ channels, as well as the Na+/Ca2+ exchanger, preventing Ca2+ overload [26–28,30–35]. DHA has also been reported to cause a modest transient increase in cytosolic Ca2+ into neutrophils [36], which could conceivably sensitize cellular Ca2+ exclusion mechanisms. The absence of an exact correlation between the magnitudes of inhibition of pneumolysin-mediated activation of NFκB and influx of Ca2+ mediated by 5 µg/ml DHA (50% and 86%, respectively) may reflect the higher concentration of neutrophils used in the NFκB assay relative to the Ca2+ influx system (5 × 106/ml and 2 × 106/ml).
Although we have focused on interference with NFκB in neutrophils, we believe that the antagonistic effects of DHA on transcription factor activation are likely to be more broadly operative, extending to other inflammatory cell types, as well as to other microbial toxins, including, but not limited to pore-forming toxins. This view is supported by observations that DHA inhibits TLR-4 dependent activation of NFκB in lipopolysaccharide-activated macrophages, probably by antioxidative mechanisms distinct from those described in the current study [37–39]. The broad spectrum anti-inflammatory potential of DHA in controlling infection-associated, over-exuberant inflammatory responses, is supported by observations that tissue levels of DHA are decreased in patients with cystic fibrosis, as well as in cystic fibrosis-knockout mice [40,41]. Interestingly, administration of DHA to cystic fibrosis-knockout mice was found to counter neutrophil pro-inflammatory activity associated with pseudomonas lipopolysaccharide-induced pneumonia [41].
Although the findings of the current study underscore the pro-inflammatory interactions of pneumolysin with neutrophils, the toxin has also been reported to interfere with the functions of these cells [42], which may also contribute to microbial persistence. Clearly the involvement, if any, of pneumolysin-mediated augmentation of the pro-inflammatory activities of airway neutrophils and monocytes/macrophages [43,44], in causing damage to epithelial barriers, thereby facilitating extra-pulmonary dissemination of the pneumococcus, remains to be conclusively established [45,46]. Nevertheless, our observations that the toxin, at pathophysiologically relevant concentrations, causes Ca2+-dependent activation of NFκB and synthesis of IL-8 by neutrophils, which are antagonized by DHA, appear to warrant further evaluation of the therapeutic potential not only of omega-3 polyunsaturated fatty acids, but possibly other types of fatty acids, in models of experimental pneumococcal disease.
References
- 1.Cockeran R, Theron AJ, Steel HC, et al. Proinflammatory interactions of pneumolysin with human neutrophils. J Infect Dis. 2001;183:604–11. doi: 10.1086/318536. [DOI] [PubMed] [Google Scholar]
- 2.Cockeran R, Durandt C, Feldman C, Mitchell TJ, Anderson R. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils. J Infect Dis. 2002;186:562–5. doi: 10.1086/341563. [DOI] [PubMed] [Google Scholar]
- 3.Strickland I, Kisich H, Hauk PJ, et al. High constitutive glucocorticoid receptor β in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med. 2001;193:585–93. doi: 10.1084/jem.193.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jounblat R, Kadioglu A, Mitchell TJ, Andrew PW. Pneumococcal behaviour and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect Immun. 2003;71:1813–9. doi: 10.1128/IAI.71.4.1813-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musher DM, Phan HM, Baughn RE. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J Infect Dis. 2001;183:827–30. doi: 10.1086/318833. [DOI] [PubMed] [Google Scholar]
- 6.Cockeran R, Anderson R, Feldman C. Pneumolysin in the immunopathogenesis and treatment of pneumococcal disease. Expert Rev Anti-Infect Ther. 2003;1:231–9. doi: 10.1586/14787210.1.2.231. [DOI] [PubMed] [Google Scholar]
- 7.García-Suárez MM, Cabal-Cima MD, Florez N, et al. Protection against pneumococcal pneumonia in mice by monoclonal antibodies to pneumolysin. Infect Immun. 2004;72:4534–40. doi: 10.1128/IAI.72.8.4534-4540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune disease. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 9.Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med. 1999;27:1409–20. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Grimminger F, Mayer K, Kiss L, Wahn H, Walmrath D, Seeger W. Synthesis of 4- and 5-series leukotrienes in the lung microvasculature challenged with Escherichia coli hemolysin: critical dependence on exogenous free fatty acid supply. Am J Respir Cell Mol Biol. 1997;16:317–24. doi: 10.1165/ajrcmb.16.3.9070617. [DOI] [PubMed] [Google Scholar]
- 11.Rose F, Kiss L, Grimminger F, et al. E. coli hemolysin-induced lipid mediator metabolism in alveolar macrophages: impact of eicosapentaenoic acid. Am J Physiol. 2000;279:L100–9. doi: 10.1152/ajplung.2000.279.1.L100. [DOI] [PubMed] [Google Scholar]
- 12.Cockeran R, Theron AJ, Feldman C, Mitchell TJ, Anderson R. Docosahexaenoic acid and eicosapentaenoic acid antagonize the proinflammatory interactions of pneumolysin with human neutrophils. Infect Immun. 2004;72:4327–9. doi: 10.1128/IAI.72.7.4327-4329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan YY, McMurray DC, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–20. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 14.Waheed AA, Shimada Y, Heijnen HF, et al. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts) Proc Natl Acad Sci USA. 2001;98:4926–31. doi: 10.1073/pnas.091090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagahama MS, Hayashi S, Morimitsu S, Sakurai J. Biological activities and pore formation of Clostridium perfringens beta toxin in HL6O cells. J Biol Chem. 2003;278:36934–41. doi: 10.1074/jbc.M306562200. [DOI] [PubMed] [Google Scholar]
- 16.Solovyova AS, Nöllman M, Mitchell TJ, Byron O. The solution structure and oligomerization behavior of two bacterial toxins. pneumolysin and perfringolysin O. Biophys J. 2004;87:540–52. doi: 10.1529/biophysj.104.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders FK, Mitchell TJ, Walker JA, Andrew PW, Boulnois GJ. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989;57:2547–52. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spreer A, Kerstan H, Bottcher T, et al. Reduced release of pneumolysin by Streptococcus pneumoniae in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob Agents Chemother. 2003;47:2649–54. doi: 10.1128/AAC.47.8.2649-2654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitag NE, Jacobs KE. Examination of Listeria monocytogenes intracellular gene expression by using green fluorescent protein of Aequorea victoria. Infect Immun. 1999;67:1844–52. doi: 10.1128/iai.67.4.1844-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–8. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez LA, Thompson J, Brown DA, et al. Activation of NFκB by PM10 occurs via an iron-mediated mechanism in the absence of IκB degradation. Toxicol Appl Pharmacol. 2000;166:101–10. doi: 10.1006/taap.2000.8957. [DOI] [PubMed] [Google Scholar]
- 22.Irani K, Xia Y, Zweier JL, et al. Mitogenic signaling mediated by oxidants in ras-transformed fibroblasts. Science. 1997;275:1649–52. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leuk Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 24.Malley R, Henneke P, Morse SC, et al. Recognition of pneumolysin by toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA. 2003;100:1966–71. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 26.Vitelli MR, Filippelli A, Rinaldi B, et al. Effects of docosahexaenoic acid on [Ca (2+) ] (i) increase induced by doxorubicin in ventricular rat cardiomyocytes. Life Sci. 2002;71:1905–16. doi: 10.1016/s0024-3205(02)01960-4. [DOI] [PubMed] [Google Scholar]
- 27.Honen BN, Saint DA, Laver DR. Suppression of calcium sparks in rat ventricular myocytes and inhibition of sheep cardiac RyR channels by EPA, DHA and oleic acid. J Membr Biol. 2003;196:95–103. doi: 10.1007/s00232-003-0628-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Zhao X, Mao ZY, Wang XM, Liu ZL. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14:2457–61. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 29.Carafoli E. The Ca2+ pump of the plasma membrane. J Biol Chem. 1992;267:2115–8. [PubMed] [Google Scholar]
- 30.Hazama H, Nakajima T, Asano M, et al. Omega-3 polyunsaturated fatty acids-modulation of voltage-dependent L-type Ca2+ current in guinea pig tracheal smooth muscle cells. Eur J Pharmacol. 1998;355:257–66. doi: 10.1016/s0014-2999(98)00484-1. [DOI] [PubMed] [Google Scholar]
- 31.Hirafuji M, Ebihara T, Kawahara F, Hamaue N, Endo T, Minami M. Inhibition by docosahexaenoic acid of receptor-mediated Ca (2+) influx in rat vascular smooth muscle cells stimulated with 5-hydroxytryptamine. Eur J Pharmacol. 2001;427:195–201. doi: 10.1016/s0014-2999(01)01274-2. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi B, Di Pierro P, Vitelli MR, et al. Effects of docosahexaenoic acid on calcium pathway in adult cardiomyocytes. Life Sci. 2002;71:993–1004. doi: 10.1016/s0024-3205(02)01792-7. [DOI] [PubMed] [Google Scholar]
- 33.Ferrier GR, Redondo I, Zhu J, Murphy MG. Differential effects of docosahexaenoic acid on contractions and 1-type Ca2+ current in adult cardiac myocytes. Cardiovasc Res. 2002;54:601–10. doi: 10.1016/s0008-6363(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 34.Denys A, Aires V, Hichami A, Khan NA. Thapsigargin-stimulated MAP kinase phosphorylation via CRAC channels and PLD activation: inhibitory activation of docosahexaenoic acid. FEBS Lett. 2004;564:177–82. doi: 10.1016/S0014-5793(04)00361-8. [DOI] [PubMed] [Google Scholar]
- 35.Xiao YF, Ke Q, Chen Y, Morgan JP, Leaf A. Inhibitory effect of n-3 fish oil fatty acids on Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Comm. 2004;321:116–23. doi: 10.1016/j.bbrc.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 36.Hardy SJ, Robinson BS, Ferrante A, et al. Polyenoic very-long chain fatty acids mobilize intracellular calcium from a thapsigargin-insensitive pool in human neutrophils. The relationship between Ca2+ mobilization and superoxide production by long- and very-long chain fatty acids. Biochem J. 1995;311:689–97. doi: 10.1042/bj3110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu W, Ishihara K, Murata M, Saito H, Shinohara K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic Biol Med. 2003;34:1006–16. doi: 10.1016/s0891-5849(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–51. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 39.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD (P) H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 40.Freedman SD, Blanco PG, Zaman MM, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–9. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 41.Freedman SD, Weinstein D, Blanco PG, et al. Characterization of LPS-induced lung inflammation in cftr-/- mice and the effect of docosahexaenoic acid. J Appl Physiol. 2002;92:2169–76. doi: 10.1152/japplphysiol.00927.2001. [DOI] [PubMed] [Google Scholar]
- 42.Paton JC, Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity and migration by pneumolysin. Infect Immun. 1983;41:212–6. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rijneveld AW, van den Dobbelsteen GP, Florquin S, et al. Roles of interleukin-6 and macrophage inflammatory protein-2 in pneumolysin-induced lung inflammation in mice. J Infect Dis. 2002;185:123–6. doi: 10.1086/338008. [DOI] [PubMed] [Google Scholar]
- 44.Rogers PD, Thornton J, Barker KS, et al. Pneumolysin-dependent and – independent gene expression identified by cDNA microarray analysis of THP-1 human mononuclear cells stimulated by Streptococcus pneumoniae. Infect Immun. 2003;71:2087–94. doi: 10.1128/IAI.71.4.2087-2094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maus UA, Srivastava M, Paton JC, et al. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol. 2004;173:1307–12. doi: 10.4049/jimmunol.173.2.1307. [DOI] [PubMed] [Google Scholar]
- 46.Hirst RA, Kadioglu A, O'Callaghan CO, Andrew PW. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol. 2004;138:195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]