Abstract
Tuberculosis (TB) is usually more severe in HIV-infected patients, and the immune derangement found in co-infected patients may differ from that in each isolated disease. Following mitogen stimulation of peripheral blood mononuclear cells (PBMC), interferon (IFN)-γ and tumour necrosis factor (TNF)-α production was evaluated in T cells by flow cytometry, and in culture supernatants by enzyme-linked immunosorbent assay (ELISA) in 33 individuals: 11 AIDS patients with tuberculosis, six asymptomatic HIV-1-infected patients, eight patients with tuberculosis and eight healthy controls. The proportion of CD4+ T lymphocytes expressing IFN-γ did not differ between the groups, whereas a trend towards increased proportions of TNF-α-expression in CD4+ T cells was observed in the TB compared to the HIV group, while intermediate values were observed in co-infected patients. Detection of IFN-γ and TNF-α in CD8+ T lymphocytes was higher in TB than in HIV individuals. Co-infected patients presented intermediate values for IFN-γ, while TNF-α detection was similar to that in HIV mono-infection. In conclusion, the proportion of T cells expressing IFN-γ was relatively preserved in co-infected patients compared to TB patients, while the percentage of T cells expressing TNF-α was decreased, mainly in CD8+ T lymphocytes. However, the marked reduction in T lymphocyte numbers in co-infected patients led to a striking reduction of both cytokines in PBMC supernatants, a finding that is consistent with the impaired response to Mycobacterium tuberculosis.
Keywords: AIDS, cytokines, tuberculosis
Introduction
Tuberculosis (TB) is among the most frequent opportunistic infections in AIDS patients. HIV-1 infection remains the most common risk factor for the development of active TB, and both reactivation of a latent Mycobacterium tuberculosis infection, and progressive TB are substantially more common in HIV-1-infected subjects. The resurgence of TB has been attributed, in part, to the HIV-1 epidemic in developed countries. Following the same trend, 60–70% of TB cases in developing countries occur in HIV-1-infected individuals [1].
Because both agents share pathogenetic disease mechanisms, one would expect a mutual interference in the disease susceptibility and course. It is recognized that HIV-1 infection leads to a higher probability of active TB development, whereas the course of HIV-related immunodeficiency is aggravated by TB [2–7]. Clinical manifestations of tuberculosis in HIV patients are usually more severe, with diffuse pulmonary involvement and frequent extra pulmonary dissemination [8]. Therefore, it is conceivable that different immunological parameters may be altered in AIDS patients with TB when compared to each isolated disease.
In a previous work, we were able to demonstrate that both CD4+ and CD8+ T lymphocyte counts are diminished in AIDS patients with TB, compared to appropriate controls. However, CD8+ T cell depletion was restricted mainly to the presence of TB, which also up-regulated the CD38 activation marker [9]. It has also been shown that co-infected patients have a decrease in proliferative response to M. tuberculosis antigens, and reduced production of interleukin (IL)-2 and interferon (IFN)-γ compared to patients with tuberculosis and without HIV [7].
IFN-γ and TNF-α play a pivotal role in the protective immune response against M. tuberculosis, with the former known to be down-regulated in HIV-1 infection and disease and the latter being associated with viral replication. We hypothesize that cytokine production in HIV-TB co-infection is distinct to what may be found in each isolated disease, supporting the higher incidence of severe and disseminated forms of TB in AIDS patients. Thus, we evaluated the ex vivo production of IFN-γ and TNF-α by CD4+ T and CD8+ T lymphocytes and in peripheral blood mononuclear cells (PBMC) supernatants from patients co-infected with HIV-TB, asymptomatic HIV-1-infected individuals and TB patients.
Materials and methods
Patients and healthy volunteers
Institutional Review Board-approved written informed consent was obtained from all participants, according to the Brazilian Ministry of Health Guidelines. Four groups of volunteers were enrolled: (1) the active TB group (TB group) consisted of HIV-1-negative patients with recently diagnosed active tuberculosis, defined by compatible medical history and radiological findings, and a positive sputum for alcohol acid-resistant bacilli or isolation of M. tuberculosis from a respiratory tract specimen; (2) the asymptomatic HIV group (HIV+ group) consisted of HIV-1 infected individuals, as determined by an enzyme-linked immunosorbent assay (ELISA) and a confirmatory Western blot. All patients had CD4+ T lymphocyte counts under 250 cells/µl (to include patients with HIV-1 induced severe immunodeficiency), had no evidence of active opportunist disease and were antiretroviral therapy naive; (3) the co-infected group (TB-HIV group) consisted of antiretroviral therapy naive, HIV-1-infected patients who presented with tuberculosis, based on clinical and laboratory findings, as defined for group 1. They had no previous opportunistic infection, and were enrolled prior to tuberculosis treatment; (4) the control group (Ctl group) consisted of healthy volunteers, without evidence of active respiratory disease.
Monoclonal antibodies (mAbs)
Peridin chlorophyll protein conjugated CD8 (CD8-PerCP, clone SK1), allophycocyanin conjugated CD3 (CD3-APC, clone JCHT1), fluorescein isothiocyanate (FITC) conjugated mouse IgG1 (mIgG1-FITC, clone MOPC-21), anti-human IFN-γ-FITC (IgG1, clone 4S.B3), anti-human TNF-α-FITC (IgG1, clone mAb 11), all from Pharmingen (San Diego, CA, USA), were used in the flow cytometry assays.
Cell preparation and stimulation
PBMC were isolated by density-gradient sedimentation over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) and washed twice with phosphate buffered saline (PBS). Cells were resuspended at a concentration of 1 × 106 cells/ml in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal calf serum (FCS) (Gibco, Grand Island, NY, USA), transferred to polypropylene tubes (Becton Dickinson, NJ, USA), and stimulated with 20 ng/ml (Sigma) for 6 h, the last 5 h in the presence of 2 µ M monensin (Sigma) to inhibit protein secretion. PBMC was resuspended for surface and intracellular staining and the supernatants (obtained in the absence of monensin) were aliquoted and kept at −70°C for TNF-α and IFN-γ determinations by ELISA.
Surface and intracellular staining
After stimulation, cells were centrifuged at 300 g at 4°C for 15 min. Cells were resuspended in 100 µl of staining buffer (PBS supplemented with 0·1% sodium azide (Sigma) and 1% fetal bovine serum (FBS, HyClone Laboratories, Logan, UT, USA), pH 7·4–7·6). CD8-PerCP and CD3-APC conjugated antibodies were added and incubated at 4°C in the dark for 15 min. Cells were washed with 2 ml of staining buffer and resuspended in 1 ml of fixation buffer [4% paraformaldehyde (Polysciences, Warrington, PA, USA) in PBS at pH 7·4–7·6]. The cells were fixed for 20 min at 4°C in darkness, centrifuged at 300 g at 4°C for 15 min, and resuspended in 1·5 ml of staining buffer. Each tube was split into three aliquots and centrifuged at 300 g at 4°C for 15 min. Samples were incubated for 30 min at 4°C in the dark with 50 µl of mAbs solution prepared with 2 µl of mAbs in 498 µl of permeabilization buffer (tube 1: mIgG1-FITC; tube 2: IFN-γ-FITC; tube 3: TNF-α-FITC), and then washed with 2 ml of permeabilization buffer [PBS supplemented with 0·1% sodium azide, 1% FBS and 0·1% saponin (Sigma)] and resuspended in 300 µl of staining buffer for flow cytometric analysis.
Flow cytometric analysis
Cell samples were analysed on a FACScalibur (Becton Dickinson Biosciences, San Jose, CA, USA). Acquisition and analyses were performed using cellquest software (Becton Dickinson). For each tube, 15 000 events were acquired in a live lymphocyte gate, based on low forward-scatter and low side-scatter light dispersion. CD8+ T lymphocytes were identified as CD3+ CD8+ cells and the T helper lymphocytes as CD3+ CD8– cells. Quadrant statistics were established using isotype mAbs stained cells and the cytokine producing cells expressed as the percentage of T cell subsets.
Supernatant cytokines
TNF-α and IFN-γ were measured in PBMC supernatants by ELISA, according to the manufacturer's instructions. Recombinant cytokines and monoclonal antibodies were obtained from PharMingen (San Diego, CA, USA). For TNF-α clone Mab1 was used as the capture antibody and clone Mab11 as the biotinylated antibody. In the case of IFN-γ the clones used were B27 and 4S.B3, respectively. Samples were tested in duplicate along with a standard curve with recombinant cytokine in each plate. The test sensitivity was 15 pg/ml for both TNF-α and IFN-γ.
Statistical analysis
The results were analysed using Statistica software (version 5·1E, StatSoft, Tulsa, OK, USA). Group comparisons were performed by use of the anova test. The variables that showed differences among the four groups were compared group to group by the Mann–Whitney U-test. A P-value of less than 0·05 was set as statistically significant.
Results
Clinical data
Thirty-three individuals were enrolled in the study: eight in the control group [five (62·5%) male; median age 36·5 years], six in the asymptomatic HIV group [three (50·0%) male; median age 29 years], eight in the TB group (all male, median age 29·5 years) and 11 in the HIV-TB group [eight male (72·7%); median age 29 years].
In the asymptomatic HIV1-infected group, the median CD4+ and CD8+ T lymphocyte counts were 134 cells/µl and 423 cells/µl, respectively. The median viral load was 96500 copies/ml (log10 = 5·1).
The clinical findings present in all TB patients were late afternoon intermittent fever (temperature ranging from 37·8°C to 38·5°C), dry cough progressing to productive cough and weight loss. All the symptoms, classically described in TB, have lasted about 3 weeks. The most frequent radiographic finding was upper lung involvement (75%), associated with cavitations or pleural effusion in 37·5% of the patients.
Tuberculosis was the first opportunistic disease in all HIV-TB patients, and nine of 11 patients in this group had both diseases diagnosed simultaneously. All the patients presented the classical TB symptoms (fever, cough and weight loss) and five patients (45·5%) reported night sweats. Patients reported the symptoms for an average of 9·2 weeks. HIV-TB patients had lymph node enlargement in at least one of the cervical, axillary and inguinal chains. Six patients (54·5%) presented with hepatomegaly and/or splenomegaly, one of them developing acute abdomen with tuberculosis peritonitis and splenic abscess. Two patients had central nervous system involvement as suggested by the computed tomography (CT) scan. One patient presented concomitant pulmonary involvement with rectal fistula. Median of CD4+ T cells counts was 65 cells/µl and median viral load was 1287 909 copies/mm3 (log 5·4).
Absolute T lymphocyte counts in the peripheral blood
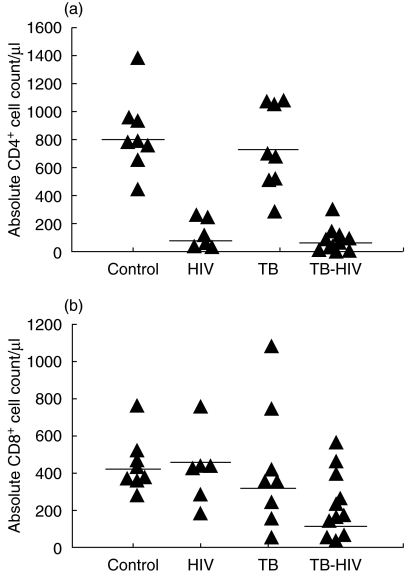
CD4+ T lymphocytes counts were statistically different among the four groups (P < 0·001, anova test). Highest CD4+ T lymphocyte counts were observed in healthy volunteers and active TB patients, contrasting to lower values in the asymptomatic HIV patients and in the HIV-TB group (Fig. 1a). No statistically significant difference was observed in the CD8+ T lymphocytes counts among the four groups (Fig. 1b).
Fig. 1.
Absolute CD4+ (a) and CD8+ (b) T lymphocyte counts in healthy controls, asymptomatic HIV-1 individuals, tuberculosis (TB) and HIV-TB patients. The lines represent the median values.
Intracellular detection of cytokines in T lymphocyte subsets
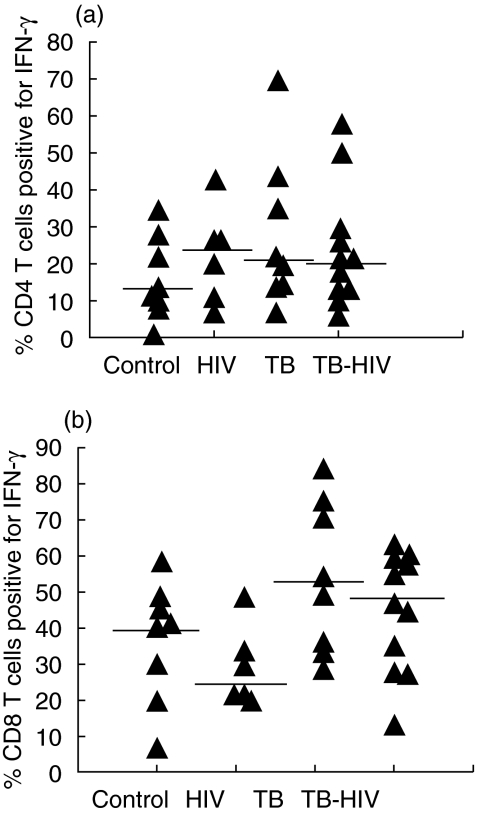
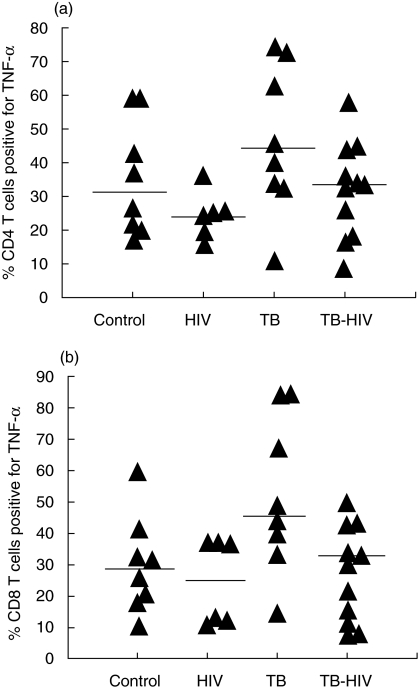
The percentage of CD4+ T lymphocytes positive-stained for IFN-γ (Fig. 2a) following PMA-Io stimulation did not differ among the four groups (P = 0·489), whereas a trend to differential production of the TNF-α could be observed (P = 0·095). A higher proportion of TNF-α positive cells was found in the TB group compared to the asymptomatic HIV group (P = 0039) and the TB-HIV group presented intermediate values between the HIV asymptomatic group (P = 0·228) and the TB group (P = 0·107) (Fig. 3a).
Fig. 2.
Detection of interferon (IFN)-γ production by intracellular staining in CD4+ T (a) and CD8+ T (b) lymphocytes in healthy controls, asymptomatic HIV-1 individuals, tuberculosis (TB) and HIV-TB patients. The cytokine producing cells are expressed as the percentage of T cell subsets. The lines represent the median values. P < 0·05 TB versus HIV asymptomatic group.
Fig. 3.
Detection of tumour necrosis factor (TNF)-α production by intracellular staining in CD4+ T (a) and CD8+ T (b) lymphocytes in healthy controls, asymptomatic HIV-1 individuals, tuberculosis (TB) and HIV-TB patients. The cytokine-producing cells are expressed as the percentage of T cell subsets. The lines represent the median values. In CD4+ T cells significant differences were observed between the TB versus HIV asymptomatic group, and in CD8+ T cells between the TB versus HIV asymptomatic and the TB versus HIV-TB groups.
IFN-γ (P = 0·053) and TNF-α (P = 0·017) detection in the CD8+ T lymphocytes were different among the four groups. The percentage of IFN-γ positive-stained cells was higher in TB patients than in HIV asymptomatic individuals (P = 0·020), and the co-infected patients showed intermediate values; however, more closed to the TB group (P = 0·364) than to the HIV group (P = 0071) (Fig. 2b). Detection of TNF-α was higher in TB patients. Interestingly, co-infected patients presented a similar percentage of CD8+ T lymphocytes positive for TNF-α to the HIV asymptomatic group (P = 0·919) and a lower percentage than the TB group (P = 0026) (Fig. 3b).
IFN-γ and TNF-α concentration in PBMC supernatant after PMA-Io stimulation
Concentrations of IFN-γ and TNF-α, measured by ELISA, in the PBMC supernatants of HIV asymptomatic patients, TB, and HIV-TB patients were statistically different (P < 0·001 for both cytokines), with higher production by the TB group (Table 1).
Table 1.
Interferon (IFN)-γ and tumour necrosis factor (TNF)-α levels in supernatants of peripheral blood mononuclear cells (PBMC) after phorbol myristate acetate–ionomycin (PMA-Io) stimulation. PBMC (2 × 106 cells/ml) were stimulated for 6 h, and the cytokines were measured by enzyme-linked immunosorbent assay. Results are expressed in pg/ml
| HIV median (range) | TB median (range) | HIV-TB median (range) | P | |
|---|---|---|---|---|
| TNF-α | 67·1 (20·1–601·9) | 897·5 (410·6–2505·7) | 58·3 (43·3–218·2) | <0·001 |
| IFN-γ | 87 (15–897) | 2713·5 (43–6796) | 294 (120–3339) | <0·001 |
Discussion
This study was conducted to evaluate the IFN-γ and TNF-α production in TB patients with AIDS. The wide range of tuberculosis clinical presentation in AIDS patients, a significant proportion with severe disease, has suggested a poor immune control of the pathogen in the context of HIV infection. This derangement could impact in the production cytokines, such as IFN-γ and TNF-α, in response to in vitro stimulation. In this study we compared the findings in co-infected patients with those found in each infection, and assessed the cytokine production, by intracellular trapping using flow cytometry and secretion into the supernatants, with the aim of detecting differences at a cellular level and resulting from differences in T lymphocytes subsets cell counts among the groups.
As expected, co-infected patients presented with more severe TB disease with lymph node involvement in all patients, including four with draining lymph nodes, five with hepatomegaly and six with splenomegaly, in contrast to the pulmonary/pleural presentation of HIV-1-negative TB patients.
We found CD4+ T cell counts to be lower in the HIV-TB than in TB patients, although similar to the HIV asymptomatic group. Also no significant difference was found between TB patients and healthy controls. These results reflect the striking difference of CD4+ T cell counts across groups. It has been often reported that most co-infected patients, without previous HIV-associated clinical manifestation present a marked fall in the CD4+ T lymphocyte counts associated with active TB [9–12], which could be reverted by appropriate TB treatment [13–15]. The lack of difference between HIV and HIV-TB groups was not expected, because in a previous work we found lower cell counts in the latter group [16]. This finding may be explained by the low number of patients in both groups, and by the low cell counts observed in the HIV-1 asymptomatic group. Indeed, a group of HIV-1 asymptomatic patients with low CD4+ T cell counts was enrolled to match pronounced HIV-induced immune deficiency seen in HIV-TB patients. Despite the fact that the CD8+ T lymphocytes counts were not significantly different among the studied groups, lower values were observed in TB-HIV co-infected patients, about half of that seen in each condition alone.
We observed a higher viral load in co-infected patients than in the HIV asymptomatic group. The effect of M. tuberculosis infection in the viral load of HIV positive individuals has been demonstrated previously [17]. The exposition of HIV-infected alveolar macrophages and lymphocytes in vitro to M. tuberculosis amplify the HIV replication [18,19]. Garrait et al. [20] have demonstrated that activated lymphocytes in pleural liquid from TB patients have increased HIV replication. In addition it was demonstrated that HIV-RNA concentration in bronchoalveolar lavage is higher in TB involved areas. The enhanced HIV-1 replication in TB patients may be due to IL-1-, IL-6- and TNF-α−producing macrophages [21,22].
Several studies have demonstrated that IFN-γ is a key cytokine in the control of intracellular M. tuberculosis, and that both CD4+ and CD8+ T lymphocytes are important sources of its production [23–27]. It has been demonstrated in murine models that the lack of IFN-γ receptor increased the risk of bacille Calmette–Guérin (BCG) strains dissemination [28] and that the M. tuberculosis protective T cell-mediated response requires IFN-γ production [29]. This protection is associated with inflammatory granuloma organization that is IFN-γ-dependent [25]. We found the ability to produce IFN-γ to be preserved in CD4+ T lymphocyte subsets in HIV-TB patients. In fact, no significant differences were observed in intracellular detection of this cytokine in CD4+ T cells among the four groups. As opposed to CD4+ T lymphocytes, the detection of IFN-γ in CD8+ T lymphocytes differed across groups, with the highest detection in the TB group and the lowest in the HIV group, supporting previous data showing an impaired IFN-γ production in HIV-infected individuals as the disease progresses [30]. Interestingly, the HIV-TB co-infected group seems to preserve the IFN-γ production, as the percentage of positive-stained cells did not differ from the TB group (P = 0·364) and showed a trend to be higher than the HIV asymptomatic group (P = 0·071). It is possible that IFN-γ production at a cellular level would be preserved in CD4+ and CD8+ T lymphocytes in the HIV-TB co-infected patients compared to the TB group. However, the amount of IFN-γ production in these patients is markedly lower than in TB patients, as may be envisaged by the levels measured in the PBMC supernatants. In this case, the lower magnitude of IFN-γ production would reflect the lower CD8+ and mainly CD4+ T cell counts found in these patients.
TNF-α is critical for cellular organization and differentiation for the granuloma formation. Kindler et al. [31] demonstrated in BCG-infected mice that granuloma arrangement coincided with local TNF-α synthesis, and the use of anti-TNF-α impaired mycobacteria destruction. Takashima et al. [32] studied TNF-α production in peripheral blood monocytes from acute, chronic, and refractory TB patients, finding a higher production in acute TB and a lower production in chronic TB, situations associated with well-differentiated and poorly organized granulomas, respectively,
We found a trend for different intracellular TNF-α detection in CD4+ T lymphocytes among the four groups studied, reaching significance only if the patient groups were compared. The TB patients presented higher proportions of TNF-α- producing CD4+ T lymphocytes than asymptomatic HIV-infected patients, while the HIV-TB group displayed intermediated production. The difference of TNF-α production was more pronounced in the CD8+ T lymphocyte subset. Again, the TB group presented a higher number of positive-stained cells than the HIV-infected individuals. In contrast to CD4+ T cells, the TNF-α production by CD8+ T cells in the HIV-TB patients was significantly lower than in the TB group. Then, the production of TNF-α in co-infected patients is down-regulated in T lymphocytes, mainly in CD8+ T subpopulation. This cellular derangement is amplified by the differences in the CD4+ and CD8+ T cell counts among the groups, as supported by our findings of profound reduction of this cytokine in PBMC culture supernatants.
Disseminated tuberculosis is also found in HIV-negative patients, mainly with other underlying diseases, including malnutrition, alcoholism and other congenital or acquired immunodeficiencies. Because we did not include disseminated tuberculosis in the TB group it is important to mention that, at least in part, the difference observed between TB and HIV-TB groups may result from disseminated tuberculosis itself, rather than HIV co-infection.
Whereas previous studies have emphasized an exaggerated proinflammatory TNF-α response in co-infected patients, our study demonstrated a defect in production of this cytokine in co-infected versus TB mono-infected patients. This difference may be explained by the focus on T cell production in this paper, while previous reports emphasized monocyte/macrophage production of TNF-α[33]. Our findings of T cell defects in TNF-α production are also consistent with the recognized role of TNF-α in granuloma formation, as granuloma formation is defective in HIV/TB co-infection. This observation suggests that TNF-α produced by T cells may be particularly critical for granuloma development.
It may be concluded, therefore, that IFN-γ and TNF-α may be regulated differentially in T lymphocytes in the co-infected patients. Compared to the TB patients, they presented a relatively preserved IFN-γ production, whereas a decreased TNF-α detection was observed, mainly in CD8+ T lymphocytes. However, the marked quantitative T lymphocytes reduction in co-infected patients led to a striking reduction in the production of both cytokines, a finding that would justify the impaired response to M. tuberculosis.
Acknowledgments
This work was supported by the Ministry of Science and Technology of Brazil, PRONEX grant 41·96·0943·00, and Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant 524088/96–9. We are indebted to P. Haslett for the critical review of the manuscript. R. M. C. Cunha received a fellowship grant from CAPES (Coordenação de Perfeiçoamento de Pessoal de Nível Superior).
References
- 1.Markowitz N, Hansen NI, Hopewell PC, et al. Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann Intern Med. 1997;126:123–32. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–28. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriki PP, Okwera A, Aisu T, Morrissey AB, Ellner JJ, Daniel TM. The influence mof human immunodeficiency virus infection on tuberculosis in Kampala, Uganda. Am Rev Respir Dis. 1991;143:185–7. doi: 10.1164/ajrccm/143.1.185. [DOI] [PubMed] [Google Scholar]
- 4.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 5.Goletti D, Weissman D, Jackson RW, et al. The in vitro induction of human immunodeficiency virus (HIV) replication in purified protein derivative-positive HIV-infected persons by recall antigen response to Mycobacterium tuberculosis is the result of a balance of the effects of endogenous interleukin-2 and proinflammatory and antiinflammatory cytokines. J Infect Dis. 1998;177:1332–8. doi: 10.1086/515276. [DOI] [PubMed] [Google Scholar]
- 6.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellener J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Gong J, Ier DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infections. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalen C, Horsburgh CR, Hom D, et al. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS. 1997;11:455–60. doi: 10.1097/00002030-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues DSS, Medeiros EA, Weckx LY, Bonnez W, Salomão R, Kallas EG. Immunophenotypic characterization of peripheral T lymphocytes in Mycobacterium tuberculosis infection and disease. Clin Exp Immunol. 2002;128:149–54. doi: 10.1046/j.1365-2249.2002.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck JS, Potts RC, Kaedjito T, Granje JM. T4 lymphopenia in patients with active pulmonary tuberculosis. Clin Exp Immunol. 1985;60:49–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Jones BE, Oo MM, Taikwel DQ, Kumar A, Maslow ER, Barnes PF. CD4 cell counts in human immunodeficiency virus-negative patients with tuberculosis. Clin Infect Dis. 1997;24:988–91. doi: 10.1093/clinids/24.5.988. [DOI] [PubMed] [Google Scholar]
- 12.McDyer JF, Hackley MN, Walsh TE, Cook JL, Seder RA. Patients with multidrug-resistant tuberculosis with low CD4+ counts have impaired Th1 response. J Immunol. 1997;158:492–500. [PubMed] [Google Scholar]
- 13.Onwubali JK, Edwards AJ, Palmer L. T4 lymphocytes in human tuberculosis. Tubercle. 1987;68:195–2000. doi: 10.1016/0041-3879(87)90055-9. [DOI] [PubMed] [Google Scholar]
- 14.Singhal M, Banavalikar N, Sharma S, Saha K. Peripheral blood T lymphocyte subpopulations in patients with tuberculosis and the effect of chemotherapy. Tubercle. 1989;70:171–8. doi: 10.1016/0041-3879(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 15.Turett GS, Telzak EE. Normalization of CD4+ lymphocyte depletion in patients with HIV infection treated for tuberculosis. Chest. 1994;105:1335–7. doi: 10.1378/chest.105.5.1335. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues DSS, Cunha RCM, Kallas EG, Salomão R. Distribution of naive and memory/effector CD4+ T lymphocytes and expression of CD38 on CD8+ T lymphocytes in AIDS patients with tuberculosis. Bras J Infect Dis. 2003;7:161–5. doi: 10.1590/s1413-86702003000200010. [DOI] [PubMed] [Google Scholar]
- 17.Toossi Z, Mayanja-Kizza H, Hirsch CS, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–8. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toossi Z, Nicolacakis K, Xia L, Ferrari INA, Rich EA. Activation of latent HIV-1 by Mycobacterium tuberculosis and its purified protein derivative in alveolar macrophages from HIV-infected individuals in vitro. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:325–31. doi: 10.1097/00042560-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 20.Garrait V, Cadranel J, Esvaint H, et al. Tuberculosis generates a microenvironment enhancing the productive infection of local lymphocytes by HIV. J Immunol. 1997;159:2824–30. [PubMed] [Google Scholar]
- 21.Cohen O, Weissman D, Fauci AS. The immunopathogenesis of HIV infection. In: Paul WE, editor. Fundamental immunology. 4. Philadelphia: Lippincott-Raven Publishers; 1999. pp. 1455–509. [Google Scholar]
- 22.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme IM, Collins FM. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn J, Chan J, Triebold K, Dalton DK, Stewart TA, Bloom BR. An essencial role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutis T, Cornelisse YE, Ottenhoff TH. Mycobacteria induce CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–95. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 27.Xing Z, Wang J, Croitoru K, Wakeham J. Protection by CD4 or CD8 T cells against pulmonary Mycobacterium bovis bacillus Calmette–Guérin infection. Infect Immun. 1998;66:5537–42. doi: 10.1128/iai.66.11.5537-5542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamijo R, Le J, Shapiro D, et al. Mice that lack the interferon gamma receptor have profoundly altered responses to infection with bacillus Calmette–Guérin and subsequent challenge with lipopolysacharide. J Exp Med. 1993;178:1435–40. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–4. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa AO, Lee FK, Freiji R, Lagrange PH, Nahmias A. Human immunodeficiency virus infection alters antigen-induced cytokine responses in patients with active mycobacterial diseases. J Infect Dis. 1998;177:1554–62. doi: 10.1086/515326. [DOI] [PubMed] [Google Scholar]
- 31.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassali P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 32.Takashima T, Ueta C, Tsuyuguchi I, Kishimoto S. Production of Tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990;58:3286–92. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanhan G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis co-activation with HIV infection. Clin Exo Immunol. 1996;103:30–4. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]