Abstract
Escherichia coli is associated with inflammation in the brain. To investigate whether astrocytes are involved in E. coil-induced inflammation, we assessed the levels of expression of proinflammatory mediators produced by E. coli-infected astrocytes. E. coli infection in primary human astrocytes and cell lines increased expression of the CXC chemokine IL-8/GRO-α, the CC chemokine MCP-1, TNF-α, and iNOS. E. coli infection activated p65/p50 heterodimeric NF-κB and concurrently decreased the signals of IκBα. Blocking the NF-κB signals by IκBα-superrepressor-containing retrovirus or antisense p50 oligonucleotide transfection resulted in down-regulation of expression of the proinflammatory mediators. Furthermore, superrepressors of IκBα, IκB kinase (IKK) or NF-κB inducing kinase (NIK) inhibited the up-regulated expression of the downstream target genes of NF-κB such as IL-8 and MCP-1, and superrepressors of TNF receptor-associated factor (TRAF)2 and TRAF5 also inhibited expression of the E. coli-induced target genes of NF-κB. These results indicate that proinflammatory mediators such as the CXC chemokine IL-8/GRO-α, the CC chemokine MCP-1, TNF-α, and iNOS can be expressed in E. coli-infected astrocytes via an NF-κB pathway, suggesting that these mediators may contribute to inflammation in the brain, including infiltration of inflammatory cells.
Keywords: astrocytes, Escherichia coli, NF-κB, proinflammatory mediators
Introduction
The gram-negative bacterium Escherichia coli causes inflammation such as meningitis or abscess in brain, although these are relatively rare situations [1,2]. Bacteria-infected tissues reveal infiltration of a variety of inflammatory cells. Numerous studies have recognized the importance of innate immune responses to bacterial infections which subsequently influence the development of adaptive immune responses [3]. Despite the importance of innate immune responses, however, little is known about the initial response of glial cells to E. coli and the pathogenic mechanisms of inflammation of central nervous system (CNS) due to E. coli infection.
A hallmark of CNS inflammation is the recruitment, activation, and entry of immune cells within the brain parenchyma through the blood–brain barrier. Once within the CNS, these cells release proinflammatory cytokines, and these cytokines may induce damage to neurones and glial cells and/or activate brain endothelial cells, microglia, and astrocytes, thus leading to an amplification of the inflammatory phenomena [4–6].
Astrocytes, the major glial cells in the CNS, maintain homeostatic microenvironment and also play an important role in immune regulation by producing chemokines, cytokines and nitric oxide (NO) [7]. Within the CNS, inflammatory responses rapidly induce marked astrocytic changes, referred to as reactive gliosis. Gliosis may be viewed as detrimental to neuronal function by forming glial scars or producing neurotoxic mediators such as tumour necrosis factor (TNF)-α[7]. The activated astrocytes can produce a repertoire of proinflammatory cytokines such as interleukin (IL)-8, and monocyte chemoattractant protein-1 (MCP-1) [8–11]. Therefore, astrocytic release of cytokines and chemokines can further exacerbate the pathological processes of degenerative or inflammatory CNS diseases.
Proinflammatory mediators, including chemokines (IL-8, growth-related oncogene (GRO)-α and MCP-1), TNF-α and inducible nitric oxide synthase (iNOS), are important mediators for the inflammatory response to many stimuli such as infection with pathogens [12,13]. These proinflammatory mediators have been shown to have multiple biological functions in inflammatory responses such as chemoattraction/activation of a variety of inflammatory cells and induction of fever [12,13]. Interestingly, astrocytes infected with some pathogenic viruses are known to be up-regulated expression of several chemokine genes [14–16], however, very little is known about these proinflammatory mediator genes expression in astrocytes following infection with E. coli, which is a potentially important mechanism underlying the initiation of inflammatory disease in the CNS. Although E. coli infection is a relatively rare cause of brain abscesses or meningitis, it is a useful paradigm and tool for study about inflammatory responses in the CNS.
Many of the genes that are activated by bacterial infection have been shown to be target genes of the transcription nuclear factor-kappa B (NF-κB) [17–21]. NF-κB is a dimeric transcription factor which is held in the cytoplasm in an inactive state by IκBs [22–24]. IκB kinases (IKK) directly phosphorylate IκBs, thereby freeing NF-κB dimer from the inactive complex to translocate to nucleus. However, the role of NF-κB in the E. coli-induced signal transduction of astrocytes has not yet been clarified. In the present study employing primary astrocyte cultures and cell lines, we examined the activation of potential proinflammatory mediator genes in astrocytes following infection with E. coli, and demonstrated that E. coli could induce chemokines (IL-8, GRO-α, and MCP-1), TNF-α and iNOS through the NF-κB pathway.
Materials and methods
Astrocyte cultures and infection
U87-MG (ATCC HTB-14) human astrocytoma cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS; Gibco BRL, Grand Island, NY, USA) and 2 mm l-glutamine.
The fetal astrocytes were isolated from 20 to 25-week-old human fetal samples. Use of the tissue samples was approved by the Institutional Review Board. The astrocytes were purified as described previously [25,26]. Astrocyte cultures yielded more than 98% positive for glial fibrillary acidic protein (GFAP) and less than 1% of the cells were microglia, based on their positive staining for HLA-DR. The primary cultures of astrocytes were maintained for up to 8 weeks after purification.
Before infection, after removal of antibiotics, the astrocytes were cultured in 6-well tissue culture plate for 24–48 h, and confluent monolayers of the cells in the culture plate (1 × 106/well) were incubated with E. coli (1 × 107/well) for the indicated times: E. coli K1 was grown to late log phase at 37°C in tryptic soy broth. The uninfected controls were included in every experiment performed. In some experiments, astrocytes were treated with iNOS inhibitor (NG-nitro-L-arginine methyl ester (L-NAME), ICN Biomedical, Aurora, OH, USA) or an inhibitor of IκB kinase (IKK) (NF-κB essential modifier (NEMO)-binding domain (NBD) peptide (200 µm, Peptron, Daejeon, Korea)), for 1 h before and during the exposure to E. coli. An NBD peptide can block association of NEMO with the IKK complex and inhibit NF-κB activation [20,27].
RT-PCR analysis, ELISA, and determination of nitrite/nitrate
Astrocytes in 6-well plates were infected with E. coli for the indicated times, and then total cellular RNA was extracted using Trizol reagent (Life Technology, Palo Alto, CA, USA). Quantitative RT-PCR by using internal standard was used to quantify mRNA levels, as described previously [20]. mRNA levels of 5 × 103 molecules/µg of total RNA were considered positive, although lower levels could be detected and quantified, however, considered to be biologically insignificant, since they would reflect average less than 1 mRNA/20 cells [28]. Synthetic standard RNA was kindly provided by Dr Kagnoff at the University of California, San Diego. PCR amplification consisted of 32–35 cycles of 1-min denaturation at 95°C, 2·5-min annealing and extension at either 60°C (IL-8 and GRO-α), 65°C (MCP-1 and RANTES), or 72°C (TNF-α and β-actin). PCR amplification for eotaxin and human iNOS consisted of 35 cycles of 45 s denaturation at 94°C, 45 s annealing at either 53°C (eotaxin) or 60°C (iNOS), and 2 min extension at 72°C.
Cytokines in culture supernatants were assayed by ELISA. Before cytokine proteins were measured, the supernatants were filtered through a 0·22-µm filter to remove any contaminants. The levels of IL-8, GRO-α, MCP-1, and TNF-α were determined by Quantikine immunoassay kits (R & D Systems, Minneapolis, MN, USA). Cytokine proteins were quantified in triplicate, and the detection limit was the same for the all five cytokines; 5 pg/ml.
To determine the levels of stable NO end products (nitrite and nitrate), the nitrate was first reduced to nitrite by incubating the samples for 60 min at room temperature with 0·05 U/ml nitrate reductase (Oxford Biomedical, Oxford, MI, USA) and 0·1 m mβ-NADH in 25 m m MOPS buffer (pH 7·0) containing 0·5 m m EDTA. The nitrite levels were then determined using the Griess reaction, as described previously [29,30].
Electrophoretic mobility shift assays
Cells were harvested, and nuclear extracts were prepared as described [19,20]. The concentrations of proteins in the extracts were determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). Electrophoretic mobility shift assays (EMSA) were performed according to the protocol of the manufacturer (Promega, Madison, WI, USA). In brief, 5 µg of nuclear extracts were incubated for 30 min at room temperature with 32P-labelled oligonucleotide probe containing a consensus NF-κB binding site. After incubation, bound and free DNAs were resolved on 5% native polyacrylamide gels as described previously [19,20].
Supershift assays were used to identify the specific members of the NF-κB family, which could be activated by infection of E. coli. EMSA was performed as described above except that rabbit antibodies (1 µg/reaction) raised against NF-κB proteins, including p50, p52, p65, c-Rel, and Rel B (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added during the binding reaction period [19,20].
Immunoblots for IκB, IKK and NOS
Confluent monolayers in 6-well plates were washed with ice-cold PBS, and lysed in a 0·5 ml/well lysis buffer[150 m m NaCl, 20 m m Tris (pH 7·5), 0·1% Triton X-100, 1 m m PMSF, 10 µg/ml aprotonin], as described previously [19,20]. Protein concentrations in the lysates were determined by the Bradford method (Bio-Rad). Proteins (15–50 µg/lane) were size-fractionated on a denaturing polyacrylamide minigel (Mini-PROTEIN II; Bio-Rad) and electrophoretically transferred to a nitrocellulose membrane (0·1-µm pore size). Specific proteins were detected, using mouse anti-human IκBα and IκBβ (Santa Cruz Biotechnology), phospho-IKKα/IKKβ (Cell Signalling Technology, Beverly, MA, USA), iNOS (Transduction Laboratories, Lexington, KY, USA), or nNOS (BD Sciences, Franklin Lakes, NJ, USA) as a primary antibody, and peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (Transduction Laboratories, Lexington, KY, USA) as a secondary antibody. Specifically bound peroxidase was detected by enhanced chemiluminescence (ECL system; Amersham Life Science, Amersham, UK) and exposure to X-ray film (XAR5; Eastman Kodak Company, Rochester, NY, USA) for 10–30 s.
Plasmids transfections and luciferase assays
A mammalian expression vector encoding a haemagglutinin (HA) epitope-tagged mutant IκBα (IκBα-AA) having substitutions of positions 32 and 36 serine residues with alanine residues (a gift of Joseph A. DiDonato, Cleveland Clinic Foundation, Cleveland, OH, USA) [31] and an expression vector encoding FLAG-tagged IKKα whose lysine at position 44 was replaced by alanine (IKKα-AA) [a gift of Dr Mercurio (Signal Pharmaceuticals, San Diego, CA, USA)][32], were used to block NF-κB activation. An expression vector for an NF-κB-inducing kinase (NIK) catalytic mutant, that has double replacement of positions 429 and 430 alanine residues to lysine residues and lacks kinase activity (NIK-AA), was a gift of Dr Karin, the University of California, San Diego [17]. Expression vectors for the superrepressor versions of TNF receptor-associated factor (TRAF)2 and TRAF5 (amino-terminal deletions) [33,34] were gifts from Joseph A. DiDonato [17]. pIL8-luciferase, p2x NF-κB-luciferase, pβ-actin-luciferase and pRSV-β-galactosidase transcriptional reporters were kindly provided by Dr Kagnoff at the University of California, San Diego, USA [17], and pMCP-1-luciferase transcriptional reporters were kindly provided by Dr Ik-Sang Kim at the Seoul National University College of Medicine, Seoul, Korea [35]. Cells in six-well dishes were transfected with 1·5 µg of plasmid DNA, using Lipofectamine Plus (Gibco BRL), according to the manufacturer's instructions. The transfected cells were incubated for 48 h at 37°C in a 5% CO2 incubator, and then were incubated with E. coli or recombinant human TNF-α (R & D Systems) as a control for 6 h. Whole cell lysates were prepared as described before [19,20,30]. Briefly, cells were lysed at 4°C for 25 min in a whole cell lysis buffer (0·1 m KPO4, 0·1 m DTT, 0·5% Triton X-100, pH 7·8), and luciferase activity was determined and normalized relative to β-galactosidase expression in accordance with the manufacture's instruction (Tropix Inc., Bedford, MA, USA).
Recombinant retrovirus and antisense p50 oligonucleotide transfection
Dominant-negative IκBα (S32A, S36A) [31] was amplified with sense (5′-aaccATGGCATACCCATACGACGTCCCA GACTACGCTttccaggcggccgagcgcccccaggag-3′) and antisense (5′-aaaaGGATCCtcataacgtcagacgctggcct-3′) primers by using high fidelity Taq polymerase (Life Technology). The capital letters represent nucleotides encoding a HA tag. The PCR products were digested with Nco-1 and BamH1 restriction enzymes and cloned into the corresponding sites in MFG retroviral vector by replacing the GFP sequence of MFG.GFP.IRES.puro. The retroviral plasmids obtained were introduced into 293 gpg retrovirus packaging cell line by transient transfection with Lipofectamine (Life Technology) [30]. After 72 h, the supernatants were harvested and used for retroviral infection. The virus titres, measured in NIH3T3 cell line by puromycin-resistant colony formation, were between 105 and 5 × 105/ml (retrovirus-IκBα-AA). The infection and selection of target cells with puromycin was performed as described previously [30].
The experiments by using antisense p50 oligonucleotide transfection were performed as described before [19]. U87-MG astrocytes were treated for 60 h with oligonucleotide (final concentration of 0·5 µm), using a cationic liposome, a commercially available transfection reagent DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethyl ammonium methylsulphate, 15 µl/ml; Boehringer-Mannheim, Mannheim, Germany) to improve stability and intracellular delivery of oligonucleotide [19]. Cells were trypsinized and plated again at a density 5 × 105 cell/ml in 24-well plates. Oligonucleotide was added to the cells at a final 0·5 µm concentration and incubated for an additional 18 h. The transfected monolayers were infected with E. coli for 24 h and the culture medium was collected. The amount of each cytokine and NOx was measured using ELISA and the Griess reaction, respectively.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for quantitative RT-PCR, and mean ± standard error of the means (SEM) for ELISA and luciferase assay. Wilcoxon's rank sum test was used for statistical analysis. A P-value less than 0·05 was considered statistically significant.
Results
E. coli infection up-regulates IL-8, GRO-α, MCP-1, TNF-α, and iNOS in astrocytes
CXC chemokine such as IL-8 and GRO-α, CC chemokine MCP-1, TNF-α, and iNOS are proinflammatory mediators that are involved in the inflammatory process. We assessed gene expression of these mediators in astrocytes following E. coli infection. As described in Table 1, quantification of mRNA using synthetic standard RNA showed that the levels of IL-8, GRO-α, MCP-1, TNF-α, and iNOS mRNA expression in primary fetal human astrocytes were 6–49 folds higher by E. coli infection than those by control. The numbers of proinflammatory mediator mRNA transcripts in U87-MG cell lines also increased in response to E. coli infection (data not shown). However, mRNA expressions of eotaxin and RANTES were not increased in the E. coli-infected astrocytes.
Table 1.
mRNA expression of cytokines and iNOS in primary human astrocytes infected with E. coli*.
| Time after infection (h) | |||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 24 | |
| IL-8 | 11·0 ± 7·9† | 390 ± 193 (35·5) | 538 ± 301 (49·0) | 434 ± 207 (39·5) | 272 ± 125 (24·8) |
| GRO-α | 2·8 ± 2·3 | 16·1 ± 6·3 (5·8) | 27·6 ± 12·7 (9·9) | 28·6 ± 9·0 (10·3) | 22·4 ± 13·9 (8·1) |
| MCP-1 | 3·8 ± 4·1 | 33·2 ± 25·7 (8·5) | 41·4 ± 21·5 (10·6) | 59·8 ± 23·6 (15·3) | 38·6 ± 11·6 (9·8) |
| TNF-α | 0·8 ± 0·6 | 13·8 ± 6·9 (16·4) | 25·2 ± 15·5 (29·9) | 22·2 ± 13·6 (26·3) | 18·0 ± 4·9 (21·3) |
| iNOS | 16·3 ± 4·4 | 133 ± 85 (8·1) | 307 ± 178 (18·8) | 218 ± 96 (13·4) | 152 ± 76 (9·3) |
| Eotaxin | < 0·5 | < 0·5 | < 0·5 | < 0·5 | < 0·5 |
| RANTES | < 0·5 | < 0·5 | < 0·5 | < 0·5 | < 0·5 |
| β-actin | 284 ± 101 | 392 ± 211 (1·4) | 430 ± 162 (1·5) | 530 ± 123 (1·9) | 324 ± 174 (1·1) |
Primary human astrocytes in 6-well plates were incubated with E. coli for the indicated times. The ratio of E. coli to astrocytes was adjusted to 10 : 1. For quantification of the expressed transcripts, total RNA was reverse-transcribed using an oligo(dT) primer and synthetic internal RNA standards, and amplified by PCR.
mean numbers ± SD of mRNA transcripts (104)/µg RNA (n = 5). Parentheses are mean fold-induction compared with uninfected control.
Astrocytes can express neuronal NOS (nNOS) [36]. Therefore, in order to determine whether the level of iNOS mRNA correlated with the iNOS protein expression and whether E. coli infection influenced nNOS expression in astrocytes, cell lysates extracted from E. coli-infected and uninfected primary human astrocytes were analysed by immunoassay. The level of iNOS protein was increased at 12 h and 24 h after E. coli infection, nNOS protein bands were not changed, and endothelial NOS (eNOS) protein was not expressed, regardless of E. coli infection (data not shown). In this system, E. coli infection of primary human astrocytes increased production of NOx (i.e. NO2– and NO3–) and the addition of iNOS inhibitor, L-NAME (0·5 m m), reduced the NOx production (control, 7·7 ± 1·2 µm; E. coli-infected; 31·8 ± 6·2 µm; E. coli+ L-NAME; 15·6 ± 3·5 µm; mean ± SEM, n = 3). These results indicate that increase of NO production by E. coli infection may be due to up-regulation of iNOS.
The magnitude of the cytokine response was proportional to the number of infected E. coli per astrocyte. Infection of the primary astrocytes (1 × 106 cells) with increasing number of E. coli for 12 h paralleled with the increased IL-8 mRNA expression: At 12 h after infection of the cultured cells with 105, 106, 107, 108 and 109 CFU of E. coli, the IL-8 mRNA transcripts increased by 1·6 ± 1·1, 17·1 ± 4·2, 36·9 ± 3·7, 35·2 ± 4·9, and 25·8 ± 6·3-folds, respectively, compared to those of uninfected controls (mean fold-increase ± SD, n = 3). In this experiment, mean number of IL-8 mRNA transcripts of the uninfected control was 2·1 × 105 transcripts/µg RNA.
E. coli infection induces activation of p50/p65 heterodimeric NF-κB and degradation of IκBα in human astrocytes
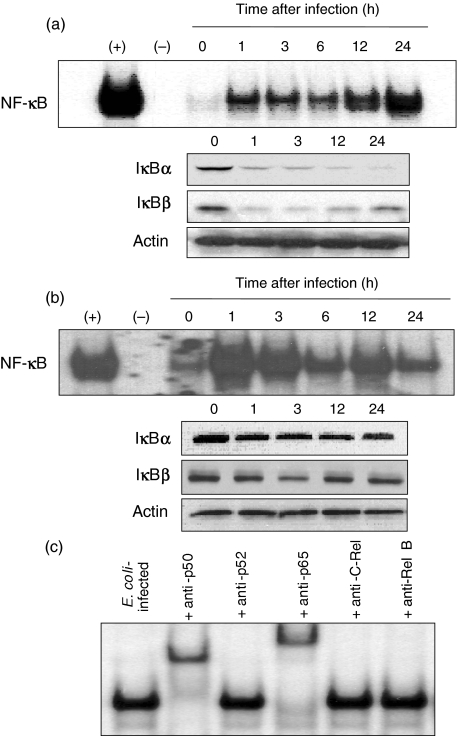
The transcription factor NF-κB has a role in the transcriptional activation of several proinflammatory genes in bacterial infections. To determine whether E. coli could activate NF-κB in astrocytes, DNA binding studies were performed using cell extracts obtained at various times after infection of human astrocytes. As seen in Fig. 1a and b, infection of the primary astrocytes and U87-MG cell lines with E. coli increased NF-κB DNA binding, as shown by EMSAs. In addition, degradation of IκBαand IκBβwas observed in E. coli-infected astrocytes, as determined by immunoblot analysis.
Fig. 1.
NF-κB activation and IκB degradation in human astrocytes infected with E. coli. (a) Primary human astrocytes or (b) U87-MG cell lines were infected with E. coli. NF-κB DNA binding activity at the indicated times was assessed by EMSA. Concurrent immunoblots for IκBα, IκBβ and actin levels in human astrocytes under the same condition are provided beneath each EMSA time point. The results are representative of five repeated experiments. (+) represents positive control in which astrocytes were treated with TNF-α (20 ng/ml) for 1 h (–) represents negative control. (c) Activation of specific NF-κB subunits in primary human astrocytes infected with E. coli. Supershift assays were performed using antibodies to p50, p52, p65, c-Rel and Rel B. Antibodies to p50 and p65 shift the entire NF-κB signals. Anti-p50, c-Rel and Rel B did not show the shifts. The results are representative of three repeated experiments.
NF-κB exists as either homo- or heterodimeric complexes [24]. Therefore, to identify the specific NF-κB subunits that comprise the NF-κB signal, a supershift assay was performed, using specific antibodies to p50, p52, p65, c-Rel, and Rel B. As shown in Fig. 1c, in primary human astrocytes after E. coli infection, the antibody to p65 was found to shift the entire signal and the antiboy to p50 also caused a significant shift. However, anti-p52, anti-c-Rel, or anti-Rel B antibodies did not shift the NF-κB signals. Similar results were also obtained in the U87-MG human astrocyte cell lines (data not shown).
Inhibition of NF-κB suppresses the expression of IL-8, GRO-α, MCP-1, TNF-α, and iNOS in astrocytes infected with E. coli
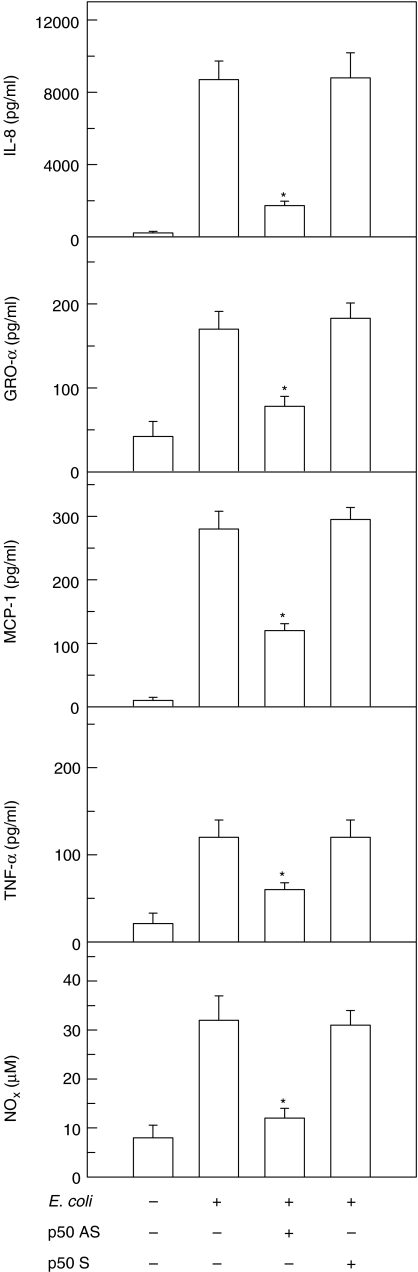
Oligonucleotides consisting of either antisense or sense p50 mRNA binding sequence are taken up by cells, and the presence of antisense oligonucleotide in cytoplasm prevents p50 mRNA transcription and NF-κB activation, resulting in the inhibition of NF-κB-dependent events [19]. Therefore, to confirm that the activated NF-κB was directly related to E. coli-induced cytokine or NOx production, p50 antisense oligonucleotide experiments were performed. As shown in Fig. 2, p50 antisense oligonucleotide significantly decreased the E. coli-induced production of CXC chemokine (IL-8 and GRO-α), CC chemokine (MCP-1), TNF-α, and NOx. On the other hand, sense oligonucleotide sequence had no significant effect. Consistent with these, as shown in Fig. 3, blocking the activation of NF-κB in primary astrocytes transfected with retrovirus-IκBα-AA significantly inhibited the expression of those proinflammatory mediators up-regulated by E. coli infection. In these experiments, transfection with retrovirus-IκBα-AA completely blocked NF-κB activity in E. coli-infected cells, however, the control retrovirus containing GFP plasmid did not change NF-κB activation (Fig. 3a), demonstrating a direct association between NF-κB activation and production of proinflammatory mediators in response to E. coli infection. We asked whether retrovirus-IκBα-AA could influence IL-8 expression in the astrocytes stimulated with TNF-α. Retrovirus-IκBα-AA almost completely inhibit expression of IL-8 induced by TNF-α stimulation (control, 0·38 ± 0·25; TNF-α stimulation, 11·87 ± 4·2; TNF-α+ retrovirus-IκBα-AA, 0·56 ± 0·32; TNF-α+ retrovirus-control plasmid, 12·80 ± 3·6; mean ± SEM (ng/ml) of three separate experiment). These results suggest that retrovirus-IκBα-AA can completely suppress IL-8 expression via TNF-α-induced NF-κB pathway.
Fig. 2.
Effect of p50 antisense oligonucleotide on E. coli-induced cytokine and nitric oxide production. U87-MG astrocytes were transfected with either p50 antisense (AS) or sense (S) oligonucleotides using DOTAP. The transfected cells were then infected with E. coli for 24 h. Protein levels of each cytokine in culture supernatants were determined by ELISA. The levels of NOx (i.e. NO2– and NO3–) were determined, using the Griess reaction and nitrate reductase. Data are mean ± SEM (n = 5). *values with E. coli+ antisense oligonucleotide sequence that are significantly different from those of E. coli alone (P < 0·05).
Fig. 3.
Expression of proinflammatory mediator genes in E. coli-infected astrocytes transfected with retrovirus containing IκBα superrepressor. (a) Primary human astrocytes were transfected with either retrovirus containing IκBα-superrepressor (retrovirus-IκBα-AA) or control virus (retrovirus-GFP). At 48 h after transfection, the cells were infected with E. coli for 3 h. NF-κB binding activity was assayed by EMSA. (b) Transfected astrocytes were then infected with E. coli for 9 h. For quantification of the expressed transcripts, total RNA was reverse-transcribed using an oligo(dT) primer and synthetic internal RNA standards, and amplified by PCR. Data are presented as percentage of increase, compared with E. coli-infected (mean ± SD, n = 5). *statistical significance with P < 0·05 in comparison with control virus-transfected cells infected with E. coli. (□) E. coli-infected; (▪) E. coli-infected cells transfected with retrovirus-IκBα-AA; ( ) E. coli-infected cells transfected with control virus (retrovirus-GFP).
) E. coli-infected cells transfected with control virus (retrovirus-GFP).
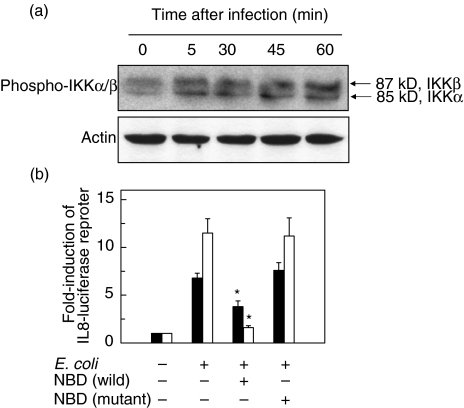
E. coli infection increases phosphorylated IKK signals in astrocytes
Major pathways for NF-κB activation are involved in the activation of IKK, which is followed by IκB degradation [23]. In the present study, E. coli infection increased the signals of phosphorylated IKKα/β in primary human astrocytes (Fig. 4) and U87-MG cell lines (data not shown). In order to study whether the activation of IKK was one of the major pathways that culminated in the expression of proinflammatory cytokines following E. coli infection, reporter gene activation using luciferase assay was performed. Addition of NBD peptide decreased the activation of IL-8 reporter genes in U87-MG cells infected with E. coli (Fig. 4b). Similar results were obtained in the primary astrocytes infected with E. coli in the presence of NBD peptide (IL-8, control, 0·19 ± 0·13; E. coli-infected, 4·76 ± 0·96; E. coli+ NBD (wild), 1·62 ± 0·98; E. coli+ NBD (mutant), 4·20 ± 1·57; mean ± SEM (ng/ml) of three separate experiments). These results demonstrate the involvement of activation of IKK as a crucial step for NF-κB activation in astrocytes following E. coli infection.
Fig. 4.
Phosphorylation of IKK in E. coli-infected astrocytes. (a) Primary human astrocytes were incubated with E. coli for the indicated times. The ratio of E. coli to the cells was adjusted to 10 : 1. Phosphorylation and protein expression of IKKα/β and actin were assessed by immunoblot. (b) U87-MG cells were transfected with pIL-8-luciferase transcriptional reporters. After 48 h, the transfected cells were incubated with E. coli (▪) or TNF-α (20 ng/ml, □) for 6 h. Data are expressed as mean fold induction ± SEM in luciferase activity relative to nonstimulated controls (n = 5). *values with E. coli + the NBD peptide that are significantly different from those of E. coli alone (P < 0·05).
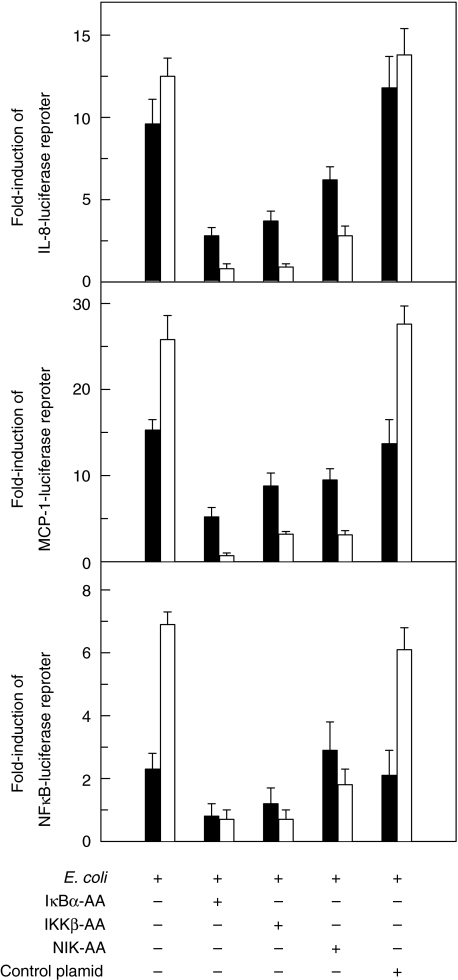
Activation of NF-κB, IL-8 and MCP-1 reporter genes induced by E. coli infection is inhibited by IκBα, IKKβ and NIK superrepressors
We asked a question of whether the activation of IκB, IKK and NIK was involved in signalling pathway of astrocytes in response to E. coli infection. As shown in Fig. 5, the activation of the IL-8, MCP-1, and NF-κB transcriptional reporters was inhibited in U87-MG astrocyte cell lines when cotransfected with IκBα, IKKβ or NIK superrepressor plasmids, but not when cotransfected with control plasmid. However, the inhibition of IL-8, MCP-1 and NF-κB transcriptional reporters by NIK superrepressor was less than that by IκBα or IKKβ superrepressors. These results suggest that NF-κB activation and IL-8/MCP-1 expression in the astrocytes by E. coli infection may be involved in other pathways, although NIK can partially be involved.
Fig. 5.
The effects of IκBα, IKKβ and NIK super-repressors on reporter gene activation in E. coli-infected U87-MG astrocytes. U87-MG cells were transfected with pIL8-, pMCP-1, or p2x NF-κB-luciferase transcriptional reporters together with IκBα-AA, IKKβ-AA, or NIK-AA expression vectors, as indicated. After 48 h, the transfected cells were infected with E. coli (▪) or TNF-α (20 ng/ml, □) for 6 h. Data are expressed as mean fold induction ± SEM in luciferase activity relative to noninfected controls (n = 7).
E. coli-induced activation of NF-κB requires TNF receptor-associated factors
Many of the NF-κB target genes, including IL-8, may be activated by signalling through TNF receptor [37]. We attempted to assess therefore whether signal transduction via TNF receptors is also required for the activation of the NF-κB target gene IL-8 in E. coli-infected cells. For these studies, an IL-8-luciferase transcriptional reporter was cotransfected into U87-MG cells with a TRAF2 superrepressor plasmid. In addition, some cultures were cotransfected with the IL-8-luciferase reporter together with a plasmid expressing a superrepressor of TRAF5, a protein which is important for the activation of NF-κB following signal transduction through the lymphotoxin-β receptor (LT-βR) [38]. Cultures were subsequently infected with E. coli or stimulated with TNF-α (20 ng/ml) as a control. As shown in Table 2, increased luciferase activity in response to E. coli infection was significantly inhibited by blocking either TRAF2 or TRAF5. To confirm that E. coli-induced TRAF activation was directly associated with the secretion of IL-8 proteins, ELISA for was also performed and the result showed (control 0·27 ± 0·15, E. coli-infected 8·33 ± 1·05, E. coli+ TRAF2 3·03 ± 0·51, E. coli+ TRAF5 3·91 ± 0·38, E. coli+ control plasmid 8·90 ± 0·98; mean ± SEM (ng/ml) of seven separate experiments). Similar results were also obtained in the E. coli–infected primary human astrocytes transfected with superrepressors of TRAF2 or TRAP5 (control 0·21 ± 0·06, E. coli-infected 4·29 ± 0·56, E. coli+ TRAF2 2·07 ± 0·95, E. coli+ TRAF5 2·53 ± 0·97, E. coli+ control plasmid 4·31 ± 0·96; mean ± SEM (ng/ml) of three separate experiments). These results suggest that NF-κB activation induced by E. coli-infection requires intracellular signalling molecules TRAFs.
Table 2.
Activation of IL-8-luciferase reporter genes in E. coli-infected astrocytes.*
| IL-8-luciferase activity | ||
|---|---|---|
| E. coli-infected | TNF-α-stimulated | |
| None | 6·21 ± 1·88† | 10·22 ± 1·11 |
| TRAF2 | 3·12 ± 0·64‡ | 1·85 ± 0·64‡ |
| TRAF5 | 4·91 ± 1·21‡ | 3·36 ± 0·46‡ |
| Control plasmid | 6·67 ± 2·77 | 11·17 ± 2·33 |
U87-MG cells were transfected with IL-8-luciferase transcriptional reporters together with expression vectors for superrepressors of TRAF2 or TRAF5, as indicated. After 48 h, cells were infected with E. coli or stimulated with TNF-α (20 ng/ml) for 6 h.
mean fold induction ± SEM in luciferase activity relative to noninfected controls (n = 7).
Significantly different from values for nontransfected cells infected with E. coli or stimulated with TNF-α, respectively (P < 0·05).
In contrast to E. coli infection, lipopolysaccharide (LPS, 10 ng/ml) stimulation did not change IL-8 expression in the primary human astrocytes transfected with superrepressors of TRAF2 or TRAP5 (control 0·29 ± 0·12, LPS stimulated 1·85 ± 0·32, LPS + TRAF2 1·62 ± 0·28, LPS + TRAF5 1·82 ± 0·25, LPS + control plasmid 1·92 ± 0·38; mean of IL-8 release ± SEM (ng/ml) of three separate experiment). These results suggest that IL-8 expression via TRAFs may reflect E. coli itself rather than LPS activity.
Discussion
E. coli infection causes inflammation such as meningitis or brain abscess [1,14]. Although E. coli infection is a relatively rare cause of brain abscesses or meningitis, it is a useful paradigm and tool for study about inflammatory responses in the CNS. In the present study, we have demonstrated that the infection of human astrocytes with E. coli up-regulated the expression of proinflammatory mediators such as IL-8, GRO-α, MCP-1, TNF-α, and iNOS.
The release of chemokines such as IL-8, GRO-α, and MCP-1 can contribute to the inflammatory cell infiltration. Especially, IL-8 and GRO-α, CXC family of chemokines, are known to be chemoattractants and activators for neutrophils [39]. MCP-1 is a member of the CC family of chemokines, and directs neutrophil and monocyte/macrophage infiltration to the site of infection. These chemokines have also been found in various inflammatory responses at CNS [8]. In addition, cytokine TNF-α is known not only to be critically involved in the systemic inflammatory process such as shock, fever, and production of acute phase proteins [40,41], but also act as a neurotoxic mediator [7]. iNOS converts l-arginine to l-citrulline to generate NO which is known to be involved in the inflammatory process in CNS [42]. Since the patients with bacterial infection in CNS show high fever and inflammatory cell infiltration such as neutrophils [43–45], it is quite obvious that these mediators contribute to the inflammatory processes in CNS. However, we did not find up-regulation of eotaxin and RANTES that are potent chemoattractants and activators of eosinophils. Since eosinophil infiltration is not a major finding of CNS infected with pathogenic bacteria [43,45], eosinophils appear to play a biologically insignificant role in inflammatory response of E. coli-infected CNS tissues.
NF-κB plays a key role in regulating the transcription of several members of proinflammatory gene family that is induced in response to inflammation or infection with pathogens [17–21]. Activation of NF-κB in the cytoplasm involves the inducible phosphorylation of IκBs, which then undergoes ubiquitin-mediated proteolysis, thereby releasing NF-κB dimers to translocate to the nucleus [22–24]. In this study, E. coli infection was found to activate NF-κB in the astrocytes, as assayed by EMSA, and degradation of IκBα and IκBβ was observed in E. coli-infected cells. Interestingly, the pattern of NF-κB expression showed prolonged activation, and IκBs disappeared rapidly and for a long period in the primary human astrocytes. In contrast, this phenomenon was not observed in the U87-MG cell line. This discrepancy may be due to the difference between a primary cell and an established cell line. Furthermore, blocking the NF-κB activation significantly decreased the expression of the proinflammatory mediators. These results indicate that phosphorylation and degradation of the inhibitory protein IκBs and subsequent dissociation of this protein from NF-κB complex are necessary for the proinflammatory mediator expression in response to E. coli infection. Suppression of NF-κB activity, however, did not completely inhibit the expression of proinflammatory mediators. Furthermore, both activator protein-1 (AP-1) and NF-κB transcription factors have been shown to be required in the activation of chemokine genes in human astrocytes infected with picornaviruses [14]. Therefore, it is highly likely that other pathways such as mitogen-activated protein (MAP) kinase pathway may be involved in the expression of the proinflammatory mediators induced by E. coli infection.
IKK has been shown to be a key intermediate in the signal transduction pathway leading to NF-κB activation following infection with pathogenic bacteria [17,19,20,37]. In this study, E. coli infection increased the signals of phosphorylated IKKα/β in astrocytes. NEMO is required for the activation of IKK by inflammatory stimuli such as TNF-α[46,47]. Treatment with an NBD peptide, which blocks the association of NEMO with the IKK complex, decreased the NF-κB target gene IL-8 expression in E. coli-infected astrocytes. These findings suggest that transcription of the proinflammatory mediators in response to E. coli infection is regulated via IKK activation. The activation of IKK requires their phosphorylation, which is mediated by NIK [48]. As shown in the present study, transfection with NIK superrepressor reduced the up-regulation of the IL-8, MCP-1, and NF-κB reporter activation in response to E. coli infection. These results suggest that the integral components of the signal transduction pathway leading to NF-κB activation such as NIK may be involved in signal transduction for the activation of the NF-κB target genes in E. coli-infected cells.
Activation of NF-κB through TNF receptor family members in response to extracellular signalling involves TRAFs that serve as adaptor molecules in these signal transduction pathways (e.g. TRAF2 and TRAF5 are involved in signalling through TNF and LT-βR, respectively) [38,49]. At upstream of the TNF-α-activated signal transduction pathway, NIK is known to interact with TRAF2, leading to NF-κB activation and IL-8 expression [50]. Recently, iNOS expression of astrocytes has been demonstrated to acquire TRAF2, TRAF6 and NIK-dependent pathway [51]. In addition, the infection of colon epithelial cells with enteroinvasive bacteria, such as Salmonella, could induce NF-κB activation, in which TRAF2 and TRAF5 were involved in this signal transduction [17]. Similar to the above, the present study also showed that interference of TRAF2 signalling by transfection with a superrepressor of TRAF2 significantly inhibited IL-8 reporter gene activity in response to E. coli infection, and that blocking TRAF5 also inhibited IL-8 reporter gene activity. Taken together, our findings suggest that E. coli infection activates a number of different intracellular signalling pathways used by TNF receptor family members, ultimately culminating in the activation of NF-κB and its target genes.
In conclusion, our data indicate that the NF-κB signal transduction pathway, including TRAF, NIK, and IKK, is involved in E. coli-induced expression of several CXC and CC chemokines, TNF-α, and iNOS in astrocytes. These proinflammatory mediators may contribute to the inflammatory process of diseases such as meningitis or brain abscess.
Acknowledgments
The experiments comply with the current laws of our country where the experiments were performed. We thank Dr Martin F. Kagnoff and Dr Joseph A. DiDonato for gifts of standard RNAs and several plasmids, Dr Hee-Young Chung for retrovirus containing IκBα superrepressor, Dr Ik-Sang Kim for pMCP-1-luciferase plasmid, and Shin-Jai Kang and Su-Jin Cho for their excellent technical help. This work was supported by a grant from National Research Laboratory program (NRL M10400000019–04J0000-01910).
References
- 1.Kim KS. Strategy of Escherichia coli for crossing the blood–brain barrier. J Infect Dis. 2002;186:S220–4. doi: 10.1086/344284. [DOI] [PubMed] [Google Scholar]
- 2.Rau CS, Chang WN, Lin YC, et al. Brain abscess caused by aerobic Gram-negative bacilli: clinical features and therapeutic outcomes. Clin Neurol Neurosurg 2002. 2002;105:60–5. doi: 10.1016/s0303-8467(02)00103-8. [DOI] [PubMed] [Google Scholar]
- 3.Liew FY, McInnes IB. The role of innate mediators in inflammatory response. Mol Immunol. 2002;38:887–90. doi: 10.1016/s0161-5890(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 4.Conti P, Barbacane RC, Reale M. Chemokines in inflammatory states. Allergy Asthma Proc. 1999;20:205–8. doi: 10.2500/108854199778339035. [DOI] [PubMed] [Google Scholar]
- 5.Ghirnikar RS, Lee YL, Eng LF. Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem Res. 1998;23:329–40. doi: 10.1023/a:1022453332560. [DOI] [PubMed] [Google Scholar]
- 6.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–8. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–38. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert P, Morino T, Miyazaki H, Ogata T, Nakamura Y, Marchini C, Ferroni S. Cascading glia reactions: a common pathomechanism and its differentiated control by cyclic nucleotide signaling. Ann NY Acad Sci. 2000;903:24–33. doi: 10.1111/j.1749-6632.2000.tb06346.x. [DOI] [PubMed] [Google Scholar]
- 12.Azuma I. Inducer of cytokines in vivo: overview of field and romurtide experience. Int J Immunopharmacol. 1992;14:487–96. doi: 10.1016/0192-0561(92)90180-s. [DOI] [PubMed] [Google Scholar]
- 13.Salazar-Mather TP, Hokeness KL. Calling in the troops: regulation of inflammatory cell trafficking through innate cytokine/chemokine networks. Viral Immunol. 2003;16:291–306. doi: 10.1089/088282403322396109. [DOI] [PubMed] [Google Scholar]
- 14.Kwon D, Fuller AC, Palma JP, Choi IH, Kim BS. Induction of chemokines in human astrocytes by picornavirus infection requires activation of both AP-1 and NF-kappa B. Glia. 2004;45:287–96. doi: 10.1002/glia.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palma JP, Kim BS. The scope and activation mechanisms of chemokine gene expression in primary astrocytes following infection with Theiler's virus. Neuroimmunol. 2004;149:121–9. doi: 10.1016/j.jneuroim.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Peterson KE, Errett JS, Wei T, Dimcheff DE, Ransohoff R, Kuziel WA, Evans L, Chesebro B. MCP-1 and CCR2 contribute to non-lymphocyte-mediated brain disease induced by Fr98 polytropic retrovirus infection in mice: role for astrocytes in retroviral neuropathogenesis. J Virol. 2004;78:6449–58. doi: 10.1128/JVI.78.12.6449-6458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elewaut D, DiDonato JA, Kim JM, Troung F, Eckmann L, Kagnoff MF. NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–66. [PubMed] [Google Scholar]
- 18.Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Cho SJ, Oh YK, Jung HY, Kim YJ, Kim N. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002;130:59–66. doi: 10.1046/j.1365-2249.2002.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JM, Oh YK, Kim YJ, Youn J, Ahn MJ. Escherichia coli up-regulates proinflammatory cytokine expression in granulocyte/macrophage lineages of CD34+ stem cells via p50 homodimeric NF-κB. Clin Exp Immunol. 2004;137:341–50. doi: 10.1111/j.1365-2249.2004.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tato CM, Hunter CA. Host–pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infect Immun. 2002;70:3311–7. doi: 10.1128/IAI.70.7.3311-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin AS. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 24.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 25.Choi C, Park YJ, Lee J, et al. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999;162:1889–95. 1999. [PubMed] [Google Scholar]
- 26.Lee J, Shin JS, Park YK, Kwon D, Choi SJ, Kim SJ, Choi IH. p38 mitogen-activated protein kinase modulates expression of tumor necrosis factor-related apoptosis-inducing ligand induced by interferon-gamma in fetal brain astrocytes. J Neurosci Res. 2003;74:884–90. doi: 10.1002/jnr.10815. [DOI] [PubMed] [Google Scholar]
- 27.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–4. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Jung HC, Im KI, Song IS, Kim CY. Synergy between Entamoeba histolytica and Escherichia coli in the induction of cytokine gene expression in human colon epithelial cells. Parasitol Res. 1998;84:509–12. doi: 10.1007/BF03356595. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori-infected human gastric epithelial cells. possible role of interferon-gamma in polarized nitric oxide secretion. Helicobacter. 2002;7:116–28. doi: 10.1046/j.1083-4389.2002.00068.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim JM, Kim JS, Jung HC, Oh YK, Chung HY, Lee CH, Song IS. Helicobacter pylori infection activates the NF-κB signaling pathway to induce iNOS and protect human gastric epithelial cells from apoptosis. Am J Physiol – Gastrointest Liver. 2003;285:G1171–80. doi: 10.1152/ajpgi.00502.2002. [DOI] [PubMed] [Google Scholar]
- 31.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-kappa B activation. Science. 1997;278:860–6. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 33.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 34.Ishida TK, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–42. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho NH, Seong SY, Huh MS, Kim NH, Choi MS, Kim IS. Induction of the gene encoding macrophage chemoattractant protein 1 by Orientia tsutsugamushi in human endothelial cells involves activation of transcription factor activator protein 1. Infect Immun. 2002;70:4841–50. doi: 10.1128/IAI.70.9.4841-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luth HJ, Holzer M, Gartner U, Staufenbiel M, Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 2001;913:57–67. doi: 10.1016/s0006-8993(01)02758-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Dziarski R, Kirschning CY, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→ TRAF→NIKIKKNF-kappaB signal transduction pathway that induces transcription of interleukin-8. Infect Immun. 2001;69:2270–6. doi: 10.1128/IAI.69.4.2270-2276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware CF, Yagita H, Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J Biol Chem. 1996;271:14661–4. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 39.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 40.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waage A, Steinshamn S. Cytokine mediators of septic infections in the normal and granulocytopenic host. Eur J Haematol. 1993;50:243–9. doi: 10.1111/j.1600-0609.1993.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 42.Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–75. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 43.De Girolami U, Anthony DC, Frosch MP. The central nervous system. In: Cotran RS, Kumar V, Collins T, editors. Robbins Pathologic Basis of Disease. 6. Philadelphia: W.B. Saunders Company; 1999. pp. 1293–357. [Google Scholar]
- 44.Pecco P, Pavesio D, Peisino MG. Rational basis of modern therapy of bacterial meningitis. Review of the literature and our clinical experience of 122 pediatric cases. Panminerva Med. 1991;33:185–90. [PubMed] [Google Scholar]
- 45.Rosenblum MK. The central nervous system. In: Rosai J, editor. Rosai and Ackerman's Surgical Pathology. 9. Edinburgh: Mosby; 2004. pp. 2461–622. [Google Scholar]
- 46.Carter RS, Pennington KN, Ungurait BJ, Ballard DW. In vivo identification of inducible phosphoacceptors in the IKKgamma/NEMO subunit of human IkappaB kinase. J Biol Chem. 2003;278:19642–8. doi: 10.1074/jbc.M301705200. [DOI] [PubMed] [Google Scholar]
- 47.Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem. 2003;278:37297–305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- 48.Dziarski R, Gupta D. Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes. J Endotoxin Res. 2000;6:401–5. doi: 10.1179/096805100101532243. [DOI] [PubMed] [Google Scholar]
- 49.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 50.Jobin C, Holt L, Bradham CA, Streetz K, Brenner DA, Sartor RB. TNF receptor-associated factor-2 is involved in both IL-1β and TNF-α signaling cascades leading to NF-κB activation and IL-8 expression in human intestinal epithelial cells. J Immunol. 1999;162:4447–54. [PubMed] [Google Scholar]
- 51.Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFα)-dependent, and involves a TNFα receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–24. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]