Abstract
Ageing is associated with evidence of immune deficiency and dysregulation. Key changes in the immune system with ageing include a progressive reduction in naive T cell output associated with thymic involution and peripheral expansion of oligoclonal memory T cells. These features are associated with evidence of impaired immune responsiveness both in vitro and in vivo, termed immune senescence. CD4+ CD25+ T cells have recently been recognized as mediators of peripheral immune regulation and play a role in the control of autoimmune and pathogen-specific immune responses. The significance of CD4+ CD25+ regulatory T cells in the context of immunosenescence is not known. We have investigated the number, phenotype and function of CD4+ CD25+ T cells in healthy volunteers over a wide age range. We demonstrate that the number of CD4+ CD25+ and CD4+ CD25high T cells in healthy volunteers increases with age. In both age groups CD4+ CD25+ T cells showed a phenotype consistent with that described for regulatory T cells. Further analysis of CD4+ CD25high T cells in young and elderly donors showed equivalent expression of intracellular CTLA-4 and surface expression of activation markers. In vitro, functional titration assays of CD4+ CD25high T cells demonstrated equivalent regulatory function in both young and elderly donors, with suppression of proliferation and cytokine production in response to polyclonal T cell stimulation. These observations demonstrate an increase in peripheral blood CD4+ CD25high regulatory T cells associated with ageing. The relevance of these expanded cells in relation to the immune senescence seen in the elderly as yet remains unclear.
Keywords: ageing, regulatory T cell
Introduction
Normal ageing is associated with the development of a number of features of impaired immune responsiveness such as a reduction in response to recall antigens and increased vulnerability to infection and malignancy [1,2]. Impaired self-tolerance is also evident, as witnessed by the increased incidence of autoimmune disease observed with ageing. However, the mechanisms that underlie these clinical effects remain poorly understood. Thymic involution in adults is associated with a marked reduction in thymic T cell output, as measured by decreased T cell receptor (TCR) excision circle (TREC)+ T cells in subjects aged over 65 years and a reduction in the number of naive CD45RA+ T cells [3,4]. It is likely, therefore, that maintenance of the peripheral T cell pool in the elderly will depend increasingly on proliferation of existing T cells through peripheral expansion of T cells with affinity for foreign and self-antigen [5]. This phenomenon may at least partly explain the contraction of the TCR repertoire and emergence of clonal T cell populations that is seen in the memory compartment in elderly subjects [6,7]. Impaired central tolerance and a reduction in the diversity of the T cell repertoire may be key factors in immune dysregulation in the elderly and against this background peripheral mechanisms for immune regulation may become increasingly important.
Naturally occurring regulatory CD4+ T cells express high levels of CD25, the interleukin (IL)-2 receptor α chain, and have been shown to suppress CD4 and CD8 T cells responses to a range of stimuli, both in vitro and in vivo[8–10]. In mice CD4+ CD25+ regulatory T cells (Treg) are involved in maintenance of peripheral T cell tolerance to self-antigens and protection against autoimmunity. Human Treg appear to enrich within CD4+ CD25high T cells and comprise approximately 1–5% of peripheral blood CD4 lymphocytes [11]. In vitro, Treg are cytokine-independent and require cell contact to mediate suppressive action [12,13]. However, Treg are able to induce other conventional CD4+ CD25– T cells to develop cytokine dependent regulatory function, termed ‘infectious tolerance’[14], and this observation may explain, in part, the complex discrepancy between in vitro and in vivo observations regarding the role for cytokines in Treg function.
To date there is little information available on how age influences the number and function of CD4+ CD25+ regulatory T cells. We have studied CD4+ CD25+ and CD4+ CD25high T cell numbers, phenotype and function in healthy volunteer donors over a wide age range. We found marked increases in CD4+ CD25+ and CD4+ CD25high T cell numbers with increasing age. In older donors aged over 60 years, the membrane phenotype of CD4+ 25high T cells matched that from younger subjects, including intracellular expression of the negative co-stimulatory molecule cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and surface membrane expression of activation markers. Direct functional assays demonstrated suppressive effects in vitro, with CD4+ CD25high T cells from both young and elderly subjects suppressing proliferation and cytokine production in response to polyclonal T cell stimulation. Together these observations demonstrate that the proportion of CD4+ CD25high Treg increases in the peripheral blood with ageing.
Materials and methods
Subjects
Healthy volunteers between the ages of 21 and 93 years were recruited into the study. The study protocol had received local ethical approval.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood samples by density centrifugation over Ficoll-Hypaque gradient (Lymphoprep, Axis Shield, Huntingdon, UK). Antibodies used for flow cytometry were as follows: phycoerythrin (PE)- and fluorescein isothiocyanate (FITC)-conjugated anti-CD4 antibodies (13B8·2, Beckman Coulter, High Wycombe, UK); PE-cychrome 5 (PC5)-conjugated anti-CD25 (B1·49·9, Immunotech); PE-conjugated anti-CD45RO (UCHL1, BD Pharmingen, Oxford, UK); FITC-conjugated anti-CD69 (FN50, BD Pharmingen); FITC-conjugated anti-CD71 (YDJ1·2.2, Immunogen); PE-Cy5-conjugated anti-HLA-DR (TU36, BD Pharmingen) and appropriate fluorochrome labelled isotype controls. For intracellular staining, PBMC were stained with surface membrane antibodies, FITC-conjugated anti-CD4 and PC5-conjugated anti-CD25, and cells were then fixed in 2% paraformaldehyde and permeabilized with 0·1% saponin, before staining with PE-conjugated CTLA-4/CD152 (BN13, BD Pharmingen) or PE-conjugated isotype control antibody. Flow cytometry was performed on a Coulter Epics XL flow cytometer (Beckman Coulter, High Wycombe, UK) using System II software (Beckman Coulter) and analysed using WinMDI software (Joseph Trotter, Scripps Research Institute, La Jolla, CA, USA).
Isolation of CD4+ CD25high and CD4+ CD25–cells
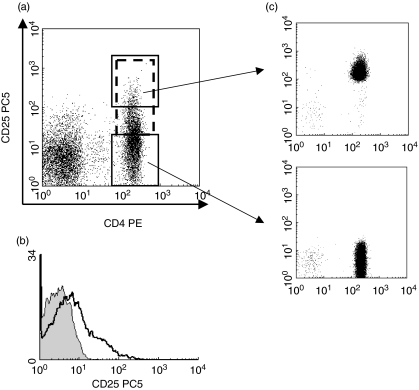
For functional assays, CD4+ CD25high and CD4+ CD25– cells were isolated by flow-sorting. Cells were stained by FITC-conjugated anti-CD4 and PE-conjugated or PC5-conjugated anti-CD25 and then purified on a Becton Dickinson FACS Vantage SE flow-cytometric cell-sorter. Consistent purity of >97% was obtained for both CD4+ CD25high and CD4+ CD25- cell fractions (Fig. 1a).
Fig. 1.
Flow cytometric analysis of CD4+ CD25+ T cell populations. Unfractionated peripheral blood mononuclear cells (PBMCs) isolated by Ficoll density gradient centrifugation were stained with phycoerythrin (PE)-conjugated anti-CD4 and PC5-conjugated anti-CD25. Cells were gated on lymphocytes via their forward- and side-scatter properties. (a) The gating strategy is shown for the analysis of CD4+ CD25+ (dotted box: including CD4+ T cells with CD25 intermediate and high expression), CD4+ CD25high (solid upper box) and CD4+ CD25– (solid lower box) T cells and for flow sorting the respective fractions. (b) Isotype controls (shaded) for each sample were run in conjunction with CD25 membrane staining (solid line). (c) Flow sorted fractions of CD4+ CD25high T cells were isolated to purity > 97% for each fraction (from three separate experiments).
Proliferation assays
RPMI-1640 medium supplemented with L-glutamine (2 m M), penicillin (100 IU/ml)/streptomycin (100 µg/ml) (all from Invitrogen, Paisley, UK) and 10% human AB serum (Sigma, Poole, UK) was used in all assays. Direct suppression ‘add-back’ experiments were performed as described previously [15]. Briefly, 1 × 104 CD4+ CD25high or CD4+ CD25– cells were incubated in the presence of phytohaemagglutinin (PHA, Sigma) at 2 µg/ml and 1 × 104 irradiated (30 Gy) autologous PBMC that had been depleted of T cells (CD3 microbeads, Miltenyi Biotec, Bisley, UK). Incubations were performed with CD4+ CD25high or CD4+ CD25– fractions alone or in co-culture (CD4+ CD25high/CD4+ 25–, CD4+ CD25–/CD4+ CD25–) at a ratio of 1 : 1. In some experiments reducing titrations of CD4+ CD25high were added to CD4+ CD25– cells (1 : 1, 1 : 2, 1 : 4, 1 : 8 and 1 : 16). All incubations were run in triplicate in 96-well plates with a final volume of 200 µl. At 72 h, 1 µCi [3H]-thymidine was added to each well and proliferation by [3H]-thymidine incorporation was assessed after a further 16 h.
Cytokine assays
CD4+ CD25high or CD4+ CD25– cells were co-cultured as above and supernatants were evaluated after 72 h for cytokine production by sandwich cytokine enzyme-linked immunosorbent assay (ELISA) for interferon (IFN)-γ (IFN-γ capture antibody and detection antibody, BD Pharmingen).
Statistics
Statistical analysis was performed using GraphPad Prism software 4·0 (GraphPad Software Inc., CA, USA). The non-parametric Spearman's rank correlation coefficient with two-tailed P-value was used to assess the significance of correlation between observed CD4+ CD25+ T cells, CD4+ CD25high T cells and subject age. Mann–Whitney analyses were performed to assess differences in young and elderly donor cell marker expression.
Results
CD4+ CD25high T cells increase in peripheral blood with increasing age
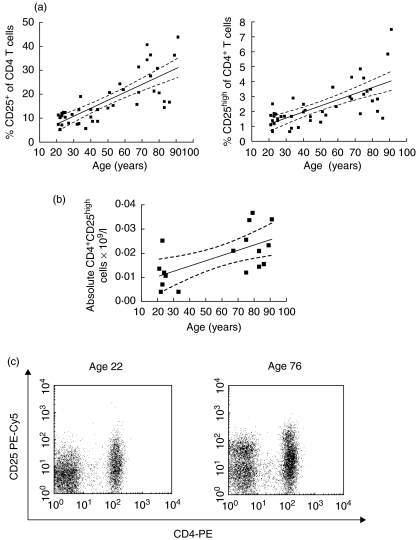
Forty-four healthy volunteer donors were recruited into the study with an age range of 21–93 years: 20–30 years (n = 14), 30–40 years (n = 5), 40–50 years (n = 5), 50–60 years (n = 5) and 60 + years (n = 15). Enumeration of CD4+ CD25+ (combined CD25int and CD25high expressing cells) and CD4+ CD25high T cells within peripheral blood mononuclear cells was performed by flow cytometry. Appropriate isotype controls were performed with each sample (Fig. 1a) and samples were run repeatedly with each batch to ensure consistency in the protocol. The percentage of CD4+ lymphocytes expressing CD25+ increased significantly with age and this effect was observed without a plateau (r = 0·808, P < 0·0001). Similarly, the percentage of CD25high expression on CD4+ T cells showed a steady rise in association with increasing subject age (r = 0·676, P < 0·0001) (Fig. 2a). In 18 subjects for whom blood counts were available the absolute number of CD4+ CD25high cells in the peripheral blood was calculated based on the peripheral blood lymphocyte count at time of sampling and this also increased significantly with increasing age (r = 0·572, P < 0·05) (Fig. 2b). No correlation was observed between subject age and the percentage of CD4+ cells in peripheral blood or the absolute peripheral blood lymphocyte count (data not shown). The proportion of CD4–CD25+ was higher in older donors than younger with median percentage of CD4–CD25+ cells in gated lymphocytes of 7·29% for donors aged > 60 and 4·93% for donors aged 20–30 (P = 0·006).
Fig. 2.
Analysis of CD4+ CD25+ and CD4+ CD25high T cells with age. The percentage of CD4+ lymphocytes which are CD25+ (a) and CD25high(b) plotted against subject age. Linear regression analysis is shown (solid line) with 95% confidence intervals (dotted lines). (c) Representative FACs plots are shown demonstrating CD4-PE and CD25-PC5 staining in a young donor aged 22 (left) and an elderly donor aged 76 (right).
CD4 CD25high T cells in elderly and young donors lack markers of T cell activation and show equivalent expression of intracellular CTLA-4
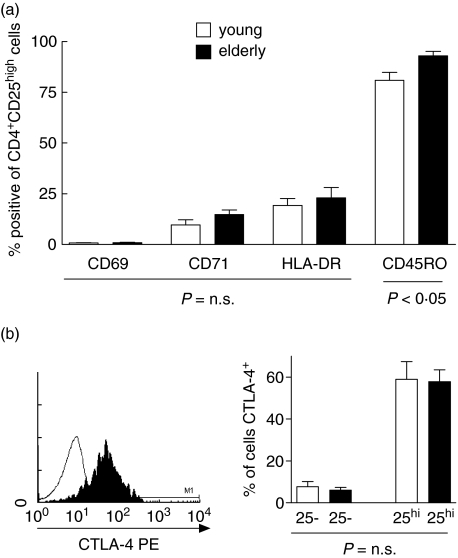
CD25 is a marker of T cell activation as well as being expressed on CD4+ T cells that mediate suppressor T cell function [16]. CD4+ CD25high T cells are therefore functionally heterogeneous and we evaluated expression of a range of phenotypic markers in order to assess potential differences in activation status and effector phenotype between young and elderly donors. The membrane phenotype of CD4+ CD25high T cells from young donors (n = 11; median age 23 years, range 21–35) and elderly donors (n = 11; median age 73 range 63–78) was determined by three-colour flow cytometry. Importantly, no demonstrable difference in the surface expression of markers of recent T cell activation (CD69, HLA-DR) and late T cell activation (CD71) was seen on gated CD4+ CD25high T cells between both age groups (Fig. 3a). The only marker that did exhibit a significant difference was CD45RO, with higher levels of CD45RO expression being seen on CD4+ CD25high T cells from elderly donors compared to those from the younger cohort (% positive, elderly 95·51%: young 83·3%, P < 0·05). Expression of intracellular CTLA-4 was assessed on CD4+ CD25high T cells from both groups and preferential expression of CTLA-4 was seen on this population in agreement with previous reports. There was no difference in the levels of expression of CTLA-4 within this fraction between young and elderly donors (% of CD4+ CD25high T cells CTLA-4 positive, elderly n = 5, 65·68%: young, n = 5, 65·46%, P = n.s.) (Fig. 3b).
Fig. 3.
Phenotype of CD4+ CD25high T cells in young and elderly subjects. (a) The membrane phenotype of CD4+ CD25high T cells was assessed by three-colour flow cytometry in both young (n = 11) and elderly (n = 11) subjects. The percentage of CD4+ CD25high T cells expressing a panel of markers (CD69, CD71, HLA-DR and CD45 RO) are shown for both young (open bars) and elderly (filled bars) volunteers. Significant differences are indicated below the columns. (b) Cells were stained with surface CD4-fluoroscein isothiocyanate (FITC) and CD25-PC5 and then fixed and permeabilized prior to intracellular staining with CTLA-4 (shaded line) and appropriate isotype control (solid). (c) Percentage of CD4+ CD25high and CD4+ CD25neg T cells expressing intracellular CTLA-4 is shown for young (n = 5) and elderly (n = 5) subjects.
CD4+ CD25high T cells in young and elderly subjects suppress immune responses in vitro
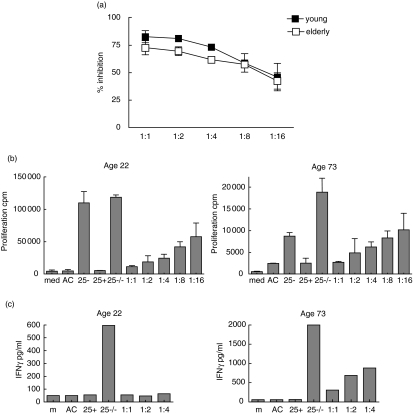
CD4+ CD25high and CD4+ CD25– T cell fractions were isolated at high purity (>97% for each fraction) (Fig. 1c) from young (n = 3, aged 21, 22 and 35) and elderly donors (n = 3, aged 63, 73 and 76). The ability of CD4+ CD25+ T cells to suppress proliferation of CD4+ CD25– T cells was determined by adding CD4+ CD25high T cells at a variable ratio to CD4+ CD25– T cells in the presence of polyclonal stimulation with PHA (2 µg/ml) and autologous irradiated CD3-depleted accessory cells (ACs) for 72 h. As shown in Fig. 4a, equivalent suppression of CD4+ CD25– T cell proliferation in response to PHA stimulation was observed in both young and elderly subjects. Furthermore, there was no significant difference in the degree of suppression mediated for each reducing titration of CD4+ CD25high T cells : CD4+ CD25– T cells, with an equivalent dose-dependent effect demonstrated for CD4+ CD25high T cell-mediated suppression in both young and elderly individuals (Fig. 4b). There was considerable variation between individuals in the proliferative response of CD4+ CD25– cells to PHA (Fig. 4b); however, this variation did not appear age-related in the subjects studied. Cytokine production of IFN-γ by CD4+ CD25–- T cells in the above PHA stimulation was also inhibited by the addition in co-culture of CD4+ CD25high T cells, and this effect was observed in both young and elderly donors (Fig. 4c).
Fig. 4.
Results of add-back suppression experiments in young and elderly subjects. Flow-sorted CD4+ CD25high or CD4+ CD25– cells were isolated from young (n = 3; age 21, 22, 35) and elderly (n = 3; age 63, 73, 76) donors. Incubations were performed with 1 × 104 CD4+ CD25high or CD4+ CD25– fractions stimulated with phytohaemagglutinin (PHA) (2 µg/ml) and 1 × 104/well irradiated (30 Gy) autologous CD3-depleted accessory cells (AC) alone or in co-culture (CD4+ CD25high/CD4+ 25–, CD4+ CD25–/CD4+ CD25–) at a ratio of 1 : 1 and with reducing titrations of CD4+ CD25high T cells 1 : 2, 1 : 4, 1 : 8 and 1 : 16. All incubations were run in triplicate in 96-well plates with a final volume of 200 µl. At 72 h, 1 µCi [3H]-thymidine was added and proliferation by [3H]-thymidine incorporation was assessed after a further 16 h. (a) % inhibition of proliferation at 72 h is shown in young (filled squares) and elderly donors (open squares); results are mean percentage for three separate donors in each age group (mean with error bars shown). Representative plots of (b) proliferation by 3H incorporation and (c) production of interferon (IFN)-γ by cytokine enzyme-linked immunosorbent assay (ELISA) are shown in a young (left) and elderly (right) donor aged 22 and 73 years, respectively.
Discussion
We have demonstrated that the number of peripheral blood CD4+ CD25high T cells increases in association with ageing. Our study included donors over 60 years of age in whom particularly high numbers were observed with a 2·4-fold increase compared to donors aged between 20 and 30 years. Human CD4+ CD25high T cells are known to be functionally heterogeneous and include cells with suppressive function and recently activated effector CD4+ T cells [16]. However, characterization of CD4+ CD25high T cells in both elderly and young donors demonstrated no differences in terms of membrane activation markers or expression of CTLA-4. The only phenotypic difference was increased expression of CD45RO in CD4+ CD25high T cells in the elderly cohort, a phenomenon well described previously for CD4+ T cells [17]. These observations suggest that the increase in CD4+ CD25high T cells in the elderly is not due to expansion of activated effector T cells and furthermore that CD4+ CD25high T cells in this age group have a phenotype consistent with that previously reported for Treg[13,16]. Currently the lack of a reliable marker that defines true Treg in the peripheral blood complicates studies in this area. Therefore to further evaluate CD4+ CD25high T cells, we have shown that CD4+ CD25high T cells from elderly donors exert suppressive function in vitro and are capable of suppressing both proliferation and cytokine production of IFN-γ. We were unable to discern any functional differences in the suppressive effects of CD4+ CD25high T cells between young and elderly donors. Together these observations indicate that there is an increase in CD4+ CD25high regulatory T cells in peripheral blood with progressive ageing.
The reasons for expansion of CD4+ CD25high regulatory T cells with age are unclear. Against a background of progressive thymic atrophy and reduced naive T cell output, it is unlikely that the expansion of CD4+ CD25high T cells numbers in the elderly is thymically derived and therefore the peripheral expansion of existing Treg may be a predominant factor. In vitro, conventional CD4+ CD25– T cells may undergo induction to a regulatory T cell phenotype in the setting of impaired co-stimulation and under the influence of immunosuppressive cytokines such as IL-10 (Tr1) [18,19]. Furthermore, under the influence of naturally occurring Treg, conventional CD4+ T cells may adopt a cytokine-dependent regulatory function, a phenomenon termed ‘infectious tolerance’[14]. In vivo the specific conditions and requirements for the peripheral generation and expansion of Treg remain unclear; however, poor co-stimulation by APCs and immunosuppressive cytokines have been suggested as important factors[20,21]. Moreover, despite their anergic phenotype in vitro, it is clear from recent reports that murine CD4+ CD25high Treg cells may undergo extensive proliferation and expansion in vivo in the setting of antigen-driven immunity [22–24]. It is possible that with ageing, broader changes within the immune environment promote Treg generation in the periphery [25,26]; however, as yet these interesting observations do not ideally explain the increases in Treg seen in human healthy donors with normal ageing. Future studies to evaluate the role of immunosuppressive cytokines in CD4+ CD25high T cell function in the elderly may be of key interest.
Finally, it is possible that the increasing CD4+ CD25high regulatory T cells numbers observed in the elderly contribute to immune deficiency and play a part in the broader decline in adaptive responses during immunosenescence. The development of markers to better delineate functional Treg will help to further dissect the significance of CD4+ CD25high T cells in the ageing immune system.
Acknowledgments
This work was supported by the Leukaemia Research Fund. Richard Gregg held a Jean Shanks Foundation award. We would like to thank David Lloyd for his assistance with FACS sorting and are grateful to the donors of the ‘Thousand Elders’ cohort for blood donation.
References
- 1.Nagami P, Yoshikawa TT. Ageing and tuberculosis. Gerontology. 1984;30:308–15. doi: 10.1159/000212650. [DOI] [PubMed] [Google Scholar]
- 2.McElhaney JE, Upshaw CM, Hooton JW, Lechelt KE, Meneilly GS. Responses to influenza vaccination in different T cell subsets: a comparison of healthy young and older adults. Vaccine. 1998;6:1742. doi: 10.1016/s0264-410x(98)00133-9. [DOI] [PubMed] [Google Scholar]
- 3.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 4.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation and HIV-1 infection. Ann Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 5.Jameson SC. Maintaining the norm: T cell homeostasis. Nat Rev Immunol. 2002;2:547–56. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 6.Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire towards greater clonality in healthy elderly individuals. J Immunol. 2002;15:1984–92. doi: 10.4049/jimmunol.169.4.1984. 169. [DOI] [PubMed] [Google Scholar]
- 7.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21:515–21. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chain (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 9.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 10.Shevach EM. Regulatory T cells in autoimmunity. Ann Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 11.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 12.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+) CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+) CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 16.Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunol. 2002;106:190–9. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Globerson A. T lymphocytes and aging. Int Arch Allergy Immunol. 1995;107:491–7. doi: 10.1159/000237091. [DOI] [PubMed] [Google Scholar]
- 18.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin-10 producing, non-proliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levings MK, Sangregorio R, Galbiati F, et al. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 20.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 21.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 22.von Boehmer H. Dynamics of suppressor T cells: in vivo veritas. J Exp Med. 2003;198:845–9. doi: 10.1084/jem.20031358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker LK, Chodos A, Eggena M, Dooms H, Abbas A. Antigen-dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisson S, Darasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198(5):7373–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghi HM, Schnelle JF, Thomas JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34:959–70. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 26.Castle S, Uyemura K, Crawford W, Wong W, Klaustemeyer W, Makinodan T. Age-related impaired proliferation of peripheral blood mononuclear cells is associated with increases in both IL10 and IL12. Exp Gerontol. 1999;34:243–52. doi: 10.1016/s0531-5565(98)00064-3. [DOI] [PubMed] [Google Scholar]