Abstract
Haem-oxygenase-1 (HO-1) has been shown to exert anti-inflammatory, anti-apoptotic and anti-proliferative effects. We investigated HO-1 expression in patients with inflammatory bowel disease (IBD) and could demonstrate a scattered expression of HO-1 in the intestinal epithelium of severely inflamed colonic mucosa of patients with IBD compared to control specimens such as diverticulitis, suggesting dysregulated expression in IBD. To further analyse potential mechanisms of HO-1 induction in the intestine we employed an in vitro epithelial cell apoptosis model and an experimental colitis model. In vitro induction of HO-1 by the HO-1 inducer cobalt protoporphyrin (CoPP) resulted in a dose-dependent down-regulation of caspase-3 activation in HT-29 cells, indicating an anti-apoptotic function of HO-1 in the intestine. In vivo, preventive HO-1 induction by CoPP in acute dextran sodium sulphate (DSS)-induced colitis led to a significant down-regulation of colonic inflammation (P < 0·01) with a concomitant reduction in interferon (IFN)-γ − but unaffected interleukin (IL)-10-secretion by isolated mesenteric lymph nodes (P < 0·01). Additionally, TUNEL staining of colonic sections demonstrated fewer apoptotic epithelial cells in the colon of CoPP treated animals. No beneficial effects were observed if HO-1 was induced by CoPP after the onset of acute colitis or in chronic DSS-induced colitis. In conclusion, the data suggest a protective role of HO-1 if it is induced before the onset of inflammation. However, as shown by the lack of effects in established acute or in chronic colitis, the induction of HO-1 may not be a promising approach for the treatment of IBD.
Keywords: apoptosis, Crohn's disease, HO-1, inflammatory bowel disease, ulcerative colitis
Introduction
The aetiology of chronic inflammatory bowel diseases is not yet completely understood. Probably environmental and genetic factors interact with the intestinal bacterial flora, which triggers an event that leads ultimately to a chronic activation of immune and nonimmune cells in the gut [1]. During this chronic inflammation mucosal tissue damage is caused partly by an enduring exposure to excessive amounts of reactive oxygen metabolites causing oxidative stress [2].
Haem-oxygenase-1 (HO-1) is the rate limiting enzyme in the conversion of haem into biliverdin/bilirubin, iron and carbon monoxide (CO); all of them can potentially function as antioxidants [3–5]. It is strongly suggested that HO-1 provides a potent cytoprotective effect, as shown in various in vitro and in vivo models of cellular and tissue injury [6–9]. HO-1 is one of the major acute phase proteins and is up-regulated by a whole variety of inducers such as endotoxin, hydrogen peroxide, prostaglandins and cytokines [interleukin (IL)-1, tumour necrosis factor (TNF)]. HO-1 knockout mice do not survive to term and the mice that do survive to adulthood are abnormal and die within a year demonstrating signs of chronic inflammation in numerous organs [10,11]. An increased expression of HO-1 has also been shown in numerous pathophysiological states including, for example, atherosclerosis [12,13], sepsis [14,15], asthma [16], pancreatitis [17] and ischaemia reperfusion injuries [18,19]
Recently the protective role of HO-1 has been demonstrated indirectly in the acute model of trinitrobenzene sulphonic acid (TNBS)-induced colitis in rats [20]. Administration of tin mesoporphyrin (SnMP), which is a known inhibitor of HO-1 prior to the induction of colitis by TNBS significantly increased the colonic inflammation, free radical production and iNOS expression, suggesting that HO-1 plays a role in attenuating experimental colitis.
To the best of our knowledge the expression of HO-1 in the inflamed and non-inflamed intestine of patients with Crohn's disease or ulcerative colitis was never investigated in comparison with other non-specific intestinal inflammations such as intestinal ischaemia or diverticulitis. Furthermore, it is not known if the direct induction of HO-1 in acute or chronic experimental colitis has protective effects. Therefore, in this study we characterized the HO-1 expression in the colon of patients with Crohn's disease, ulcerative colitis and non-specific colonic inflammation. By employing an in vitro epithelial cell apoptosis model as well as the acute and chronic model of dextran sodium sulphate (DSS)-induced colitis, we investigated the effects of HO-1 induction on the extent of epithelial cell apoptosis in vitro and in vivo and analysed the therapeutic effects in the experimental colitis model in a preventive as well as a therapeutic approach.
Methods
Immunohistochemistry
Colonic specimens of 24 patients, seven controls (three diverticulitis, three ischaemic and one radiation colitis), 10 Crohn's disease (CD), and seven ulcerative colitis (UC) were included in the analysis. Specific histological features [21] together with their distribution in the intestinal wall were used to distinguish between inflammatory bowel disease (IBD) and non-IBD associated colitis and to grade the intensitiy of inflammation. Immunohistochemistry was performed on paraffin-embedded thin sections of patient specimen by using an avidin–biotin peroxidase method with diaminobenzidine (DAB) chromogen. After antigen retrieval from the tissue sections (water bath of formalin-fixed, paraffin-embedded 2–3 µm tissue sections for 20 min at 240 W in citrate buffer, pH 6·0) the pretreated slides were blocked at room temperature with bvovine serum albumin (BSA) and next incubated at 4°C overnight with the primary antibody (rabbit, polyclonal antibody H-105, clone SL 10789, Santa Cruz, USA) at a dilution of 1 : 150. Slides were then washed in phosphate buffered saline (PBS) and incubated for 1 h at 37°C with the secondary antibody (Vecta stain kit, Universal, Vector Laboratories, Burlingame, CA, USA). Antibody binding was visualized with 0·05% DAB (Ventana Medical System, Frankfurt, Germany) and 0·01% hydrogen peroxide. The material was rinsed in PBS and counterstained with haematoxylin.
Cell culture conditions
The colonic carcinoma epithelial cell line HT-29 was cultured in DMEM (PAN Biotech GmbH, Passau, Germany) containing 10% fetal calf serum (FCS). The cells were incubated at 37°C in air with 5% CO2.
Caspase-3 activity assay
HT-29 cells were incubated with the monoclonal Fas-activating antibody CH11 (250 ng/ml) (Upstate, NY, USA) for 24 h with or without a 24 h preincubation period with 2, 20 or 200 µM cobalt protoporphyrin (CoPP). Caspase-3 activation was determined from cytosolic extracts of HT-29 cells with or without CoPP treatment. The colorimetric activity assays were performed with a commercially available caspase assay kit (Biomol Research Laboratory, Plymouth Meeting, USA) according to the manufacturer's recommendations. In brief, following the respective treatment, cells were collected and briefly spun down. Cells were lysed on ice (50 mM HEPES pH 7·4, 0·1% CHAPS, 1 m M DTT, 0·1 m M EDTA), nuclei were removed (6500 g, 10 min), and the cytosolic preparations were quickly frozen and stored at −80°C until usage (max. 2 weeks). Equal amounts of cytosolic protein were added to the assay buffer (50 m M HEPES pH 7·4, 100 m M NaCl, 0·1% CHAPS, 10 m M DTT, 1 m m EDTA, 10% gycerol, caspase-3 substrate Ac-DEVD-pNA) in 96-well enzyme-linked immunosorbent assay (ELISA) plates. The caspase-3 activity was quantified with an ELISA-plate reader (OD405nm).
Animals
Female, inbred Balb/c mice (18–20 g) were obtained from Charles River, Sulzfeld, Germany and were provided with food and water ad libidum. The animal studies were approved by the local Institutional Review Board.
Induction of colitis and design of treatment
Acute colitis was induced by giving 3% DSS (mol. wt 40 000, ICN, Eschwege, Germany) orally in drinking water for 7 days followed by 1 day of normal drinking water.
Cobalt protoporphyrin (CoPP) was prepared in dim light because of the light sensitivity of the compound. We chose a dose of 125 µg CoPP, because Gerbitz et al. have demonstrated recently that this amount of CoPP is sufficient to induce a strong induction of HO-1 in the spleen, liver as well as in the intestine [22]. CoPP was dissolved in sodium hydroxide (150 m M) and the pH was subsequently adjusted to pH 7 by adding equal amounts of hydrochloric acid. The final pH of 7·4 was achieved by further dilution with PBS. The final concentration of CoPP was 500 µg/ml. A volume of 250 µl (equal to 125 µg CoPP) or vehicle was given by intraperitoneal injection on days 1 and 3 (n = 5/group; two independent experiments) or on days 3 and 5 of DSS-application (n = 5/group; three independent experiments). The animals were sacrificed on day 8, the colon removed and the distal parts were submitted for histological examination and evaluation of colonic myeloperoxidase activity.
For induction of chronic colitis mice received four cycles of DSS treatment, as described previously [23,24]. Each cycle consisted of 3% DSS in drinking water for 7 days, followed by a 10-day interval with normal drinking water. CoPP, 125 µg, was given by intraperitoneal injection on days 1 and 3 of each cycle of 3% DSS-application. Four weeks after the last DSS-cycle the animals were killed (n = 15/group).
Assessment of histological score
From the distal third of the colon 1 cm of colonic tissue was removed and used for histological analysis, as described previously [23,24]. Three sections were evaluated, each obtained at 100 µm distance. Mice were scored individually, each score representing the mean of three sections. Histological examination was performed by an investigator (F.O.) blinded to the source of treatment. Histology was scored as follows:
Epithelium (E). 0: normal morphology; 1: loss of goblet cells, 2: loss of goblet cells in large areas; 3: loss of crypts; 4: loss of crypts in large areas.
Infiltration (I). 0: no infiltrate; 1: infiltrate around crypt basis; 2: infiltrate reaching to lamina muscularis mucosae; 3: extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant oedema; 4: infiltration of the lamina submucosa.
The total histological score represents the sum of the epithelium and infiltration score and ranges from 0 to 8.
Isolation and incubation of mesenterial lymph node cells
Mesenterial lymph nodes (pooled from each group of mice) were collected under sterile conditions in cold cell culture medium (RPMI-1640, 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin from Gibco-BRL (Eggenstein, Germany) and β-mercaptoethanol 3 × 10−5 m (Sigma, Deisenhofen, Germany). Lymph nodes were disrupted mechanically and filtered through a cell strainer (70 µm); 2 × 105 cells/well were incubated in 200 µl culture medium over 24 h and spontaneous cytokine secretion was measured in the supernatants by ELISA (all from Endogene, Woburne, MA, USA), using four wells per condition.
Measurement of myeloperoxidase activity
Colonic myeloperoxidase (MPO) activity was determined using a modified standard method, as described previously [25]. Briefly, colonic tissue was homogenized in 1 ml of 50 mmol/l potassium phosphate buffer (pH 6·0) containing 0·5% (wt/vol) hexadecyltrimethylammonium hydroxide and centrifuged at 7500 g at 4°C for 20 min. Ten µl of the supernatant was transferred into phosphate buffer (pH 6·0) containing 0·17 mg/ml 3,3′-dimethoxybenzidine and 0·0005% H2O2. MPO activity of the supernatant was determined by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine and expressed as units per gram of total protein. Total protein content of the samples was analysed using a bicinchoninic acid protein assay kit (Sigma).
Terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick-end labelling staining (TUNEL staining)
TUNEL stainings were performed using the in situ cell death detection kit, AP (Roche, Penzberg, Germany) according to the manufacturer's recommendations. Briefly, colonic tissue sections of 4 µm were mounted on glass slides, deparaffinized, hydrated and treated for 10 min with proteinase K (20 µg/ml). After rinsing, TUNEL reaction mixture (terminal deoxynucleotidyl transferase from calf thymus and nucleotide mixture containing fluoresceinated deoxy-UTP) was added to the samples. Slides were incubated in a humidified chamber for 60 min at 37°C, rinsed and incubated with antifluorescein antibody (Fab fragment from sheep, conjugated with alkaline phosphatase). Then, substrate solution (nova red) was added, and slides were incubated for 15 min at room temperature. Apoptotic cells were identified by the presence of a distinct red staining of the nucleus.
Statistics
Results are expressed as mean ± s.e.m. Statistical analysis was performed using Student’s-t-test or the Mann–Whitney rank sum test. P < 0·05 was considered statistically significant.
Results
Up-regulation of HO-1 in patients with inflammatory bowel disease, non-specific inflammation and non-inflamed controls
Expression of HO-1 was detected in colonic mucosa from control and IBD patients. Mainly epithelial cells, mononuclear cells and scattered endothelial cells demonstrated HO-1 immunoreactivity (Fig. 1a). In patients with diverticulitis and intestinal ischaemia the HO-1 expression correlated with the histological grade of inflammation. There was an increased HO-1 expression in macrophages and epithelial cells in colonic mucosa of patients with more severe gut inflammation compared to non-inflamed mucosa (Fig. 1b). Similar patterns of HO-1 expression in histologically mildly inflamed CD and UC were observed (Fig. 1c). In contrast to controls with severe inflammation due to diverticulitis or ischaemia, in CD and UC patients with a high histological inflammatory activity HO-1 expression markedly decreased, with a notable heterogeneous distribution of positively stained epithelial cells (Fig. 1d).
Fig. 1.
Haem-oxygenase-1 (HO-1) immunohistochemistry in normal and inflamed colonic specimens of patients with IBD and controls. (a) Normal colonic mucosa; there is weak HO-1 expression in the cytoplasma of goblet cells of colon crypts (magnification × 400). (b) Inflamed colonic mucosa of a patient with diverticulitis, demonstrating strong HO-1 expression in epithelial cells at the luminal region of colonic crypts (arrow) and also intense immunostaining in macrophages (arrow; magnification × 400). In colonic specimens of patients with Crohn's disease (CD) and ulcerative colitis (UC) there was a similar HO-1 expression in mild inflamed colonic mucosa. (c) Representative slide of a patient with ulcerative colitis (magnification × 400) but in severe inflammation there was a decreased HO-1 immunostaining in epithelial cells with focal heterogenous distribution. (d) Representative histology of a patient with Crohn's disease (arrow, magnification × 200). There was no difference regarding HO-1 expression in macrophages.
Induction of HO-1 by CoPP prevents apoptosis by down-regulation of caspase-3 activation
One of the pathophysiological events, which may contribute to intestinal inflammation by dysregulation of the epithelial barrier function is increased apoptosis of colonic epithelial cells [26,27]. We therefore analysed as to whether the activation of caspase-3 induced by the anti-Fas antibody CH-11 can be down-regulated by the induction of HO-1 in the colonic carcinoma epithelial cell line HT-29. As shown in Fig. 2, CH-11-induced caspase-3 activation was dose-dependently down-regulated by the preincubation with different amounts of CoPP.
Fig. 2.
Colorimetric assay for caspase 3 activity in CH11 stimulated HT-29 cells treated with two different doses of cobalt protoporphyrin (CoPP) CH11 monoclonal antibody (mAb) (250 ng/ml) was used for the induction of apoptosis. CoPP preincubation dose-dependently down-regulated caspase-3 activation. The viability of HT-29 cells in the presence of CoPP was assessed by trypan blue exclusion. n = 3 experiments. P < 0·05 200 µ M CoPP versus 2 µM CoPP.
Up-regulation and induction of HO-1 in DSS-induced colitis in Balb/c mice
To analyse further the effects of HO-1 induction by CoPP we employed the DSS-induced colitis model in Balb/c mice. Similar to the pattern observed in colonic inflammation in humans, HO-1 immunoreactivity was only weakly expressed in epithelial cells and scattered mononuclear cells in the non-inflamed colon. At day 7 after the induction of DSS-colitis HO-1 expression was strongly up-regulated in the epithelial cells as well as in mucosal infiltrate, consisting mainly of neutrophils and macrophages (data not shown). Furthermore, colonic HO-1 expression could be induced in healthy mice by intraperitoneal administration of CoPP (Fig. 3a,b).
Fig. 3.
(a) Immunohistochemical staining of haem-oxygenase-1 (HO-1) in a colon specimen of a healthy Balb/C mouse. HO-1 immunoreactivity was observed in the epithelial cell layer and in scattered mononuclear cells (magnification × 200 and × 400). (b) Immunohistochemical staining of haem-oxygenase-1 (HO-1) of a colon of a Balb/c mouse 2 days after the administration of a single dose of cobalt protoporphyrin (CoPP) (125 µg) intraperitoneally. HO-1 activity was induced mainly in epithelial cells but not in mononuclear cells (magnification × 200 and × 400).
Induction of HO-1 before the onset of intestinal inflammation in acute DSS colitis (preventive HO-1 induction)
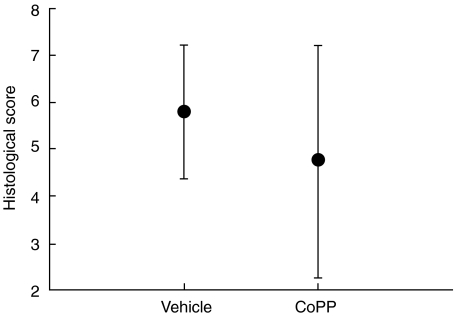
In preliminary experiments we evaluated a single administration of 125 µg CoPP on day 1 of DSS administration only, which demonstrated a non-significant amelioration of intestinal inflammation (data not shown). However, when we performed an induction of HO-1 by intraperitoneal injection of 125 µg CoPP on days 1 and 3 of DSS application (cumulative dose 250 µg CoPP), the colonic inflammation as determined by a histological score was significantly ameliorated compared to the control animals (Fig. 4). Additionally, the animals demonstrated a lower weight loss compared to the controls, which were treated with the vehicle (weight loss 9·1% ± 2·8% CoPP group versus 14·0% ± 5·0% vehicle group). Using colonic myeloperoxidase (MPO) activity as a quantitative index of granulocyte infiltration, the induction of HO-1 reduced significantly DSS-induced colonic MPO activity (36 ± 3 units/g protein CoPP group versus 63 ± 6 units/g protein; P < 0·01). The decrease of colonic inflammation in CoPP-treated animals was also accompanied by a significantly decreased secretion of interferon (IFN)-γ (1·8-fold) from stimulated mesenteral lymph node cells (Fig. 5). The secretion of IL-10 was not significantly altered, indicating a shift towards a more protective cytokine balance (IL-10 secretion: 109·5 ± 35·8 pg/ml CoPP versus 77·1 ± 44·0 pg/ml vehicle). Furthermore, by employing TUNEL staining, less apoptotic epithelial cells are detectable in CoPP-treated animals compared to vehicle-treated mice (Fig. 6a,b).
Fig. 4.
Haem-oxygenase-1 (HO-1) induction by cobalt protoporphyrin (CoPP) in acute dextran sodium sulphate (DSS)-induced colitis − preventive setting. Treatment on day 1 and day 3 with 125 µg CoPP intraperitoneally significantly ameliorates the histological degree of intestinal inflammation. Mice were killed on day 8. Extent of colonic inflammation was estimated histologically (max. grade 8, mean ± s.e.m.) in a blinded fashion (n = 10/group, two separate experiments). *P < 0·05 versus DSS/vehicle.
Fig. 5.
Secretion of interferon (IFN)-γ by isolated mesenterial lymph node cells from cobalt protoporphyrin (CoPP) and vehicle-treated animals on day 1 and day 3 in acute DSS-induced colitis. Mesenteral lymph node cells were stimulated with anti-CD3 antibody in the presence of IL-2 and cytokine concentrations in supernatants were determined after 24 h. Cells from pooled mesenterial lymph nodes were isolated and incubated in quadruplicate cultures for 24 h. Supernatants were used for cytokine measurement by enzyme-linked immunosorbent assay (ELISA). Each group consisted of five mice. IFN-γ secretion is significantly down-regulated in CoPP-treated animals (*P < 0·05 versus vehicle).
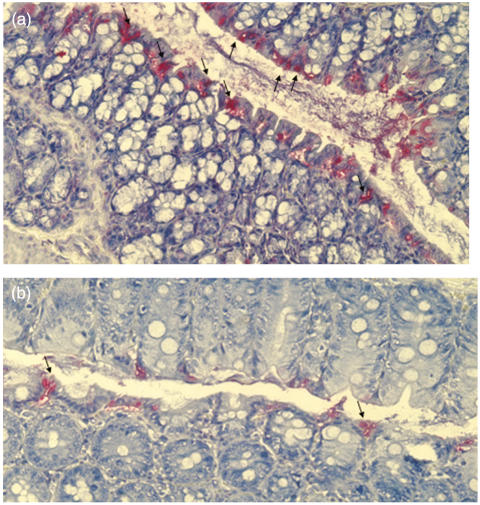
Fig. 6.
TUNEL staining of colonic sections of cobalt protoporphyrin (CoPP) and vehicle treated animals on day 1 and day 3 in acute dextran sodium sulphate (DSS)-induced colitis. Representative histological section of a vehicle-treated animal demonstrate numerous apoptotic epithelial cells (arrows) (a, magnification × 200) compared to a CoPP-treated mouse (arrows) (b, magnification × 200).
Induction of HO-1 after the onset of inflammation in acute DSS colitis (therapeutic HO-1 induction)
As we have demonstrated previously, the extravasation of leucocytes in DSS-induced colitis is already significantly increased on day 2 of DSS application, indicating the onset of colonic inflammation [28]. Therefore, to mimic a more clinically relevant treatment protocol, we induced HO-1 by intraperitoneal application of CoPP on days 3 and 5. In this setting we did not observe beneficial effects on either the extent of colonic inflammation (Fig. 7) nor on the secretion of IFN-γ (data not shown).
Fig. 7.
Haem-oxygenase-1 (HO-1) induction by cobalt protoporphyrin (CoPP) in acute dextran sodium sulphate (DSS)-induced colitis − therapeutic setting. Treatment on day 3 and day 5 with 125 µg CoPP intraperitoneally has no effect on the histological severity of intestinal inflammation as measured by a histological score on day 8 in acute DSS-induced colitis (n = 15/group; three separate experiments).
Effects of HO-1 induction in chronic DSS-induced colitis
To further evaluate the effects of HO-1 induction in experimental colitis, we induced chronic colitis in Balb/c mice by applying four cycles of DSS, as demonstrated previously [23,24,29]. Similarly to the preventive approach used in acute DSS-induced colitis, CoPP was intraperitoneally injected on day 1s and 3 of each DSS cycle. However, a trend towards a protection of colonic inflammation was observed in the CoPP group (histological score: 3·0 ± 1·2 CoPP versus 4·2 ± 1·6 vehicle; n = 15/group, four and two animals died during the course of colitis in the vehicle group and in the CoPP-treated group, respectively). The secretion of IFN-γ and IL-10 from the stimulated mesenterial lymph node cells were also not significantly different (data not shown).
Discussion
In this study we investigated the expression of HO-1 in the normal and inflamed human intestine. We then explored further the effects of HO-1 induction on the course of acute and chronic DSS-induced colitis in mice. The results demonstrate an up-regulation of HO-1 expression in patients with Crohn's disease and ulcerative colitis, which is comparable to that of patients suffering from intestinal ischaemia or diverticulitis. However, only in the severely inflamed mucosa, differences in the expression pattern of HO-1 in patients with inflammatory bowel disease (IBD) and non-specific inflammatory controls could be detected. In these cases, the epithelial cells displayed a more scattered expression and numerous epithelial cells not having HO-1 immunoreactivity at all were observed. These findings, along with the only published study demonstrating that the inhibition of HO-1 by tin mesoporphyrin (SnPP) aggravates the acute TNBS-induced colitis in rats [20], suggested that by inducing HO-1 a protective effect in chronic intestinal inflammation may be achieved [30].
We used the established model of acute and chronic DSS-induced colitis in Balb/c mice. In this model proinflammatory cytokines such as TNF, IFN-γ, IL-12 as well as reactive oxygen and nitrogen species play a role in the initiation and perpetuation of intestinal inflammation [24,25,31–36]. Additionally, in the DSS model an increased amount of apoptosis, mainly of epithelial cells, is observed which probably leads to a breakdown of the epithelial barrier function [37]. In our experiments we used two approaches to investigate potential protective effects of HO-1. In the preventive approach, induction of HO-1 using CoPP as an inductor was given together with the DSS administration on day 1 and was repeated on day 3, whereas in the therapeutic approach CoPP was administrated after the onset of intestinal inflammation on days 3 and 5. Interestingly, we observed an anti-inflammatory effect of HO-1 induction only in the preventive but not in the therapeutic approach. Furthermore, we could not detect protective effects of HO-1 induction in chronic DSS-induced colitis.
The protective effects of the preventive approach could be mediated by HO-1 in several ways. Haem oxygenase-1 (HO-1) cleaves the porphyrin ring of haem into carbon monoxide, Fe2+ and biliverdin, which is then converted into bilirubin by the bilirubin reductase [38]. The free iron, which is released of haem, is used in the intracellular metabolism as well as being sequestered by the iron chelator ferritin [39,40]. It has been shown that induction of HO-1 inhibits the expression of different adhesion molecules, such as ICAM, VCAM and E-selectin, which is mediated either by bilirubin and/or by a decrease in free intracellular Fe2+, thus limiting the migration of inflammatory cells [41,42]. Additionally, in several in vivo and in vitro models an anti-apoptotic effect of HO-1 induction has been demonstrated [43–45], which is probably due mainly to the generated CO and heavy chain ferritin [18,46–48].
On day 2 following the start of DSS administration significant increases in leucocyte adhesiveness and extravasation can be demonstrated in acute colitis [28]. This is paralleled by increases in both the histological inflammation score and myeloperoxidase activities. This might explain why the additional induction of HO-1 on day 3 after the start of DSS did not show any effect, implying that ongoing leucocyte–endothelium interactions and leucocyte extravasation is not reversible by the induction of HO-1. In the published studies of various animal models of ischaemia–reperfusion injury, graft-versus-host disease or organ transplantation HO-1 induction was induced only in a preventive fashion before the manifestation of inflammation, thus indicating that HO-1 counteracts early events in the initiation of inflammation [9,22,49,50].
Moreover, despite application of CoPP before each cycle of DSS similar to the preventive approach in acute DSS-induced colitis, HO-1 induction did not significantly influence intestinal inflammation in chronic DSS-induced colitis, hinting at the involvement of different inflammatory pathways in acute and chronic DSS-induced colitis, as has been shown previously with experimental blockade of different cytokines, nitric oxide or antibiotic treatment in this model [24,32,51].
To investigate further the mechanisms by which HO-1 fulfils its protective function in the preventive approach in the colitis model in vivo, we performed in vitro experiments using a colonic carcinoma epithelial cell line (HT-29). Similar to results published with freshly isolated murine islets cells and a pancreatic cell line [52,53], in HT-29 cells CoPP-induced up-regulation of HO-1 resulted in a down-regulation of Fas-mediated caspase-3 activation. These in vitro results suggest that one protective mechanism of HO-1 in DSS-induced intestinal inflammation is the protection of epithelial cells from apoptosis, which we could demonstrate also by employing TUNEL staining in CoPP-treated mice and which has been shown in other models, such as the protection of rat hearts from ischemia–reperfusion injury [18,54]. Epithelial cell apoptosis has been demonstrated before in DSS-induced colitis and is accompanied by a significant expression of the pro-apoptotic molecules Fas, FaL, Bax and p53 in epithelial cells [37]. Therefore, as well as the above-described, the reasons for the failure of protection of HO-1 induction after the onset of inflammation or in chronic colitis may not be sufficient to prevent already ongoing apoptotic events, which again trigger inflammatory responses in the intestine.
In conclusion, these results demonstrate a protective effect of HO-1 induction in the acute DSS-induced colitis model, if HO-1 is induced before the onset of inflammation. However, HO-1 induction has no effect in already established DSS-induced acute colitis or in chronic DSS-induced colitis, indicating that the induction of HO-1 may not be a promising approach in chronic inflammatory bowel disease such as Crohn's disease or ulcerative colitis.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to H.H., S.F., M.F and G.R. (SFB 585).
References
- 1.Fiocchi C. Inflammatory bowel disease. Etiol Pathogen Gastroenterol. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Simmonds NJ, Allen RE, Stevens TR, Van Someren RN, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186–96. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- 3.Oberle S, Schwartz P, Abate A, Schroder H. The antioxidant defense protein ferritin is a novel and specific target for pentaerithrityl tetranitrate in endothelial cells. Biochem Biophys Res Commun. 1999;261:28–34. doi: 10.1006/bbrc.1999.0941. [DOI] [PubMed] [Google Scholar]
- 4.Yesilkaya A, Altinayak R, Korgun DK. The antioxidant effect of free bilirubin on cumene-hydroperoxide treated human leukocytes. General Pharmacol. 2000;35:17–20. doi: 10.1016/s0306-3623(01)00084-2. [DOI] [PubMed] [Google Scholar]
- 5.Choi AM, Otterbein LE. Emerging role of carbon monoxide in physiologic and pathophysiologic states. Antioxid Redox Signal. 2002;4:227–8. doi: 10.1089/152308602753666271. [DOI] [PubMed] [Google Scholar]
- 6.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–6. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 7.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of haem oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103:1047–54. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otterbein LE, Lee PJ, Chin BY, et al. Protective effects of haem oxygenase-1 in acute lung injury. Chest. 1999;116:S61–3. doi: 10.1378/chest.116.suppl_1.61s-a. [DOI] [PubMed] [Google Scholar]
- 9.Soares MP, Lin Y, Anrather J, et al. Expression of haem oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–7. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 10.Poss KD, Tonegawa S. Reduced stress defense in haem oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–24. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of haem oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152:711–20. [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of haem oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–16. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–94. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 15.Yet SF, Pellacani A, Patterson C, et al. Induction of haem oxygenase-1 expression in vascular smooth muscle cells. A link to endotoxic shock. J Biol Chem. 1997;272:4295–301. doi: 10.1074/jbc.272.7.4295. [DOI] [PubMed] [Google Scholar]
- 16.Horvath I, Loukides S, Wodehouse T, Kharitonov SA, Cole PJ, Barnes PJ. Increased levels of exhaled carbon monoxide in bronchiectasis: a new marker of oxidative stress. Thorax. 1998;53:867–70. doi: 10.1136/thx.53.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato H, Siow RC, Bartlett S, et al. Expression of stress proteins haem oxygenase-1 and -2 in acute pancreatitis and pancreatic islet betaTC3 and acinar AR42J cells. FEBS Lett. 1997;405:219–23. doi: 10.1016/s0014-5793(97)00191-9. [DOI] [PubMed] [Google Scholar]
- 18.Akamatsu Y, Haga M, Tyagi S, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant-associated ischemia reperfusion injury. FASEB J. 2004;18:771–2. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Shan P, Jiang D, et al. Small interfering RNA targeting haem oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–84. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 20.Wang WP, Guo X, Koo MW, et al. Protective role of haem oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586–94. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Riddell RH, Saito H, Soma Y, Hidaka H, Kudo H. Morphologic criteria applicable to biopsy specimens for effective distinction of inflammatory bowel disease from other forms of colitis and of Crohn's disease from ulcerative colitis. Scand J Gastroenterol. 1999;34:55–67. doi: 10.1080/00365529950172844. [DOI] [PubMed] [Google Scholar]
- 22.Gerbitz A, Ewing P, Wilke A, et al. Induction of haem oxygenase-1 before conditioning results in improved survival and reduced graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2004;10:461–72. doi: 10.1016/j.bbmt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Obermeier F, Dunger N, Strauch UG, et al. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217–24. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojouharoff G, Hans W, Obermeier F, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herfarth H, Brand K, Rath HC, Rogler G, Scholmerich J, Falk W. Nuclear factor-kappa B activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000;120:59–65. doi: 10.1046/j.1365-2249.2000.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu MT, Palladino AA, Arnold ET, Kwon RS, McRoberts JA. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology. 2000;119:1524–36. doi: 10.1053/gast.2000.20232. [DOI] [PubMed] [Google Scholar]
- 27.Dignass AU, Baumgart DC, Sturm A. Review article: the aetiopathogenesis of inflammatory bowel disease − immunology and repair mechanisms. Aliment Pharmacol Ther. 2004;20:9–17. doi: 10.1111/j.1365-2036.2004.02047.x. [DOI] [PubMed] [Google Scholar]
- 28.Farkas S, Herfarth H, Rossle M, et al. Quantification of mucosal leucocyte endothelial cell interaction by in vivo fluorescence microscopy in experimental colitis in mice. Clin Exp Immunol. 2001;126:250–8. doi: 10.1046/j.1365-2249.2001.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermeier F, Dunger N, Deml L, Herfarth H, Scholmerich J, Falk W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol. 2002;32:2084–92. doi: 10.1002/1521-4141(200207)32:7<2084::AID-IMMU2084>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Naito Y, Takagi T, Yoshikawa T. Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:177–84. doi: 10.1111/j.1365-2036.2004.01992.x. [DOI] [PubMed] [Google Scholar]
- 31.Krieglstein CF, Cerwinka WH, Laroux FS, et al. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;194:1207–18. doi: 10.1084/jem.194.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma) − and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–45. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki Y, Andoh A, Fujiyama Y. The free radical scavenger edaravone suppresses experimental dextran sulfate sodium-induced colitis in rats. Int J Mol Med. 2003;12:125–9. [PubMed] [Google Scholar]
- 34.Hans W, Scholmerich J, Gross V, Falk W. Interleukin-12 induced interferon-gamma increases inflammation in acute dextran sulfate sodium induced colitis in mice. Eur Cytokine Netw. 2000;11:67–74. [PubMed] [Google Scholar]
- 35.Carrier J, Aghdassi E, Platt I, Cullen J, Allard JP. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989–99. doi: 10.1046/j.1365-2036.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- 36.Kruidenier L, van Meeteren ME, Kuiper I, et al. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med. 2003;34:753–65. doi: 10.1016/s0891-5849(02)01426-0. [DOI] [PubMed] [Google Scholar]
- 37.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47:1447–57. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]
- 38.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of haem to bilirubin by microsomal haem oxygenase. Proc Natl Acad Sci USA. 1968;61:748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenstein RS, Munro HN. Translational regulation of ferritin synthesis by iron. Enzyme. 1990;44:42–58. doi: 10.1159/000468746. [DOI] [PubMed] [Google Scholar]
- 40.Eisenstein RS, Garcia-Mayol D, Pettingell W, Munro HN. Regulation of ferritin and haem oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci USA. 1991;88:688–92. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soares MP, Seldon MP, Gregoire IP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–63. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 42.Wagener FA, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential effects of haem oxygenase isoforms on haem mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther. 1999;291:416–23. [PubMed] [Google Scholar]
- 43.Tanaka S, Akaike T, Fang J, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003;88:902–9. doi: 10.1038/sj.bjc.6600830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobiasch E, Gunther L, Bach FH. Heme oxygenase-1 protects pancreatic beta cells from apoptosis caused by various stimuli. J Invest Med. 2001;49:566–71. doi: 10.2310/6650.2001.33721. [DOI] [PubMed] [Google Scholar]
- 45.Ozawa N, Goda N, Makino N, Yamaguchi T, Yoshimura Y, Suematsu M. Leydig cell-derived haem oxygenase-1 regulates apoptosis of premeiotic germ cells in response to stress. J Clin Invest. 2002;109:457–67. doi: 10.1172/JCI13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato K, Balla J, Otterbein L, et al. Carbon monoxide generated by haem oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–94. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 47.Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by haem oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–26. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berberat PO, Katori M, Kaczmarek E, et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724–6. doi: 10.1096/fj.03-0229fje. [DOI] [PubMed] [Google Scholar]
- 49.Kato H, Amersi F, Buelow R, et al. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–8. [PubMed] [Google Scholar]
- 50.Attuwaybi BO, Kozar RA, Moore-Olufemi SD, et al. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J Surg Res. 2004;118:53–7. doi: 10.1016/j.jss.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Hans W, Scholmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12:267–73. doi: 10.1097/00042737-200012030-00002. [DOI] [PubMed] [Google Scholar]
- 52.Pileggi A, Cattan P, Berney T, et al. HO-1 upregulation protects the pancreatic cell line betaTC3 from cytokines and Fas-induced apoptosis. Transplant Proc. 2001;33:266–7. doi: 10.1016/s0041-1345(00)02007-8. [DOI] [PubMed] [Google Scholar]
- 53.Pileggi A, Molano RD, Berney T, et al. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50:1983–91. doi: 10.2337/diabetes.50.9.1983. [DOI] [PubMed] [Google Scholar]
- 54.Katori M, Buelow R, Ke B, et al. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287–92. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]