Abstract
Detection of self-reactive antibodies has an established role in the diagnosis and monitoring of many human autoimmune diseases. Autoantibodies with restricted reactivity to cytoplasmic compartments and structures are an occasional incidental finding following routine examination of serum for antinuclear antibody reactivity. A prerequisite for rational exploitation of self-reactive antibodies, in either clinical or research settings, is the establishment of the molecular identity of the target autoantigen(s). Here we report on the identification of a novel autoantigen that co-localizes with a subset of cytoplasmic microbodies marked by ABCD3 (PMP-70) and/or PXF (PEX19). Immunoscreening a HeLa cell cDNA expression library with a human autoimmune serum identified two clones that encode fragments of limkain b1 (LKAP). We demonstrate that mouse polyclonal antibodies raised against a bacterially expressed fragment of limkain b1 mark the same cytoplasmic structures as human serum, as does an EGFP:LKAPCT429 fusion protein expressed in HeLa cells. An immunoblot screen against a bacterially expressed MBP:LKAPCT429 fusion protein substrate, using a cohort of 16 additional human sera that display Hep 2 cell cytoplasmic staining patterns similar to the prototype serum, identified three additional sera reactive to limkain b1. This is the first report establishing the molecular identity of a peroxisomal autoantigen. Preliminary results suggest that limkain b1 may be a relatively common target of human autoantibodies reactive to cytoplasmic vesicle-like structures.
Keywords: autoantibody, autoantigen, Limkain b1, LKAP, peroxisomes
Introduction
Annotation of the human genome has identified fewer than 30 000 genes contributing to the definition, generation and maintenance of the functional form. However, the estimated number of proteins encoded by these genes is likely to be several orders of magnitude higher. For many thousands of these proteins, the structure, function and interacting partners have been characterized. For the vast majority of these genes, the processing of primary transcripts to define isoforms is yet to be investigated, tissue distribution and subcellular and/or extracellular distributions of the protein remain unresolved, interacting partners yet to be identified, and function yet to be established. The task of defining the structure and function of an unknown protein is aided by the fact that some proteins are related to other proteins or contain similar functional motifs. Databases cataloguing consensus sequence patterns and protein families, used in concert with a means of identifying the protein (such as antibodies raised against the protein/antigen), are a prerequisite for rationally based functional investigation of novel proteins.
Naturally occurring human autoantibodies have proved useful in the prosecution of characterization and functional investigations of novel proteins of unknown function [1]. The unifying property of antibodies is their ability to bind to the target antigen which allows the identification and tracking of the molecule. In addition, the affinity of these probes for their targets can be utilized in assays addressing the mechanisms of action and function of the target molecule in situ and in model systems. Autoantibodies have tended to be superior to antibodies raised against self or non-self antigens with regard to their usefulness in biological studies. This is due in part to the nature of autoantibodies, which most often recognize epitopes that are highly conserved in evolution, allowing their exploitation as molecular probes in a variety of animal model systems. For example, human anti-Sm (SNRPD1, 2 and 3) autoantibodies recognize autoepitopes of Sm antigen in frogs and sea urchins [2]; human antiribosomal P2 (RPLP2) antibodies are reactive to Artemia protein [3]; and human antienolase (ENO1-3) antibodies recognize enolase in yeast [4]. The autoepitopes, conserved in nature across the species, often form part of critical functional domains of the target molecule. This property of autoantibodies can be exploited in biological assays where the antibody may act as a suppressor with regard to the function of the target molecule.
In this study we utilize human serum collected from a 67-year-old female patient that produces a cytoplasmic speckling staining pattern on HEp-2 cell substrate. Screening a cDNA expression library resulted in the molecular identification of limkain b1 as a novel autoantigen target. We validate this finding and demonstrate that limkain b1 localizes to a subset of PXF (peroxisomal farnesylated protein, known previously as PEX19) and/or ABCD3 (ATP-binding cassette subfamily D member 3, known previously as PMP-70) marked microbodies. Employing a bacterially expressed fragment of limkain b1 fused to maltose-binding protein as a substrate we identify a further three sera with reactivity to this peroxisomal autoantigen out of the cohort of 16 sera, selected at random on the basis of producing a cytoplasmic speckling staining pattern on HEp-2 cell test slide substrates.
Materials and methods
Antibodies
Human sera used in these investigations were originally referred for antinuclear antibody (ANA) investigations. Ethics approval from the Alfred Hospital was granted for use of discarded tissue. The sera were stored at either 4°C or −20°C. Antibodies used for co-localization studies include: rabbit polyclonal anti-PMP-70 (diluted 1 : 500; Zymed, CA, USA) and murine monoclonal antibodies to human EEA1 (IgG1), transferrin receptor (IgG2a), Rab 5 (IgG2a), Rab 11 (IgG2a) and PEX19 (IgG1) (each diluted 1 : 10; BD Transduction Laboratories, Australia), LAMP-1 (IgG1, diluted 1 : 100; BD Biosciences, Australia) and lysobisphosphatidic acid (LBPA, diluted 1 : 100 [5]). Mouse polyclonal antibodies reactive to MBP:LKAPCT429 fusion protein were generated by immunizing BALB/c mice by subcutaneous injection with 40 µg of purified fusion protein in Freund's complete adjuvant (Difco Laboratories, USA), with booster immunizations 14, 28 and 42 days later. Mice were bled out 10 days post-final boost, and antibody was affinity purified against the fusion protein bound to Sepharose 4B and MBP reactivity absorbed (see Yong et al. [6]) Antibody conjugates included: antihuman immunoglobulin conjugated to Alexa 594, antimouse immunoglobulin conjugated to Alexa 488, antirabbit immunoglobulin conjugated to Alexa 568 (all diluted 1 : 1000; Molecular Probes, USA), antihuman immunoglobulin conjugated to FITC (undiluted, Kallestad, USA), HRP-conjugated rabbit immunoglobulins to human IgA, IgG and IgM, κ and λ and mouse immunoglobulin (diluted 1 : 1000; Dako, Australia).
Indirect immunofluorescence
Cell substrates included fixed HEp-2 cells on commercial test slides (Kallestad) and HeLa cells grown on coverslips, fixed and then permeabilized as described by Waite et al. [7]. As required, HEp-2 cells were treated with Proteinase K (Gibco-BRL, USA) at a final concentration of 8000 U/ml) under the manufacturer's recommended buffer conditions for 30 min at room temperature, followed by extensive washes in 0·5 m M EDTA and 10 m M Tris pH 8 prior to use. Cells were incubated with autoimmune serum singly or in combination with other antibodies for 30 min (in the case of affinity purified autoantibodies the incubation was overnight at 4°C), washed in phosphate buffered saline (PBS) for 10 min, then incubated with appropriately conjugated antibodies for 30 min and washed again. DNA was counterstained with 5 mM Hoechst 33342 dye for 5 min. Proteinase K-treated cell substrates were tested with antibodies reactive to EEA1 to confirm their functionality in indirect immunofluorescence assays. All slides were mounted with FluorSave (Calbiochem, CA, USA) and viewed at an excitation wavelength of 440–500 nm using a Leica DMRB fluorescent microscope (Wetzlar, Germany) and selected images were collected with Image Pro Plus (version 4·0; Media Cybernetics, Silver Spring, MD, USA). Co-localization was determined by overlaying images in Adobe Photoshop (version 5·02).
Antibody screening of cDNA library and DNA sequencing
A HeLa cell cDNA library (UNI-ZAP XR, Stratagene) was immunoscreened with the prototype serum (JA). In brief, a total of 1·5 × 105 plaques were plated to a density of 50 000 plaques/plate. Expression of recombinant fusion protein, screening, plaque purification and rescue of pBluescript SK- phagemids was performed as described in Tsukada et al. [8]. Automated DNA sequencing was performed by the Baker Heart Research Institute Sequencing Facility utilizing Applied Biosystems equipment. Oligonucleotides synthesized for DNA sequence analysis (Geneworks, Australia) included T3, T7, 5′-TGAAGCTCACAAGTCTG TATTTGTTTGC-3′, 5′-GCAGGAGCTTCTCCGCCTGAC CGAC-3′ and 5′-CTCCCATTTGGAATATAGAATTAGG-3′ sequencing primers.
Construction of transgenes, transfections and purification of fusion protein
Polymerase chain reaction (PCR)-based strategies, utilizing clone JA4 as the template, were used to construct vectors encoding fusion proteins MBP:LKAPCT429, EGFP:LKAPCT429, and EGFP:LKAPCT429-TKL. The PCRs were performed in 50 µl volumes with 25 pmol/µl of each primer, 0·2 pmol/µl template with 2·5 U Taq polymerase (Gibco-BRL) under standard conditions (10 m M Tris-Cl pH 8·3, 50 mM m KCl, 1·5 m M MgCl2, 0·2 m M of each dNTPs) with 30 cycles (denaturation 94°C 45 s; annealing 60°C 45 s; elongation 72°C 60 s). Primers used in the reactions were 5′-CGGAAT TCGATACTTTACAAGTATTGGAATG-3′, 5′-GCTCTAGAT TAAAGCTTGGTTATAGGTGC-3′, CGGAATTCCTGATAC TTTACAAGTATTGG-3′, 5′-GCTCTAGATTAAAGCTTGG TTATAGGTGC-3′, 5′-CGGAATTCCTGATACTTTACAATA TTGG-3′ and 5′-GCTCTAGATTATATAGGTGCTAAGGA AAAG-3′. The PCR products were digested with appropriate restriction enzymes, purified and ligated into appropriately digested expression vectors, pMaL-c2 (New England BioLabs, USA) and pEGFP_C3 (Clontech) employing conventional molecular biology techniques [9]. pMaL-c2:MBP:LKAPCT429, encodes a maltose binding protein fused to the distal 429 carboxyl amino acids of LKAP (isoform I) referred to as MBP:LKAPCT429. MBP:LKAPCT429 was expressed and purified on amylose resin according to the manufacturer's instructions (New England BioLabs). pEGFP_C3:LKAPCT429 encodes EGFP fused to the distal 429 carboxyl amino acids of LKAP (isoform I) referred to as EGFP:LKAPCT429. pEGFP_C3:LKAPCT429-TKL is similar to pEGFP_C3:LKAPCT429, except that it lacks the last three carboxyl terminal amino acids of LKAP (T, K and L) and is referred to as EGFP:LKAPCT429-TKL. The pEGFP_C3-based vectors were transfected into HeLa cells utilizing Effectine Reagent (Qiagen, USA) according to the manufacturer's instructions. Sixteen hours post-transfection HeLa cells were passaged into fresh media, cultured for a further 48 h on coverslips, then fixed (see ‘Indirect immunofluorescence’ section above).
Immunoblotting and affinity purification of autoantibodies from nitrocellulose membrane
MBP:LKAPCT429 (1 µg/lane) was subjected to 10% (w/v) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions (in the presence of 5% (v/v) 2-β-mercaptoethanol), transferred to nitrocellulose membrane and immunoblotted with human and mouse sera (all diluted 1 : 100 in BLOTTO; 5% (w/v) skimmed milk powder in PBS, as described previously [4]. Antibody reactivity was detected with the addition of appropriate HRP-conjugated immunoglobulins (diluted 1 : 1000 in BLOTTO) and visualized using enhanced chemiluminescence (Pierce, USA). MBP:LKAPCT429 (at 10 µg/lane) was also subjected to SDS-PAGE under reducing conditions, transferred to a nitrocellulose membrane, blocked with BLOTTO and then reacted with JA serum (diluted 1 : 100 in BLOTTO). The membrane was washed several times in PBS/0·05% Tween-20 (v/v), and the remaining bound antibody was eluted in 1 ml of 100 m M glycine (pH 3). The eluant was neutralized immediately by addition of 0·5 M Tris Cl (pH 8). Antibodies present in the eluant were concentrated 10-fold employing a microconcentrator with a 5 kDa cut-off (Millipore, Australia) and used in indirect immunofluorescence.
Results
Reactivity of autoimmune sera cohort
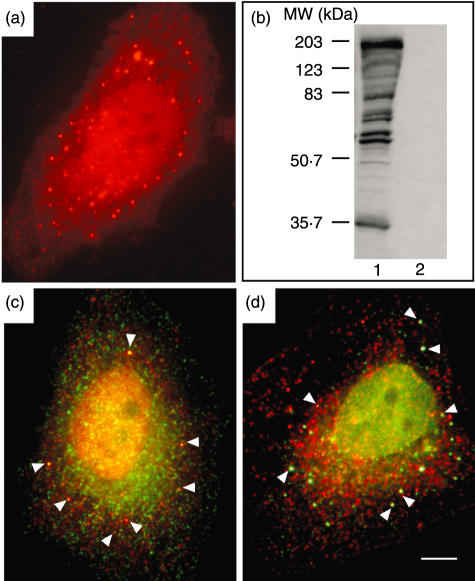
The pathology service routinely screens about 2200 sera for ANA reactivity yearly. Approximately 0·07% of these sera display cytoplasmic vesicle-like reactivity on a HEp-2 test slide substrate [7]. A cohort of 17 sera were selected at random for use in this study from samples referred for ANA over a 10-year period on the basis of immunofluorescence to cytoplasmic vesicle-like reactivity, with no significant co-localization with markers for early endosome compartments (EEA1, Rab5, transferrin receptor (CD71); recycling endosomal compartments (Rab11); late endosome compartments (LBPA) and late endosome and lysosomal compartments (LAMP1/CD107a). From this sera cohort the serum designated JA was selected for initial characterization on the basis of the cytoplasmic vesicle-associated autoantigen being protein in nature by demonstration of ablation of serum antibody reactivity to the cytoplasmic antigens on proteinase K treated HEp-2 cells. The serum JA was collected from a 67-year-old Caucasian female. The serum displayed a distinctive pattern of staining by indirect immunofluorescence on a HEp-2 cell substrate characterized by reactivity to polymorphous cytoplasmic structures (titre 1 : 102 400), in addition to strong reactivity to nuclear antigens (titre 1 : 1600) (Fig. 1a). The serum immunoblotted several HeLa cell antigens with molecular weights ranging from ∼34 to ∼190 kDa (Fig. 1b). A subset of vesicles labelled by JA serum co-localized with a subset of peroxisomes marked with PXF (after peroxisomal farnesylated protein) and ABCD3 (after ATP-binding cassette, subfamily D (ALD), member (3) (Figs 1c,d).
Fig. 1.
(a) Indirect immunofluorescence on fixed HEp-2 cells in interphase with patient serum JA detected with Alexa 594 (red)-labelled antihuman immunoglobulin. (b) Immunoblotting of 50 µg of HeLa cell protein extract with JA serum (lane 1) and normal human serum (lane2). Protein molecular weight size markers are as indicated. Overlayed dual indirect immunofluorescence performed on HEp-2 substrate with (c) JA serum detected with Alexa 594 (red)-labelled antihuman immunoglobulin and anti-PXF detected with Alexa 488 (green)-labelled antimouse immunoglobulin and (d) JA serum detected with Alexa 488 (green)-labelled antihuman immunoglobulin and anti-ABCD3 detected with Alexa 568 (red)-labelled antirabbit immunoglobulin. Arrows indicate selected areas of co-localization.
cDNA library screening and characteristics of clones JA.4 and JA.5
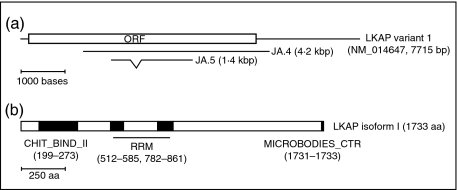
JA serum was used to immunoscreen a HeLa cDNA expression library. Five positive clones were purified to homogeneity and blast searches were employed to identify the cDNAs. The nucleotide sequences of two clones, JA.4 and JA.5, were found to have sequence identity to an as-yet uncharacterized gene given the interim name limkain b1 (mRNA reference sequences NM_014647 and NM_019081) and gene symbol LKAP. The NCBI mRNA reference sequence for LKAP isoform 1 (NM_014647) is 7715 bases in length, harbouring an open reading frame from base 194 to 5395 encoding a protein of 1733 amino acids (Fig. 2). The JA.4 clone harbours a cDNA insert of approximately 4·2 kbp which corresponds to bases 1436 to 5906 of LKAP isoform I reference sequence (see Fig. 2a). JA.5 has an insert of approximately 1·4 kbp, with identity to bases 2056–3915 of the LKAP isoform I cDNA. However, JA.5 lacks sequences equivalent to bases 2526 to 2770 of the LKAP isoform I cDNA. The bases absent from JA.5, compared to NM_014647, are all contained within designated exon 15 of the LKAP gene. This observation is consistent with JA.5 containing a fragment of an RT-mRNA that is derived from a processed splice variant of LKAP in which exon 15 has been excised.
Fig. 2.
Schematic representations of LKAP depicting: (a) the relationship of the previously reported NM_014647 LKAP isoform 1 reference sequence to clones JA.4 and JA.5 (open box represents open reading frame (ORF) of the reference sequence; note JA.5 lacks NM_014647 bases 2526–2770 as indicated), and (b) LKAP isoform I protein features (shaded boxes) indicating the presence of a chitin-binding type 2 domain profile (CHIT_BIND_II), tandem RNA recognition motif signatures (RRM) and a microbodies C-terminal targeting signal (MICROBODIES_CTER); note regions of low complexity at: 117–128, 291–306, 307–324, 601–617, 1670–1695 based on SMART predictions.
Predicted characteristics of limkain b1
The structure, subcellular localization, interacting partners and function of LKAP have not been reported in the literature to date. The LKAP gene is located at 16p13.13 (locus ID: 9665) and two isoforms have been described. Based on the limkain b1 isoform I coding sequence, which matches most closely the sequence of JA.4 and JA.5 cDNAs, the mRNA codes for a protein of 1733 amino acids. Utilizing the Simple Modular Architecture Research Tool (SMART) [10] sequence motifs for a chitin-type 2 binding domain (Chit-bind-II) at amino acids 199–273, tandem RNA-binding motifs (RRM, RPN-1) at amino acids 514–580 and 784–856 and a carboxyl terminal microbody targeting signal (MICROBODIES_CTER, TKL) amino acids 1731–1733 were identified in LKAP (see Fig. 2b). In addition there are several short stretches of low complexity sequences spanning the protein. Limkain b1 isoform 1 has a predicted molecular weight of 191·8 kDa with a theoretical pI of 8·08. JA serum reacted to a number of HeLa cell proteins including, one of an approximate molecular weight of 190 kDa (see Fig. 1b).
Validation of JA serum reactivity to limkain b1
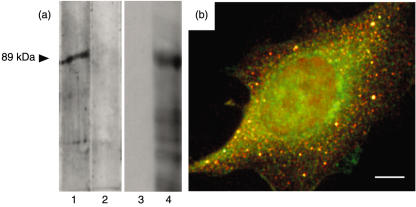
The reactivity of JA serum to MBP:LKAPCT429, a recombinant protein composed of the maltose binding protein fused to the distal 429 carboxyl amino acids of LKAP isoform I, was examined by immunoblotting. The JA serum reacted strongly to MBP:LKAPCT429 and there was no evidence of normal human serum reacting to the fusion protein (Fig. 3a lanes 1 and 2). MBP:LKAPCT429 was also used to immunize mice to raise polyclonal antibodies reactive to LKAP (see Fig. 3a, lanes 3 and 4). Co-localization of reactivities of mouse polyclonal antibodies (reactive to MBP:LKAPCT429) and the human serum JA could be demonstrated by dual indirect immunofluorescence (Fig. 3b).
Fig. 3.
(a) Immunoblotting of 1 µg of affinity purified MBP:LKAPCT429 fusion protein with JA serum (lane 1) normal human serum (lane 2), mouse preimmune serum (lane 3) and serum from mouse immunized with MBP:LKAPCT429 (lane 4). Normal human serum, JA serum and mouse preimmune serum did not react with MBP:ΔlacZ (data not shown). (b) Overlayed dual indirect immunofluorescence performed on HEp-2 substrate with JA serum detected with Alexa 594 (red)-labelled antihuman immunoglobulin and mouse polyclonal antibodies raised against MBP:LKAPCT429 detected with Alexa 488 (green)-labelled antimouse immunoglobulin. Mouse preimmune serum was non-reactive to HEp-2 substrate (data not shown).
Subcellular localization of limkain b1
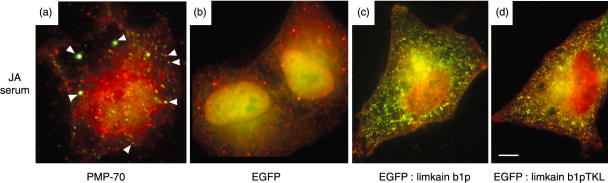
The subcellular localization of limkain b1 was determined by co-localization of affinity purified antibodies from JA serum (reactive to MBP:LKAPCT429) with rabbit polyclonal anti-ABCD3 by dual indirect immunofluorescence (Fig. 4a). In addition, a vector expressing an enhanced green fluorescent protein (EGFP) fused to LKAPCT429 (EGFP:LKAPCT429) was transfected into HeLa cells and employed in transient expression studies in cells that were then fixed and subjected to indirect immunofluorescence staining with human JA serum. Transfection of HeLa cells with an EGFP expression vector alone showed weak diffuse expression in the nucleus and cytoplasm (Fig. 4b). In contrast, cells expressing EGFP:LKAPCT429 displayed a discrete vesicle staining pattern for the EGFP fusion protein that had significant overlap with JA serum staining (Fig. 4c). As noted earlier, the carboxyl terminal amino acid sequence of LKAP contains a microbodies_CTR signal (T-K-L, amino acids 1731–1733). To determine whether the localization of EGFP:LKAPCT429 fusion protein to peroxisomes was dependent on the C-terminal T-K-L amino acids, we generated an additional vector that expresses a fusion protein identical to EGFP:LKAPCT429, except that it lacks the three carboxyl terminal amino acids of LKAP (T,K, and L), named EGFP:LKAPCT429-TKL. We found the EGFP:LKAPCT429-TKL pattern of localization was similar to that of EGFP:LKAPCT429 (Fig. 4d). This supports an assertion that targeting of the EGFP:LKAPCT429 fusion protein to peroxisomes is not dependent on a microbodies_ CTR signal.
Fig. 4.
(a) Overlay of dual indirect immunofluorescence on a HEp-2 substrate with antibodies affinity purified from JA serum (reactive to MBP:LKAPCT429) detected with Alexa 488 (green)-labelled antihuman immunoglobulin and anti-ABCD3 detected with Alexa 568 (red)-labelled antirabbit immunoglobulin. Arrows indicate examples of regions of co-localization of affinity purified human antibodies with rabbit antibodies reactive to ABCD3. Fluorescent studies of HeLa cells transfected with vectors expressing (b) EGFP (c) EGFP:LKAPCT429 and (d) EGFP:LKAPCT429-TKL, as indicated, and reacted with JA serum (detected with Alex 594 (red) antihuman immunoglobulins) and EGFP (green) visualized by immunofluorescence.
Incidence of limkain b1 autoantibodies in serum producing vesicular staining patterns
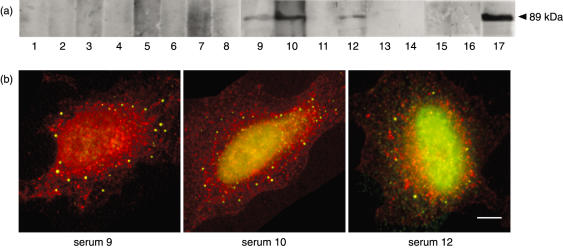
To determine the frequency of reactivity to LKAP present in the remaining 16 sera from this cohort, these sera were reacted to MBP:LKAPCT429 (see Fig. 5a). Although the MBP:LKAPCT429 fusion protein contains only 429 of the 1733 amino acids of limkain b1, it does contain at least one autoreactive epitope detectable by the prototype serum. Three additional sera were also found to be reactive to MBP:LKAPCT429. The reactivity of these sera could also be demonstrated to partially co-localize with peroxisomes containing ABCD3. Therefore, at least four sera within this small cohort of 17 sera that display vesicle-like reactivity are reactive to limkain b1.
Fig. 5.
(a) Immunoblot of 1 µg of bacterially expressed and affinity purified MBP:LKAPCT429 size fractionated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and reacted to a selected cohort of 16 patient sera (lanes 1–16) and the prototype human serum JA (lane 17). (b) Overlayed dual indirect immunofluorescence performed on HEp-2 substrate with patient serum 9, 10 and 12 (as indicated) detected with Alexa 488 (green)-labelled antihuman immunoglobulin and rabbit polyclonal antibodies raised against ABCD3 detected with Alexa 568 (red)-labelled antirabbit immunoglobulin.
Discussion
Naturally occurring autoantibodies to cytoplasmic structures including the Golgi, mitochondria, the MTOC and ribosomes are well documented [11,12]. There are fewer reports of autoantibodies to the class of small cytoplasmic vesicle-like structures such as endosomes, lysosomes, peroxisomes and proteasomes [12]. To date, the molecular identity of only eight autoantigens of this class has been established (Table 1). Here we report on the molecular identity of the first autoantigen of this class that localizes to a subset of peroxisomes, limkain b1. We found that within a small cohort of 17 sera, with cytoplasmic vesicle reactivity, four immunoblotted a bacterially expressed fusion protein incorporating a portion of limkain b1. This finding may under-represent the sera within this cohort that react to the autoantigen, as the bacterially expressed fusion protein harboured only 429 of the 1733 amino acids of limkain b1. There have been earlier reports of autoantibodies reactive to peroxisomes; however, the molecular identities of the target autoantigen(s) were never established [13].
Table 1.
Known molecular identities of a subclass of autoantigens with discrete cytoplasmic distributions
| Gene1 | Gene/protein aliases2 | Protein localization | References |
|---|---|---|---|
| –3 | Lyso-bis phosphatidic acid (LBPA) | Late endosomes | Kobayashi et al. 1998 [5] |
| EEA1 | Early endosome antigen 1 (EEA1) | Early endosomes, cytoplasmic | Mu et al. 1995 [14] |
| Waite et al. 1997 [7] | |||
| Selak et al. 1999 [15] | |||
| IGF2R | Insulin-like growth factor 2 receptor (IGF2R) cation-independent mannose 6-phosphate receptor (CI-MPR), CD222 | Lysosomes, Plasma membrane | Tarrago et al. 1999 [16] |
| LAMP2 | Lysosomal associated membrane protein 2 CD107b | Lysosomes | Kain et al. 1995 [17] |
| Endosomes | |||
| Plasma membrane | |||
| LKAP4 | Limkain b1 KIAA0430, A-362G6·1 | Subset of peroxisomes | This report |
| PMSA3 | α3-HC9, proteasome subunit C8 | Proteasomes | Feist et al. 1996 [18] |
| RSN | Reed–Steinberg microtubule-associated protein (RSN) cytoplasmic linker protein 170 (CLIP-170) | Early endosomes | Griffith et al. 2001 [19] |
| Cytoplasmic | |||
| TFRC | Transferrin receptor (TFCR) CD71 | Early endosomes, recycling endosomes, plasma membrane | Larrick et al. 1984 [20] |
| TNRC6 | Trinucleotide repeat containing 6 (TNRC6) GW182, EDIE | Cytoplasmic | Eystathioy et al. 2002 [21] |
Approved HUGO symbol (see http://www.gene.ucl.ac.uk/nomenclature/).
As catalogued in GeneCards: (see http://bioinformatics.weizmann.ac.il/cards).
LBPA is not protein in nature.
Gene symbol not yet approved by HUGO/GDB nomenclature committee.
Partial cDNA sequences of LKAP were first entered into the database in 1997 via direct submission by Miyamoto et al. (Accession no. AB012134) and annotated as encoding a novel large protein, named limkain b1, that associates with LIM kinase 2. To date, there has been no publication describing the association of limkain b1 with LIM kinase 2 (LIMK2). Independent of the annotation of the human genome, limkain b1 encoding cDNAs were identified as part of the human unidentified gene encoded (HUGE) protein database project cataloguing cDNA clones encoding relatively large proteins from human brain [22,23]. The identification of autoantibodies reactive to limkain b1 provides the first evidence of the association of LKAP with a subset of peroxisomes. We have also determined that LKAP is present on a distinct set of peroxisomes that are marked with the presence of PXF and ABCD3. However, we observed some LKAP staining of distinct cytoplasmic structures that do not co-localize with either PXF or ABCD3. Whether these structures/compartments are peroxisomes lacking PXF and ABCD3 will require further investigation. We also found incomplete overlap in peroxisome detection by staining with anti-PXF and anti-ABCD3. For the HeLa cell substrate employed, we detected more PXF-stained vesicles than ABCD3-stained vesicles. Many proteins that localize to peroxisomes contain a conserved targeting signal (MICROBODIES_CTR) in the last three amino acids in the carboxyl terminus [24]. The LKAP carboxyl terminus sequence TKL (Thr-Lys-Leu) fits the MICROBODIES_CTR consensus sequence. However, we demonstrated that removal of the last three carboxyl terminal amino acids from a EGFP:LKAPCT429-TKL does not affect co-localization of the fusion protein with a set of ABCD3 peroxisomes.
LIMK2 has been reported to regulate actin cytoskeletal organization through phosphorylation of the actin filaments depolymerizing factor cofilin [25]. Recently it has been argued that peroxisomes are tethered to and achieve mobility through interaction with actin filaments [26]. This suggests that interactions between LIMK2 and LKAP may occur at the sites of peroxisome attachment to actin filaments. However, the putative, unpublished interaction of LIMK2 and LKAP requires verification. LKAP contains a chitin-type 2 binding domain motif spanning amino acids 199–273. Chitin is a polysaccharide present in the cell wall of fungi and exoskeleton of insects. In mammals, protein containing chitin-binding domains are thought to participate in defence against nematodes and other pathogens [27]. Peroxisomes are primary sites for hydrolysing of insoluble chitin [27,28]. Tandem RNA-binding domain motifs (RRM, RPN-1) at amino acids 514–580 and 784–856 are present in LKAP. To date we have not addressed whether these are bona fide RNA binding sites. We have no evidence of LKAP localization in the nucleus, therefore if the RNA binding domains are functional, interaction with RNA species would be restricted to the cytoplasm and peroxisomes.
Autoantibodies reactive to small cytoplasmic compartments/structures, apart from AMA, have demonstrated limited clinical utility to date, although there are a number of reports suggesting potential clinical application for detection of LPBA and EEA1. Kobayashi et al. [5] argue that detection of LBPA may have significance for cardiolipin antibody detection, and Salek et al. [15,29] found some limited association of EEA1 autoantibodies with neurological disease; however, specific tests for detection of these autoantibodies are not performed routinely in clinical laboratories. Two of the four sera reactive to LKAP also had ANA. The JA serum was reactive to replication protein A subunits 1 and 2 and serum from patient 12 produced a speckled nuclear staining pattern and was reactive to Ro (SS-A) and La (SS-B). Both JA and sample 12 sera were negative for reactivity to double-stranded DNA, other extractable nuclear antigens and rheumatoid factor. Patient JA and 12 had a history of interstitial lung disease and muscle aches and pains, respectively. The other two patients had unremarkable clinical presentations and were negative for the aforementioned additional autoantigen reactivities. There were no reports of peroxisomal biogenesis disorders in the patients reactive to LKAP. However, the limited sample size and sparsity of clinical information on the patients with circulating autoantibodies reactive to LKAP preclude determination of the clinical relevance of this class of autoantibodies.
Many of the human autoantibodies identified to date have established diagnostic application. The potential clinical utility of autoantibodies to small cytoplasmic vesicle-like structures has yet to be exploited due to the lack of clinical correlation studies and the dearth of knowledge regarding the molecular identity of the cognate autoantigens. Identification of these autoantigens is a prerequisite for further investigation of the potential diagnostic utility of these autoantibodies. In our small cohort of 17 sera with autoantibody reactivity to small cytoplasmic vesicle-like structures, we identified four sera with autoantibodies that co-localize with a subset of PXF marked peroxisomes that are not reactive to our LKAP fusion protein. This observation suggests that autoantibodies to limkain b1 may be the first in a series of peroxisomal autoantibodies.
Acknowledgments
K. Dunster thanks the Alfred Pathology Service (Alfred Hospital) for support of postgraduate studies at Monash University. This work forms part of K. Dunster's MBioMedSc studies.
References
- 1.Kakinuma T, Toh B-H, Sentry J. Human autoantibodies as reagents in biochemical research. Mod Rheumatol. 2003:15–21. doi: 10.3109/s101650300002. [DOI] [PubMed] [Google Scholar]
- 2.Riedel N, Wolin S, Guthrie C. A subset of yeast snRNAs contains functional binding sites for the highly conserved Sm antigen. Science. 1987;235:328–31. doi: 10.1126/science.2948278. [DOI] [PubMed] [Google Scholar]
- 3.Elkon K, Bonfa E, Llovet R, Danho W, Weissbach H, Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci USA. 1988;85:5186–9. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitlits VM, Sentry JW, Matthew ML, Smith AI, Toh BH. Autoantibodies to evolutionarily conserved epitopes of enolase in a patient with discoid lupus erythematosus. Immunology. 1997;92:362–8. doi: 10.1046/j.1365-2567.1997.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–7. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 6.Yong TJ, Gan YY, Toh BH, Sentry JW. Human CKIalpha (L) and CKIalpha (S) are encoded by both 2.4- and 4. 2-kb transcripts, the longer containing multiple RNA-destablising elements. Biochim Biophys Acta. 2000;1492:425–33. doi: 10.1016/s0167-4781(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 7.Waite RL, Sentry JW, Stenmark H, Toh BH. Autoantibodies to a novel early endosome antigen 1. Clin Immunol Immunopathol. 1998;86:81–7. doi: 10.1006/clin.1997.4455. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada Y, Ichikawa H, Chai Z, et al. Novel variant of p230 trans-Golgi network protein identified by serum from Sjogren's syndrome patient. Eur J Cell Biol. 2000;79:790–4. doi: 10.1078/0171-9335-00114. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. Cold Spring Harbour: Cold Spring Harbour Laboratory Press; 1989. [Google Scholar]
- 10.Letunic I, Goodstadt L, Dickens NJ, et al. Recent improvements to the SMART domain-based sequence annotation resource. Nucl Acids Res. 2002;30:242–4. doi: 10.1093/nar/30.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strassburg CP, Jaeckel E, Manns MP. Anti-mitochondrial antibodies and other immunological tests in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:595–601. doi: 10.1097/00042737-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Stinton LM, Eystathioy T, Selak S, Chan EK, Fritzler MJ. Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblysomes, exosomes, and GW bodies. Clin Immunol. 2004;110:30–44. doi: 10.1016/j.clim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Holm R, Gaarder PI, Helgeland L, Falkenhaug EI. A human autoantibody to peroxisomes. Clin Exp Immunol. 1985;61:305–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Mu FT, Callaghan JM, Steele-Mortimer O, et al. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine ‘fingers’ and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–11. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 15.Selak S, Chan EK, Schoenroth L, Senecal JL, Fritzler MJ. Early endosome antigen. 1. An autoantigen associated with neurological diseases. J Invest Med. 1999;47:311–8. [PubMed] [Google Scholar]
- 16.Tarrago D, Aguilera I, Melero J, Wichmann I, Nunez-Roldan A, Sanchez B. Identification of cation-independent mannose 6-phosphate receptor/insulin-like growth factor type-2 receptor as a novel target of autoantibodies. Immunology. 1999;98:652–62. doi: 10.1046/j.1365-2567.1999.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kain R, Matsui K, Exner M, et al. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: the lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med. 1995;181:585–97. doi: 10.1084/jem.181.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feist E, Dorner T, Kuckelkorn U, et al. Proteasome alpha-type subunit C9 is a primary target of autoantibodies in sera of patients with myositis and systemic lupus erythematosus. J Exp Med. 1996;184:1313–8. doi: 10.1084/jem.184.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith KJ, Ryan JP, Senecal JL, Fritzler MJ. The cytoplasmic linker protein CLIP-170 is a human autoantigen. Clin Exp Immunol. 2002;127:533–8. doi: 10.1046/j.1365-2249.2002.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larrick JW, Hyman ES. Acquired iron-deficiency anemia caused by an antibody against the transferrin receptor. N Engl J Med. 1984;311:214–8. doi: 10.1056/NEJM198407263110402. [DOI] [PubMed] [Google Scholar]
- 21.Eystathioy T, Chan EK, Tenenbaum SA, Keene JD, Griffith K, Fritzler MJ. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol Biol Cell. 2002;13:1338–51. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohara O, Nagase T, Ishikawa K, et al. Construction and characterization of human brain cDNA libraries suitable for analysis of cDNA clones encoding relatively large proteins. DNA Res. 1997;4:53–9. doi: 10.1093/dnares/4.1.53. [DOI] [PubMed] [Google Scholar]
- 23.Kikuno R, Nagase T, Waki M, Ohara O. HUGE: a database for human large proteins identified in the Kazusa cDNA sequencing project. Nucl Acids Res. 2002;30:166–8. doi: 10.1093/nar/30.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould SJ, Keller GA, Schneider M, et al. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 1990;9:85–90. doi: 10.1002/j.1460-2075.1990.tb08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumi T, Matsumoto K, Nakamura T. Mitosis-dependent phosphorylation and activation of LIM-kinase 1. Biochem Biophys Res Commun. 2002;290:1315–20. doi: 10.1006/bbrc.2002.6346. [DOI] [PubMed] [Google Scholar]
- 26.Mathur J, Mathur N, Hulskamp M. Simultaneous visualization of peroxisomes and cytoskeletal elements reveals actin and not microtubule-based peroxisome motility in plants. Plant Physiol. 2002;128:1031–45. doi: 10.1104/pp.011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tjoelker LW, Gosting L, Frey S, et al. Structural and functional definition of the human chitinase chitin-binding domain. J Biol Chem. 2000;275:514–20. doi: 10.1074/jbc.275.1.514. [DOI] [PubMed] [Google Scholar]
- 28.Chang KL, Tai MC, Cheng FH. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J Agric Food Chem. 2001;49:4845–51. doi: 10.1021/jf001469g. [DOI] [PubMed] [Google Scholar]
- 29.Selak S, Fritzler MJ. Altered neurological function in mice immunized with early endosome antigen 1. BMC Neurosci. 2004;5:2. doi: 10.1186/1471-2202-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]