Abstract
Recent reports have described reduced populations of CD27+ memory B cells and increased percentages of undifferentiated B cells in peripheral blood of patients with common variable immunodeficiency (CVID). This work has prompted two attempts to classify CVID based on rapid flow cytometric quantification of peripheral blood memory B cells and immature B cells. Evidence to support the hypothesis that such in vitro B cell classification systems correlate with clinical subtypes of CVID is being sought. For the classification to be useful in routine diagnosis, it is important that the flow cytometric method can be used without prior separation of peripheral blood mononuclear cells (PBMC). We have examined 23 CVID patients and 24 controls, using both PBMC and whole blood, and find an excellent correlation between these methods. The reproducibility of the method was excellent. We classified the CVID patients by all three of the existing classifications, including secretion of immunoglobulin by B cells in vitro as described by Bryant, as well as the more recent flow cytometric classification methods. Only one patient changed classification as a result of using whole blood.
Keywords: CD27+, CD21, immunodeficiency, memory B cells, whole blood
Introduction
Common variable immunodeficiency (CVID) comprises a clinically heterogeneous group of primary antibody immunodeficiency states, rather than a single gene defect as found for the X-linked primary antibody deficiencies [1]. The basic immunological defects underlying this syndrome are unknown. Attempts at classifying the disease based on in vitro assessment of B cell proliferation, differentiation and immunoglobulin (Ig) production have been made, but have not yielded clear clinical correlates [2–5]. CVID patients are characterized by low levels of Igs, especially IgG and IgA and by B lymphocyte percentages ranging from very low to normal. Saiki et al. [2] and Ariga et al. [3] both used Staphylococcus aureus Cowan 1 (SAC) plus or minus IL2 to examine the proliferative and differentiation ability of B cells from 15 and 7 CVID patients, respectively. Both groups found that CVID B cells could be classified into (a) CVID patients with no B cells (b) those whose B cells did not proliferate to SAC or produce Ig (c) those who proliferated normally to SAC ± IL-2, but produced no IgM or IgG (d) those who had normal proliferation but produced IgM only, and, in the study of Ariga [3], a fifth group (e) was identified whose B cells proliferated normally to SAC ± IL-2 and produced normal levels of IgG and IgM.
The most useful immunological classification system to date was that described by Bryant et al. [4]. CVID B cells were classified functionally, according to their capacity to produce IgG, IgM and IgA in vitro upon stimulation of peripheral blood lymphocytes (PBL) with IgM-coated beads and IL-2 [4]. In this work four groups were described: those with <1% B cells and three groups (A, B, C) with none, IgM only or full immunoglobulin production in vitro. All CVID patients fall into one of these four groups. Different groups within this classification appear to correlate with distinct immunological parameters [5]. Group A patients include those with granulomatous disease and splenomegaly whereas group B patients include those with both X and autosomal forms of hyper-IgM syndrome [5]. However, this classification has not been adopted widely, as the in vitro B cell phenotypes were not predictive and, perhaps more importantly, the technology did not lend itself easily to international standardization. Finally, a number of reports have shown that Epstein-Barr virus (EBV) can overcome the B cell fault in some CVID patients resulting in Ig secretion in vitro[6,7]. What these classification attempts have shown is the defect in CVID in terms of B cells is heterogeneous. Although useful, probably all of these classification systems need to be re-analysed and patient diagnoses reassessed in the light of recent genetically identified immunodeficiencies, many of which had been previously categorized as CVID. Examples include patients with XLP, ICOS and AID mutations [8–11].
Recently, a number of reports [12–15] have described reduced populations of CD27+ memory B cells and increased percentages of undifferentiated B cells in peripheral blood of patients with primary antibody deficiencies. This work has prompted attempts from both Warnatz et al. in Freiburg [12] and Piqueras et al. in Paris [13] to classify CVID based on rapid flow cytometric quantification of peripheral blood (PBL) memory B cells and immature B cells. Both groups have suggested an in vitro B cell classification system that highlights defects at different stages in B cell differentiation and correlates with clinical subtypes of CVID.
The classification of Warnatz et al. [12] used flow cytometry to analyse memory B cell populations in PBL from CVID patients. Patients with less than 0·4% class-switched CD27+IgM–IgD– memory B cells were termed group I, while group II CVID patients had greater than 0·4% class-switched CD27+IgM–IgD– memory B cells amongst their PBLs. These results correlated with the capacity of PBL to produce immunoglobulins in vitro upon stimulation with Staphylococcus aureus Cowan I (SAC) plus interleukin-2 because the production of IgG in vitro is entirely dependent on the presence of switched memory B cells. Group I patients were further subdivided into those with an increased proportion of CD21– peripheral B cells (> 20%; group Ia) and patients with normal percentages of CD21– B cells (< 20%; group Ib). A significant clustering of CVID patients with splenomegaly and autoimmune cytopenias were found in group Ia.
Piqueras et al. [13] have proposed a classification based on the quantitative repartition of naive/memory B cells based on the dual expression of IgD and CD27. CVID patients were categorized into three groups: Group MB2 are those with normal memory B cells; Group MB1 are those with defective switched memory (IgD–CD27+) but normal nonswitched memory B cells (IgD+CD27+) and Group MB0 CVID patients have almost no memory B cells. In addition, activation markers including CD25 and CD21 were down-regulated on B cells of group MB1 patients. As with the Warnatz scheme, this classification also correlates with some clinical aspects of CVID. Group MB0 had a higher prevalence of splenomegaly, lymphoid proliferation and granulomatous disease. Splenomegaly was also frequent in CVID group MB1 patients, however, autoimmunity was observed with similar prevalence in all three groups.
The published classifications (which will be referred to as Warnatz and Piqueras classifications, respectively) are undergoing validation at present (K. Warnatz et al. in preparation), but these use prior separation of peripheral blood lymphocytes (PBL) to quantify memory and immature B cells [12,13]. We determined whether the flow cytometric method is applicable to whole blood (WB) samples, assessed the reproducibility and compared this to the other three published methods [4, 12, 13] for the diagnosis and classification of patients with CVID.
Classifications using these types of methods depend on defining tight cut-offs and being able to show that the proportions of immune phenotypes are stable with time and not secondary to the complications of CVID, including intercurrent infections. Only large collaborative studies will be able to show this, and so it is essential that the methods involved are simple and reliable. Quality assurance of such assays will play an important role if these classifications are to be used predicatively.
Materials and methods
Patients and healthy donors
Ethical permission to study B cells in CVID patients and healthy donors (HD) was obtained from the Central Oxfordshire Research Ethics Committee.
Informed consent was obtained from 23 CVID patients who met the International (PAGID and ESID) diagnostic criteria; only those with normal numbers of circulating B cells were tested. CVID patients included those with granulomatous and autoimmune disease. Although not all CVID patients discussed in this report were tested individually for XLP, ICOS or HIGM mutations, their clinical histories and laboratory phenotypes strongly supported the CVID diagnosis. Amongst the Oxford patients, the mean duration of disease was 10·4 years (range: newly diagnosed – 48 years). Twenty-four HD were recruited from hospital staff. All patients were stable on immunoglobulin substitution and none were on additional medication.
Lymphocyte counts
Full blood counts, and lymphocyte counts were measured using a routine haematology analyser (Cobras, Argos. Roche).
Preparation of PBL
Heparinized blood, 20–30 ml, was obtained immediately prior to the next immunoglobulin infusion. Peripheral blood mononuclear cells (PBL) were isolated by Ficoll-Hypaque density gradient centrifugation. Whole blood was diluted 1:3 in RPMI containing penicillin, streptomycin and l-glutamine before being carefully overlaid onto 10 ml of ficoll-Hypaque and centrifuged at 2000 r.p.m. for 25 min with the brake off. The PBL interface was carefully removed then washed twice in phosphate buffered saline (PBS). The final supernatant could then be decanted and the cell pellet re-suspended in 5 ml RPMI. This was gently overlaid onto 2 ml of foetal calf serum (FCS), centrifuged at 1500 r.p.m. to reduce cell-bound IgG, and resuspended in RPMI containing 10% FCS. The cells were adjusted to a concentration 2·5 × 106/ml before 200 µl/well were plated out onto a 48-well plate for stimulation with SAC + Il-2. Alternatively, they were adjusted to a concentration of 2·5 × 105/50 ml in RPMI containing 10% FCS for use in monoclonal antibody staining by flow cytometry.
Preparation of whole blood lymphocyte populations
Whole blood (WB) must be washed to remove free antibody, as this can inhibit antibody staining. One ml of whole blood was added to 3 ml of PBS, vortexed and then centrifuged at 200 g for 5 min. The supernatant was aspirated and the cells resuspended in 3 ml PBS; this washing step was repeated twice. After the final centrifugation and aspiration, 1 ml PBS was added to the cell pellet and the whole blood preparation was ready for B cell staining.
Staining of PBMC and whole blood lymphocytes with monoclonal antibodies to B cells
PBMC at a concentration of 2·5 × 105 in 50 µl of RPMI 1640 containing 10% FCS, were stained for 20 min at 4°C with 10 µl of a mixture of CD27-FITC (Dako, Denmark) or CD21-FITC (Pharmingen, Oxford, UK) anti IgD-PE (Southern Biotechnology, UK) CD19-PC7 (Coulter Immunotech, UK) and anti-IgM-Cy5 (Jackson Laboaratories, UK). Next, 1·5 ml of FACSlyse (Becton Dickinson, Oxford, UK) was added to the WB tubes and incubated for 5 min in the dark. PBMC tubes had 1·5 ml of PBS added. Cells were washed twice by centrifuging for 5 min at 1200 r.p.m. After the final wash, the supernatant was decanted and cells resuspended in 400 µl of 1% formaldehyde before being read on the FACScalibur. We have found that, once stained, samples can be stored for up to 24 h before being read on the FACScalibur.
FACS analysis and statistical analysis
Cells were assessed using four-colour acquisition on a FACSCalibur (Becton Dickinson, CA, USA) and data analysed using cellquest software (Becton-Dickinson). PBL were examined using forward- versus side-scatter gating and B cells analysed using side-scatter versus CD19 gating. Statistical analyses of numerical data were performed where appropriate using Excel or Prism GraphPad 4 programs (GraphPad Prism, USA)
Assessment of Ig syntheses in vitro
To assess immunoglobulin synthesis in vitro, 5 × 105 PBMC from patients and controls were stimulated in RPMI-1640 (Sigma, Poole, UK) plus 10% fetal calf serum (FCS: Sigma) for 8 days at 37°C with or without Staphylococcus aureus strain Cowan (SAC, 1 : 10 000) + 20 U/ml interleukin (IL)-2 (Calbiochem, UK). On day 8, supernatants were removed from wells, replicate wells were pooled and supernatants frozen at – 0°C until they were needed for use in the ELISAs. Control cultures were kept in medium without B cell stimulants. Results were expressed as ng/ml of immunoglobulin isotype produced in the stimulated culture minus the control cultures.
ELISA method
Separate ELISAs were developed to detect and quantify IgG, IgA and IgM in supernatants from controls and patients. Supernatants from stimulated cultures were added to the central 60 wells of the ELISA plate (after plate optimization, J Jones, MSc Thesis, London). ELISA plates were coated using anti human IgG or IgA or IgM (Jackson Laboratories, UK) 4 h prior to use. Next, 100 µl of patient (1:50 dilution) and control (1:200 dilution) samples were added in duplicate to ELISA plates before being incubated at 4°C for 2 h. Alkaline phosphate-conjugated antihuman immunoglobulin was added for a further 2 h incubation at 4°C before addition of an alkaline phosphatase substrate. Plates were monitored regularly, read at 405 nm, and stopped once the second standard curve point reached an optical density of 1·0–1·2. Results were expressed as ng/ml of Ig isotype produced in the stimulated culture minus the control cultures. Based on the results of Ig synthesis, we assigned CVID patients into groups A, B or C as defined by Bryant et al. [4] (see Table 1).
Table 1.
Characteristics of CVID Patients and Controls.
| IgM/D+2 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM/D+27– | IgM/D+27– | IgM/D+27+ | IgM/D+27+ | IgM/D–27+ | IgM/D–27+ | CD27+ | Warnatz | Piqueras | |||||||||||
| No | Sex | Age | Age Onset | Age Diag | CD19 % | Lymph's mm2 | naive % PBL | naive % B | IgD mem % PBL | IgD mem % B | Switched % PBL | Switched % B | % B % B | CD21-ve % B | Bryant | PBMC | WB | PBMC | WB |
| 1 | M | 53 | 27 | 28 | 7.4 | 2399 | 7.30 | 98.50 | 0.10 | 1.30 | 0.003 | 0.04 | 1.34 | 18.20 | A | 1b | 1b | MBO | MBO |
| 2 | M | 73 | 63 | 71 | 4.0 | 702 | 3.8 | 96.3 | 0.13 | 3.3 | 0.01 | 0.22 | 3.52 | 1.94 | A | 1b | 1b | MBO | MBO |
| 3 | M | 67 | 62 | 63 | 1.7 | 2155 | 1.5 | 85 | 0.17 | 9.8 | 0.03 | 1.7 | 11.5 | 10.9 | A | 1b | 1b | MB1 | MB1 |
| 4 | F | 20 | 13 | 14 | 5.3 | 1282 | 4.8 | 91 | 0.1 | 2.2 | 0.03 | 0.5 | 2.7 | 2.02 | A | 1b | 1b | MBO | MBO |
| 5 | M | 68 | 65 | 65 | 4.2 | n.d | 3.8 | 89.5 | 0.2 | 4 | 0.10 | 3.2 | 7.2 | 64.7 | A | 1a | 1a | MBO | MBO |
| 6 | F | 50 | 39 | 41 | 30.4 | 746 | 29.6 | 82 | 0.3 | 8.7 | 0.10 | 2.5 | 11.2 | 20.4 | A | 1a | 1b | MBO | MBO |
| 7 | M | 55 | 35 | 35 | 5.3 | 730 | 4.9 | 92.4 | 0.2 | 4.7 | 0.10 | 1.5 | 6.2 | 35.07 | A | 1a | 1a | MB0 | MBO |
| 8 | M | 40 | 33 | 36 | 2.2 | 1828 | 1.7 | 76.1 | 0.2 | 7.63 | 0.10 | 5.61 | 13.2 | 24.3 | A | 1a | 1b | MB0 | MB1 |
| 9 | F | 76 | 60 | 60 | 6.0 | 637 | 5.2 | 86.5 | 0.3 | 5.37 | 0.10 | 1.94 | 7.3 | 16.6 | A | 1b | 1b | MB0 | MBO |
| 10 | F | 50 | 2 | 39 | 1.1 | 559 | 0.9 | 65.7 | 0.1 | 8.57 | 0.10 | 2.9 | 11.5 | 32.14 | A | 1a | 1a | MB1 | MB1 |
| 11 | F | 33 | 31 | 32 | 3.6 | 2940 | 2.6 | 71.7 | 0.9 | 24.6 | 0.10 | 2.3 | 26.9 | 3.49 | A | 1b | 1b | MB1 | MB1 |
| 12 | M | 18 | 16 | 16 | 19.0 | 1039 | 16.7 | 88.3 | 1.9 | 9.7 | 0.20 | 1.3 | 11 | 1.74 | C | 1b | 1b | MB1 | MB1 |
| 13 | F | 71 | 71 | 71 | 8.3 | 1468 | 5 | 60.6 | 2.6 | 31.5 | 0.37 | 4.64 | 36.1 | 39.83 | B | 1a | 1a | MB1 | MB1 |
| 14 | F | 40 | 39 | 39 | 8.6 | 695 | 6.5 | 75.1 | 1.5 | 17.9 | 0.36 | 4.13 | 22.6 | 4.7 | B | 1b | 1b | MB1 | MB1 |
| 15 | F | 22 | 17 | 17 | 9.9 | 769 | 7.7 | 79 | 1.3 | 13.6 | 0.28 | 3.61 | 17.2 | 17.8 | A | 1b | 1a | MB1 | MB1 |
| 16 | F | 54 | 43 | 49 | 11.2 | 1378 | 8.4 | 75 | 2.3 | 21 | 0.33 | 3.6 | 24.6 | 1.5 | ND | 1b | 1b | MB1 | MB1 |
| 17 | F | 72 | 64 | 66 | 12.2 | 917 | 8.8 | 71.9 | 1.6 | 12.7 | 0.36 | 2.96 | 16.7 | 21.88 | B | 1a | 1a | MB1 | MB1 |
| 18 | F | 25 | 2 | 6 | 6.0 | 1569 | 4.1 | 68.3 | 1.2 | 19 | 0.58 | 9.61 | 29.5 | 9.68 | B | 11 | II | MB2 | MB2 |
| 19 | M | 23 | 1 | 3 | 14.8 | 2011 | 11 | 73.9 | 2.8 | 19 | 0.70 | 4.82 | 23.8 | 7.65 | C | 11 | II | MB1 | MB1 |
| 20 | M | 39 | 38 | 38 | 4.7 | 2231 | 2.7 | 57.2 | 1.3 | 27.7 | 0.66 | 14.1 | 41.8 | 5.38 | C | 11 | II | MB2 | MB2 |
| 21 | M | 19 | 16 | 16 | 12.7 | 2054 | 9.6 | 75.6 | 1.3 | 10.5 | 1.37 | 10.75 | 21.5 | 1.86 | A | 11 | II | MB2 | MB2 |
| 22 | M | 44 | 13 | 26 | 6.3 | 1460 | 4 | 64.8 | 1.7 | 26.8 | 0.58 | 6.4 | 33.2 | 4.29 | B | 11 | II | MB1 | MB1 |
| 23 | F | 58 | 50 | 50 | 12.0 | 2041 | 6.7 | 65.5 | 1.1 | 19 | 0.60 | 9.8 | 28.8 | 56.71 | B | 11 | 1a | MB2 | MB1 |
Details of 23 common variable immunodeficiency (CVID) patients used in the study. The percentages of CD19+ B cells, CD27– (naive) and CD27+ (memory) B cells, and CD21+ B cells were derived from peripheral blood mononuclear cells (PBMC). The means for each characteristic defined using PBMCs are compared to the means obtained when whole blood (WB) preparations were used to calculate percentages. We have assigned the patients to the classifications suggested by Bryant [4], Warnatz [12] or Piqueras [13]. Results are expressed as means ± s.d.; n.d. = not done.
Results
Comparison of whole blood and lymphocyte separation methods
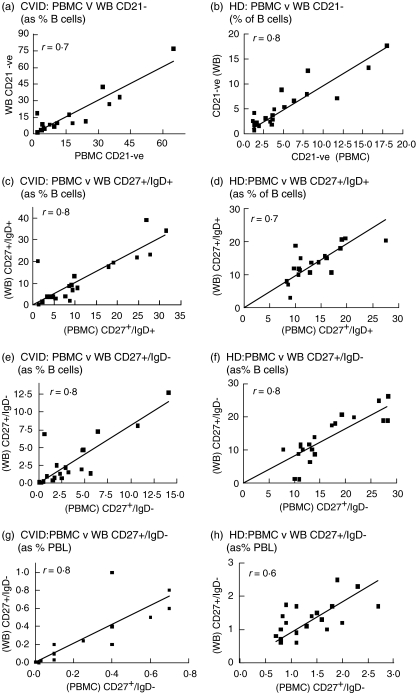
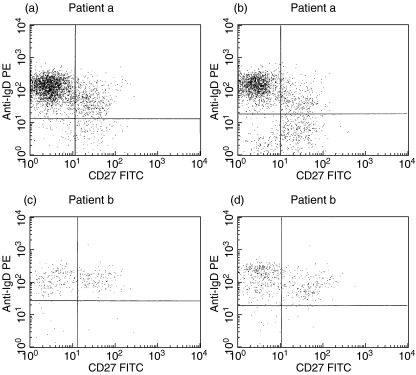
The details of 23 CVID patients are given in Table 1. The percentages of CD19+/CD27- (naive) B cells, CD19+/CD27+ (memory) B cells and CD21+ B cells shown in the table were derived from PBMC preparations. The mean values for each PBMC population were compared to mean values obtained from the WB method and no significant differences were observed. This close correlation between the two separation methods is reflected in the correlation graphs in Fig. 1. Comparison of individual FACS dot plots from either PBMC or WB preparations for two CVID patients (named a and b) are shown in Fig. 2. FACs plots of stained B cell populations prepared by WB or PBMC methods were highly comparable. Reproducibility of normal and CVID blood samples prepared by the WB method showed that mean interassay CVs for three subjects over three different staining assays ranged from 11·9 to 14·1% (Table 2).
Fig. 1.
Comparison of percentages of B cell subpopulations obtained from peripheral blood mononuclear cells (PBMC) and whole blood (WB) methods. CD19+/CD21– B cells from common variable immunodeficiency (CVID) and healthy donors (HD) as a percentage of B cells are shown in (a, b) and CD19+/CD27+/IgD+ and CD19+/CD27+/IgD– B cells calculated as percentage of B cells are shown in (c, e) for CVID blood and in (d, f) for HD blood. (g, h) Comparison of CD19+/CD27+/IgD– B cells calculated as percentage of PBL. Spearman's r correlation values are shown and all were significant.
Fig. 2.
FACS dot plots of CD19 gated lymphocytes stained with anti-CD27 and anti-IgD antibodies. CD19+ populations from two representative common variable immunodeficiency (CVID) patients analysed on separated peripheral blood mononuclear cells (PBMC) (a, c) or on lymphocytes from whole blood preparations (WB) (b, d) are compared.
Table 2.
| No. | Sex | Age | Age onset | Age diagn. | CD19 % | Lymphs mm2 | IgM/D+27– naive % PBL | IgM/D+27– naive % B | IgM/D+27+ IgD mem % PBL | IgM/D+27+ IgDmem % B | IgM/D-27+ Switched % PBL | IgM/D-27+ Switched % B | CD27+% B | CD21–% B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Means | CVID n = 23 | |||||||||||||
| PBMC | 11M/12F | 48 ± 20 | 33 ± 22 | 39 ± 21 | 1398 ± 666 | 8.2 ± 7n.s. | 6.5 ± 7n.s. | 78.9 ± 11n.s. | 1 ± 0.9n.s. | 12 ± 9n.s. | 0.28 ± 0.3n.s. | 2.62 ± 3n.s. | 16.3 ± 11n.s. | 16 ± 17n.s |
| WB | 8.7 ± 8 | 7.1 ± 7 | 81.1 ± 14 | 1.1 ± 1 | 12 ± 11 | 0.3 ± 0.3 | 3.5 ± 6 | 16.2 ± 14 | 14.8 ± 19 | |||||
| Means | HD n = 24 | |||||||||||||
| PBMC | 10M/14F | 38 ± 12 | 1892 ± 434 | 8.6 ± 3 | 5.6 ± 2n.s. | 65 ± 11n.s. | 1.2 ± 0.5n.s. | 15 ± 6n.s. | 1.4 ± 0.5n.s. | 17.5 ± 7n.s. | 29.8 ± 8n.s. | 4.9 ± 5n.s. | ||
| WB | 8.5 ± 3 | 5.9 ± 2 | 68.7 ± 11 | 1.1 ± 0.7 | 13.7 ± 5 | 1.2 ± 0.5 | 13 ± 8 | 26.3 ± 14 | 5.4 ± 5 |
Numbers of lymphocytes and percentages of B cell subpopulations form CVID patients were compared to 24 healthy donors (HD). Where possible, the mean for each characteristic is shown for PBMC and WB from CVID and HD. Statistics were performed using the Mann-Whitney U-test in GraphPad prism 4.
Assigning classification to CVID patients
We have assigned the patients to the classifications suggested by Bryant [4], Warnatz [12] or Piqueras [13], as shown in Table 1. The Warnatz classification assigns CVID patients into (Group I) patients where < 0·4% of PBMCs are switched (CD27+ IgD-) memory B cells and (Group II) patients where >0·4% of the PBMCs are switched memory B cells. Warnatz subdivided Group I further into patients with > 20% CD21+ B cells, termed Group Ia and Group Ib; these are are those with <20% CD21+ B cells. Piqueras et al. also classified their CVID patient cohort based on quantification of peripheral blood memory B cells subsets; however, they expressed the B cell subpopulations as a percentage of total B cells in peripheral blood. The most severe phenotype assigned to patients was termed MB0, with less than 11% CD19+/CD27+ B cells in the peripheral blood. An MB1 phenotype is where ≤ 8% (CD27+ IgD-) switched memory B cells are present with normal or increased percentages of non-switched (CD27+/IgD+) cells present. MB2 defines those patients who have near normal distribution of naive/memory B cells in their blood. The Bryant classification depends on the nature of immunoglobulin secreted in vitro after stimulation of B cells: Group A, none detectable; Group B, IgM only; Group C, both IgG and IgM.
Comparison within Freiburg and Paris classifications
No healthy control changed classifications as a result of using whole blood. One patient changed classification completely: patient 23 changed from Group II/MB2 using the PBMC method to Group I/MB1 with the WB method; patient 8 moved slightly within the classifications, from MB1/Ia using PBMC separations to MB0/Ib using the WB technique, as follows:
patient no. 23: PBMC II/MB2, whole blood I/MB1;
patient no. 6: PBMC Ia/MB1, whole blood Ib/MB0.
Comparison between Freiburg and Paris classifications
In this small cohort, 21/23 patients correlated as expected between Warnatz and Piqueras classifications when either separation technique was used. Seventeen of 23 were Group I and MB0/1 and three of 23 were Group II and MB2 (Table 1). Within the separation technique used, patient 23 also correlated between Warnatz and Piqueras classification systems.
Two of 23 patients, however, did not correlate between the two schemes. Patients 19 and 22 were clearly Group II with 0·7% and 0·58% of PBLs of the CD19+/27+/IgD- phenotype. However, both were classified as MB1 in the Piqueras classification system [13].
Comparison with the Bryant classification system
These are given in Table 1. Ninety-three per cent of Bryant Group A patients fell into Group MB0/1 of Piqueras and Group 1 of Warnatz. However, one Bryant Group A patient (patient 21) was clearly MB2 and Group II. Fifty per cent (three of six) Bryant Group B patients were MB0/1 and Group I (a or b) and 50% were MB2 and Group II patients; one of these, patient 23, had a split classification, being MB1 and Group II. Sixty-six per cent of Bryant Group C patients were Group II and MB2; however, one was clearly Group Ib and MB1.
Discussion
A number of immunological abnormalities in CVID patients have been identified, including primary B cell defects [2,16, 17], T cell abnormalities [18,19], heightened macrophage activation [20] and abnormal cytokine production [21–23]. The immunophenotypes found in CVID are heterogeneous, as are the clinical phenotypes, but no satisfactory method of classifying the underlying syndromes has yet been found.
Recently, a number of studies have re-examined populations of B cells in peripheral blood of CVID patients. B cells developing in the bone marrow enter the blood as immature B cells where they mature into long-lived CD19+ IgM+ IgD+ CD27- naive B cells. On encountering antigen, these naive B cells enter germinal centres and emerge as either plasma cells or CD19+ CD27+ memory B cells. CD27+ memory B cells divide into two subpopulations defined by the expression of IgD on the cell surface. CD19+/27+ IgM+/IgD+ B cells are thought to be non-switched B cells while CD19+/27+ IgM+/IgD- B are switched memory B cells [26,27]. Using CD27 and IgD expression on peripheral blood B cells as markers of functional human B memory has resulted in the definition of three subpopulation of B cells: cB1 (IgD+ 27-) are naive B cells, cB2 (IgD+ 27+) are unswitched memory B cells and have been shown to undergo limited somatic hypermutation and produce high-affinity IgM and some IgG. cB3 (IgD- 27+) are switched memory B cells, producing IgG, IgM and IgA [26].
Reports [12–15] describing low populations of CD27+ memory B cells and increased percentages of immature B cells in peripheral blood of patients with CVID have resulted in two largely overlapping ex vivo B cell classification schemes being recommended [12,13] to classify CVID using a rapid flow cytometric method. There is some evidence that classifying CVID patients in this way correlates with clinical subtypes of CVID [5,6]. In the Warnatz classification, Group Ia patients had increased incidence of splenomegaly and autoimmune disease. In the Piqueras classification, 59% of Group MB0 had splenomegaly, 48% had lymphoid proliferation and 44% had granulomatous disease. Forty-two per cent of MB1 patients also had splenomegaly, but autoimmunity was similar in all three groups.
The WB described here is fast, requires very little blood and is reproducible. Percentages of naive and memory cells obtained from patients and controls were not significantly altered using WB or PBMC methods. In this small cohort we confirm (Table 1) the previous finding that MB0/MB1 grouped together correlated with Warnatz Groups 1a and 1b, but not if MB0 and MB1 were used individually (Table 1). Warnatz et al. have previously shown IgG production in vitro to be dependent on the presence of switched memory B cells [12]. In general, the findings from this small cohort of CVID patients would support this. Ninety-three per cent of Bryant Group A patients (no IgG, IgM or IgA production in vitro) were within the MB0/MB1 and Group Ia/b categories, which have reduced numbers of switched and non-switched memory B cells. No Groups B or C patients were found within the MB0 category, which is the most severe of all the phenotypes defined by CD27 and IgD expression.
The WB method described in this report to quantify CD27+ memory cells in peripheral blood would ensure easy follow-up of patients and allow monitoring of their memory B cell phenotype over time and in response to medications.
References
- 1.Chapel H, Geha R, Rosen F. For the IUIS PID Classification Committee. Primary immunodeficiency Diseases; an update. Clin Exp Immunol. 2003;132:9–15. doi: 10.1046/j.1365-2249.2003.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saiki O, Ralph P, Cunningham-Rundles C, Good RA. Three distinct stages of B cell defect in common variable immunodeficiency. Proc Natl Acad Sci USA. 1982;79:6008–14. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariga T, Okano M, Takahashi Y, Sakiyama Y, Matsumoto S. Analysis of B cell dysfunction in patients with common variable immunodeficiency by using recombinant interleukin 2. Tohoku J Exp Med. 1987;152:53–61. doi: 10.1620/tjem.152.53. [DOI] [PubMed] [Google Scholar]
- 4.Bryant A, Calver NC, Toubi E, Webster ADB, Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to IgM and interleukin 2. Clin Immunol Immunopathol. 1990;56:239–48. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- 5.Spickett GP, Farrant J, North ME, Zhang J-G, Morgan L, Webster ADB. Common Variable immunodeficiency: How many diseases? Immunol Today. 1997;18:325–32. doi: 10.1016/s0167-5699(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 6.De Gast GC, Wilkins SR, Webster ADB , Rickinson A and Platts-Mills TAE. Functional ‘immaturity’ of isolated B cells from patients with hypogammaglobulinaemia. Clin Exp Immunol. 1980;42:535–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong G, Ralph P, Nakoinz I, Saiki O, Cunningham-Rundles C. Rescue of IgM, IgG and IgA production in common variable immunodeficiency by T cell independent stimulation with Epstein-Barr virus. J Clin Immunol. 1985;5:122–9. doi: 10.1007/BF00915010. [DOI] [PubMed] [Google Scholar]
- 8.Eastwood D, Gilmour KC, Nistala K, et al. Prevalence of SAP gene defects in male patients diagnosed with common variable immunodeficiency. Clin Exp Immunol. 2004;137:584–8. doi: 10.1111/j.1365-2249.2004.02581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimbacher B, Hutloff A, Schlesier M, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–8. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 10.Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–75. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 11.Minegishi Y, Lavoie A, Cunningham-Rundles C, et al. Mutations in activation-induced cytidine deaminase in patients with hyper IgM syndrome. Clin Immunol. 2000;97:203–10. doi: 10.1006/clim.2000.4956. [DOI] [PubMed] [Google Scholar]
- 12.Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, Eibel H, Schleiser M, Peter HH. Severe deficiency of switched memory B cells (CD27+IgM– IgD–) in subgroups of patients with common variable immunodeficiency (CVID) – A new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 13.Piqueras B, Lavenu-Bombled C, Galicier L, Bergeron-van der Cruyssen F, Mouthon L, Chevret S, Debre P, Schmitt C, Oksenhendler E. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;5:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 14.Brouet JC, Chedeville A, Fermand JP, Royer B. Study of the B cell memory compartment in common variable immunodeficiency. Eur J Immunol. 2000;30:2516–20. doi: 10.1002/1521-4141(200009)30:9<2516::AID-IMMU2516>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Jacquot S, Macon-Lemaitre L, Paris E, et al. B cell co-receptors regulating T cell dependent antibody production in common variable immunodeficiency: CD27 pathway defects identify subsets of severely immunocompromised patients. Int Immunol. 2001;13:871–6. doi: 10.1093/intimm/13.7.871. [DOI] [PubMed] [Google Scholar]
- 16.Denz A, Eibel H, Illge H, Kienzle G, Schleiser M, Peter HH. Impaired up-regulation of CD86 B cells of type A common variable immunodeficiency. Eur J Immunol. 2000;30:1069–77. doi: 10.1002/(SICI)1521-4141(200004)30:4<1069::AID-IMMU1069>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Levy Y, Gupta N, LeDeist F, Garcia C, Fischer A, Weill JC, Reynaud C-A. Defect in IgV gene somatic hypermutation in common variable immunodeficiency syndrome. PNAS. 1998;95:13135–40. doi: 10.1073/pnas.95.22.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boncristiano M, Majolini MB, D’Elios MM, et al. Defective recruitment and activation of ZAP-70 in common variable immunodeficiency patients with T cell defects. Eur J Immunol. 2000;30:2632–8. doi: 10.1002/1521-4141(200009)30:9<2632::AID-IMMU2632>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Stagg AJ, Funauchi M, Knight SC, Webster ADB, Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1994;96:48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aukrust P, Lien E, Kristoffersen AK, Muller F, Haug CJ, Espevik T, Froland SS. Persistent activation of the tumour necrosis factor system in a subgroup of patients with common variable immunodeficiency-possible immunologic and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]
- 21.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 22.Sneller MC, Strober W. Abnormalities of lymphokine gene expression in patient with common variable immunodeficiency. J Immunol. 1990;144:3762–9. [PubMed] [Google Scholar]
- 23.Antrobus P, Foster D, Chapel HM, Ferry BL. TNF Cytokine levels in CD4 and CD8 T cells in subgroups of common variable immunodeficiency patients (CVID) Proceedings of IX Meeting of the European Society for Immunodeficiencies. 2000:3–11. [Google Scholar]
- 24.Loder F, Mutschler B, Ray R, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–85. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy RR, Hayakawa K. B cell development pathways. Ann Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Agematsu K, Ochs HD, Sugane K. Functional analysis of human memory B-cell subpopulations: IgD+ CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol. 2003;108:128–37. doi: 10.1016/s1521-6616(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 27.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cell. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]