Abstract
Toll-like receptors (TLRs) constitute an archetypal pattern recognition system. Their sophisticated biology underpins the ability of innate immunity to discriminate between highly diverse microbial pathogens and self. However, the remarkable progress made in describing this biology has also revealed new immunological systems and processes previously hidden to investigators. In particular, TLRs appear to have a fundamental role in the generation of clonal adaptive immune responses, non-infectious disease pathogenesis and even in the maintenance of normal mammalian homeostasis. Although an understanding of TLRs has answered some fundamental questions at the host–pathogen interface, further issues, particularly regarding therapeutic modulation of these receptors, have yet to be resolved.
Keywords: IL-1 receptor, innate immunity, proinflammatory, Toll-like receptor
Introduction
Until the 1990s immunology was dominated by advances in adaptive immunity, a biological system restricted to vertebrate species. This led to developments in transplantation biology and vaccine design, together with an improved understanding of the pathogenesis of infectious and autoimmune disease. In contrast, innate immunity, a phylogenetically more ancient biological system, remained poorly characterized. Although some facets of this system such as phagocytosis, free radical killing and activation of complement had been explored, the fundamental means by which pathogens are first recognized by the host (and distinguished from commensal organisms or self-antigens) were entirely unknown.
In 1991 a remarkable sequence similarity between Toll, a transmembrane protein involved in Drosophila embryogenesis, and the human interleukin-1 receptor was described [1,2]. A mammalian Toll-like receptor homologue (TLR1) was then cloned and mapped to chromosome 4 before an immune role for both Drosophila toll and mammalian Toll-like receptors (TLRs) was confirmed [3–5]. A total of 13 mammalian TLR paralogues (11 are expressed in humans) have now been described, each responsible for the ‘pattern-recognition’ of distinct invariant microbial structures [6] (Table 1). Critically, the TLR members of the IL-1 receptor family not only play a pivotal role in innate defence, but also facilitate the non-clonal high-fidelity discrimination of self from non-self originally predicted by Janeway [7]. In this review, we will describe how advances in TLR biology have revealed previously hidden sophistication within innate defences and created new fundamental questions even beyond the field of immunology.
Table 1.
List of common abbreviations found in the Toll-like receptor (TLR) field.
| dsRNA | Double-stranded RNA |
| ECSIT | Evolutionarily conserved signalling intermediate in Toll pathways |
| HSP | Heat-shock protein |
| IKK | IκB kinase |
| IRAK | Interleukin-1 receptor-associated kinase |
| JAK | Janus kinase |
| Jnk | Jun N-terminal Kinase |
| LPS | Lipopolysaccharide |
| LTA | Lipoteicoic acid |
| Mal | myD88-adapter-like (= TIRAP) |
| MAPK | Mitogen-activated protein kinase |
| mDC | Myeloid derived dendritic cell |
| MIF | Macrophage-inhibitory factor |
| MyD88 | Myeloid differentiation factor 88 |
| NF-κB | Nuclear factor-κB |
| PAMP | Pathogen-associated molecular pattern |
| pDC | Plasmacytoid dendritic cell |
| PGN | Peptidoglycan |
| PRR | Pattern recognition receptor |
| RIP | Receptor interacting protein |
| SIGGR | Single immunoglobulin IL-1R-related molecule |
| shRNA | Short hairpin RNA |
| siRNA | Short interfering RNA |
| SIRS | Systemic inflammatory response syndrome |
| SOCS | Suppressor of cytokine signalling |
| ssRNA | Single-stranded RNA |
| STAT | Signal transducer and activator of transcription |
| TANK | TRAF-family member-associated NFκB-activator |
| TAK1 | Transforming growth factor β-activated kinase-1 |
| TAB1 | TAK-1 binding protein-1 |
| TBK1 | TANK-binding kinase 1 |
| TIR | Toll/IL-1 receptor (domain) |
| TIRAP | TIR domain-containing adapter protein (= Mal) |
| TICAM | Toll-IL-1 receptor homology domain (TIR)-containing adapter molecule |
| TLR | Toll-like receptor |
| TNFR | Tumour necrosis factor (TNF) receptor |
| TRAF | TNFR-associated factor |
| TRAM | TRIF-related adapter molecule (= TICAM-2) |
| TRIF | TIR domain-containing adapter inducing interferon (IFN)-β (= TICAM-1) |
Complex distribution of TLR pattern recognition
Not only do TLRs exhibit marked differential tissue activity, but their absolute levels within a discrete cell type can also be highly dynamic (Table 2). Critically, both expression axes provide an integral part of receptor function and prevent host injury from inappropriate triggering of powerful down-stream inflammatory responses or autoimmune phenomena.
Table 2.
Location, modulation, ligands and function of mammalian Toll-like receptors (TLRs).
| TLR | Location | Modulation | Ligands | Functions | Refs |
|---|---|---|---|---|---|
| TLR1 | Ubiquitous | Soluble bacterial factors (Mycobacteria, Neisseria,Borrelia); Tri-acyl peptides | Heterodimers with TLR2 | [23,57] | |
| TLR2 | Surface membrane and phagolysosomes Myeloid,mast, and NK cells; mDCsT cells (including gamma–delta) | Enhanced expression with LPS, dsRNA Enhanced expression with IL-2, IL-15, IL-1beta, IFN-gamma, TNF-alpha | Bacterial: lipoprotein stereoisomers, PGN, LTA, phenol-soluble modulin (S.epidermidis), porins (Neisseria); LPS (Leptospira, Pseudomonas Helicobacter); lipoarabino- mannan (M. tb)Fungal: zymosan Viral: HCV core and NS3 proteins, measles virus, human CMV, HSV-1 Parasitic: trypanosomal, treponemal phospholipids Endogenous: HSP70, HSP60, defensins; Cys3pam | Co-operation with Dectin-1 T helper 2 responsesCo-stimulatory receptor modulation Metalloproteinase inductionAlpha-defensin production from NK cells Modulation of apoptosis, cell repair Adrenal responses | [9,27,33,34,44,54,58,72,87,91,137,141,143,145,149] |
| TLR3 | Intracellular in mDCs, NK cells | Viral/helminth: dsRNA Synthetic: polyinosinic–polycytidylic acid [poly (I:C)] siRNA, shRNAEndogenous: mRNA | Anti-viral: type I interferon production/cross-presentation Pathogenesis of West Nile virus | [10,14,41,43,152,173] | |
| TLR4 | Surface membrane of monocytes, mast cells and neutrophils Golgi in gut epithelial cells Regulatory/gamma–delta T cells Endothelial cells | Up-regulated IFN-gammaUp-regulated by RSVUp-regulated by bacterial superantigens Modulated by MIF | Bacterial: LPS; Pseudomonas exoS; C. pneumoniae, H.pylori HSP 60Viral: RSV F protein, MMTV envelope proteinParasitic: T. cruzi lipidsEndogenous: HSP 70, HSP 90, fibronectin, heparin, hyaluronic acid, fibrinogen, beta-defensin 2. Synthetic: taxol (murine); MPL (LPS mimetic) | Cell surface and intracellular recognition of LPSCo-ordination of neutrophil functionsDC maturation Apoptosis Endothelial functions (e.g. leucocyte roll)Atherosclerosis | [12,16–18,24,25,52,53,55,60–65,142,156] |
| TLR5 | Surface epithelial, NK cells; mDCs monocytes | Bacterial: flagellinSynthetic: discontinuous 13 amino acid peptide | T helper 2 responses Lung/gut mucosal immunity | [11,150,161] | |
| TLR6 | Surface myeloid, mast, B cells | Diacyl lipopeptides (mycoplasma) | Heterodimers with TLR2 | [23,57] | |
| TLR7 | Endosomal:pDCs, ?mDCs, B cells, eosinophils | Viral: ssRNA (e.g. influenza, VSV, HIV)Synthetic: imidazoquinolines | Eosinophil survival Defence against viral infection | [42] | |
| TLR8 | Endosomal: NK, T, myeloid cells | Viral: ssRNASynthetic:imidazoquinolines | Human not murine antiviral defence | [42] | |
| TLR9 | Endosomal: pDCs, B/NK cellsSurface of tonsillar cells | Down-regulated by CSF-1 | Bacterial and viral DNA (HSV 1 and 2; murine CMV)Malaria schizontsHost chromatin | B cell cross-priming Pathogenesis of autoimmunityVaccine adjuvant | [26,39,45,86] |
| TLR10 | Expressed in B cells, pDCs | Unknown | ?TLR2 dimerization? role in asthma | [23,154] | |
| TLR11 | Murine uroepithelium | Unknown | Murine uroepithelial defence, ?humans | [13] |
Differential tissue and cellular expression of TLRs
Differential expression of TLRs is particularly marked at epithelial surfaces, with important consequences for both innate defence and microbial pathogenesis. For example, while TLRs 2, 3, 4 and 5 have specific roles in bronchial and gastrointestinal epithelial defence, murine TLR11 appears to facilitate urogenital immunity [8–13]. Other tissues, such as the blood–brain barrier and endothelium, also have distinct TLR expression patterns with specific biological consequences. While TLR3 appears to play a critical role in the penetration of the blood–brain barrier by West Nile virus, TLR 2 and 4 expression on endothelial cells leads to specific sensitivity to bacterial ligands and directs the movement of rolling leucocytes within the microcirculation and their subsequent migration into inflamed tissues, such as in the lung [14–17]. The distribution of TLRs on the gut epithelium is particularly sophisticated, with different anatomical compartments of the gastrointestinal tract exhibiting specific TLR expression patterns [18]. In addition, the polarized or intracellular anatomical location of TLR4 and TLR5 may allow discrimination of commensal microorganisms from invasive pathogens, although it is now thought that some constitutive contact between gastrointestinal microbiota and TLRs may be required to maintain gut integrity [11,12,19–21].
The differential expression of TLRs on myeloid and lymphoid cells also underpins many key immune responses [22,23]. Neutrophils, natural killer cells, mast cells, eosinophils, B-cells and monocytes all have characteristic TLR expression patterns with explicit immunological consequences [24–31]. Indeed, the discovery of TLRs on T cells, including those with gamma–delta receptors, has suggested the capacity for unexpected functional sophistication [32–34]. Importantly, TLR expression discriminates myeloid from plasmacytoid dendritic cells (DCs) and also defines their respective states of differentiation and activation [22,31,35]. The profound consequences that TLR-mediated signalling provokes in this context, particularly with respect to development of adaptive immunity, have been the subject of several seminal reviews [36,37].
The expression profile of TLRs in immune cells (and particularly DCs) also best illustrates the fundamental cellular compartmentalization of these receptors. While certain TLRs are trafficked to the cell surface for engagement of extracellular pathogens (TLRs 1, 2, 4, 5 and 6), others are almost exclusively found at intracellular locations (TLRs 3, 7, 8, 9). This key design feature within innate immunity allows targeted and powerful TLR-mediated effects to operate against intracellular pathogen-derived products, which closely resemble endogenous host antigens. Several elegant techniques have been designed to demonstrate this division, including the fluorescent tagging of TLRs or down-stream signalling elements (such as the adapter protein MyD88) and the tracking of various TLR chimeras to distinct subcellular compartments [38–40]. The intracellular location of TLRs 3, 7, 8 (non-functional in mice) and 9 means that their ligands (viral dsRNA, ssRNA and prokaryotic DNA) require internalization to the endosome before signalling pathways can become activated [39,41,42]. However, this protective compartmentalization may break down where there is tissue injury, when even endogenous mRNA may become immunoactive [43]. Moreover, as is exemplified by the intracellular location of TLRs 2, 4 and 5 in some circumstances and the cell surface expression of TLR9 in others, this seemingly fundamental division is not absolute [12,38,44,45]. Detailed structural analyses of cytoplasmic anchoring sequences and the identification of chaperoning proteins (such as gp96) have now provided an insight into the mechanisms responsible for determining the subcellular destination of different TLRs [12,46]. Finally, it should be noted that complete viral and bacterial responses may not occur unless TLR signals are received from both epithelial surfaces, where contact first occurs with a pathogen, and the leucocytes in which antigens from the pathogen are subsequently processed [47,48].
Dynamic expression of TLRs
In addition to the diverse tissue and cellular locations of TLRs, it is also now clear that their expression is not static. Not only does the global expression of TLRs gradually alter with age, but a more rapid modulation in levels of TLR mRNA or protein can occur following the exposure of cells to environmental stress, pathogenic microbes or host mediators such as cytokines [49–55]. Notably, functionally distinct TLRs may even influence one another's level of expression. In one model, the activation of neutrophils via TLR4 led to a co-ordinated up-regulation in TLR2 expression on co-incubated endothelial cells via free oxygen radical release [15]. Even following rapid trafficking to the cell surface (see above), TLR location remains fluid within the context of membrane lipid rafts [56]. This allows an ongoing dynamic interaction with other cell surface structures (including other TLRs) to facilitate extension of pattern-recognition repertoires or more complex immune functions [57,58]. The pattern-recognition of lipopolysaccharide (LPS) appears to require the formation of a particularly complex macromolecular structure [59].
Complexity of TLR ligands
Unexpectedly, sophistication in host TLR biology has been found to be matched by complexity in their putative ligands. First, it has been suggested that TLRs not only recognize pathogen-associated molecular patterns (PAMPs) but also host proteins, particularly following tissue injury. Endogenous TLR ligands include extracellular matrix proteins (fibronectin, fibrinogen and hyaluronic acid) and other mediators of host proinflammatory responses such heparin sulphate, beta-defensins or heat-shock proteins [60–65]. Some reports have suggested that endotoxin contamination may be responsible for the activity of some of these ligands, particularly where recombinant products are used [66]. However, even where this confounder has been excluded, endogenous proteins have been found to have a highly sophisticated influence on host immune responses [67]. Conversely, the removal of TLR2 lipopeptide components from LPS by phenol re-extraction has also improved the interpretation of the resulting TLR signature [68]. Although such contamination has been responsible for the repeated misidentification of TLR2 as the pattern-recognition receptor for some species of LPS, this area remains complex and controversial [69–72].
Paradoxically, a complete reliance on highly pure TLR ligands may also not be desirable. This is because whole microbes can trigger quite different TLR response patterns to those induced by their individual component structures [73]. Indeed, different molecular domains of the same structural component from some pathogens can activate distinct TLRs. For example, the C- and N-terminals of the ExoS virulence factor from Pseudomonas induce differential activation of TLRs 2 and 4 [74]. Alternatively, different components of group B streptococci have been shown to induce parallel but distinct activation of different TLR pathways [75]. The net effect of TLR challenge with whole bacteria or fungi is the induction of a complex cumulative gene activation programme within cells such as macrophages and neutrophils [76,77]. This co-ordinates cellular responses as diverse as proinflammatory cytokine release, oxidative microbial killing and apoptosis [78].
Further complexity is introduced by the description of dose-dependent and time-dependent TLR ligand effects. While low doses of LPS provoke the T helper 2 responses seen in some asthma models, higher doses appear to induce DC-driven T helper 1 effects [79]. Not only does infection induce temporally distinct activation of TLRs, but their chronic stimulation can also invoke complex cellular processes [80]. While myeloid cells can become tolerant to repeated stimulation, thereby blunting proinflammatory responses (discussed below), the persistent presence of some ligands can overcome peripheral T cell tolerance [81]. Under other circumstances, repeated TLR ligation may lead to a more generalized disruption of lymphoid tissues and may be utilized by some pathogens to subvert normal innate defences [82,83].
Finally, the precise nature of mammalian TLR–ligand interactions remains less well delineated than in Drosophila[84]. Indeed, it has proved remarkably difficult to confirm direct physical contact. Nevertheless, the extraordinary high fidelity of TLR-mediated pattern-recognition is highly suggestive of sophisticated physical interactions. TLRs have been shown to be capable of differentiating subtle structural variants of individual PAMPs, including different species of LPS or RNA, plasmid DNAs of different methylation status and contrasting lipopeptide stereoisomers [71,72,85–87]. The demonstration of competitive antagonism between TLR ligands provides additional evidence for direct receptor–ligand interaction [88]. Manipulation of ligand structure, analysis with atomic force microscopy and the use of sophisticated transfection models have contributed towards the resolution of this issue [89–92]. In addition, X-ray analysis of the leucine-rich repeat (LRR) sequences in the extracellular portion of TLRs predicts a capacity for sophisticated and direct engagement of microbial PAMPs [93]. The basic framework for TLR pattern-recognition is likely to consist of a horseshoe-shaped solenoid that contains an extensive beta-sheet on its concave surface with numerous ligand-binding insertion points.
High-fidelity TLR-mediated pattern recognition can be exploited by some pathogens. Salmonella adapts itself to the host microenvironment by adjusting the acylation state of its lipid A to down-regulate proinflammatory potential [94]. Pseudomonas species can also modulate the structure of their lipid A, through the addition of ethanolamine, aminoarabinose and palmitate moities, to resist killing and recognition while the presence of hexa-acylated LPS in patients with cystic fibrosis may increase proinflammatory injury [95,96]. Finally, bacteria, fungi and viruses can suppress host responses by induction of interleukin (IL)-10 through TLR-dependent pathways in addition to the selective production of phenotypes resistant to TLR recognition [97–99].
Complexity in TLR signalling pathways
A detailed description of the signalling pathways down-stream from TLRs has been the subject of several reviews and is also outlined in Figs 1 and 2 [100,101]. As with the biology of TLR–ligand interactions, some general themes regarding signal transduction are beginning to emerge. It is now clear that two intracellular adapter proteins called MyD88 and TRIF (TICAM-1) provide a central ‘platform’ for propagating the complex signals derived from TLRs via a direct cytoplasmic association with kinases (e.g. IRAKs, RIPs) and transcription factors [102–106]. Two additional gating adapters called TIRAP (Mal) and TRAM (TICAM-2) appear to be necessary for signal transduction from TLRs 2 and 4 [107–109]. The multiple homophilic interactions that occur between these varying signalling elements are conducted through a system of common modular structural moieties, including TIR and ‘death’ domains [100].
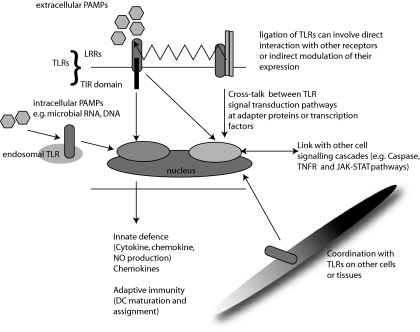
Fig. 1.
Complexity and consequences of Toll-like receptor (TLR)-mediated pattern recognition. Each TLR has an extracellular domain [containing multiple leucine-rich repeats, (LRRs)], and a Toll-interleukin (IL)-1 receptor (TIR) domain. Sophisticated interactions between different TLRs can occur both at the cell surface and at common nodes within signal transduction pathways. Cross-talk between TLRs in distinct cellular locations or on distant cell types can facilitate co-ordinated innate and adaptive immune responses and the modulation of other cellular processes such as apoptosis.
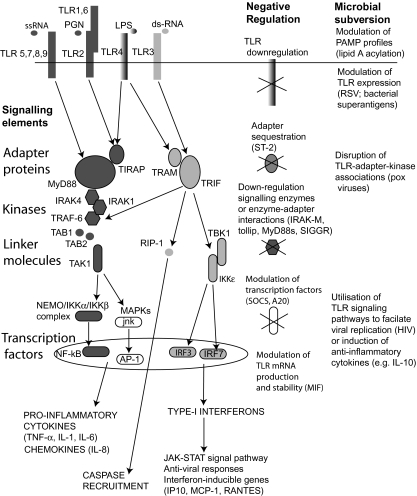
Fig. 2.
Toll-like receptor (TLR) signalling cascade and the locations for negative regulation and microbial subversion of these pathways. Two central adapter proteins MyD88 and TRIF propagate TLR signal transduction by interacting with TLRs via their respective Toll-interleukin (IL)-1 receptor (TIR) components and recruiting down-stream enzymes (e.g. IRAK4, TRAF6) through their ‘death’ domains. Subsequent modulation of transcriptional control elements is less well defined but occurs through linker molecules such as TAB1, TAK1 and TBK1. The powerful proinflammatory properties of Gram-negative lipopolysacharide (LPS) may be explained by TLR4-mediated recruitment of both major adapter proteins. Abbreviations are explained in Table 1 [100–120,126–132].
While some TLRs have the capacity to activate multiple adapters and their associated down-stream signalling pathways, others are more restricted. For example, while TLR4 ligation can result in the recruitment of all of the above adapter molecules, TLR3-mediated induction of NF-κB translocation may be entirely dependent on TRIF–RIP kinase interactions [110]. Further down-stream, while there may be considerable cross-talk between different TLR signal transduction pathways at transcription factors (such as IRF3, IRF7 and NF- κB), there is still the capacity for different TLR ligands to generate highly distinct protein phosphorylation patterns and gene activation programmes [111]. The resulting pathogen-specific cellular responses may explain observed differences in host immunity to Gram-positive and Gram-negative pathogens. In addition, different signalling molecules may be involved in different components of the host response to individual pathogens [112]. The specific kinetic characteristics inherent in different signalling pathways may enable the necessary co-ordination of such responses. For example, IRF-3 activation through TRIF is more rapid following TLR3 than TLR4 ligation, facilitating a rapid beta-interferon response to viral products, while NF-κB activation appears to be under duel phase control from early MyD88-mediated signalling and late MyD88-independent signalling [113].
These complex signalling cascades also offer multiple ‘brake points’ for the negative regulation of innate immune activation (Fig. 2). Some negative regulators such as ST-2 appear to sequester adapter proteins (MyD88 or TIRAP/Mal), limiting their availability for down-stream signalling [114]. Other suppressor proteins, such as Tollip, MyD88s (a splice variant of MyD88), IRAK-M or SIGGR either target phosphorylation/kinase activity of key signalling intermediaries (e.g. IRAK-1, IRAK-4, TRAF-6), or disrupt the modular interaction domains [115–118]. Interestingly, there is the capacity to direct inhibitory molecules to specific transcription factors. For example, the protein SOCS has a powerful suppressive effect on type I interferon production while leaving the proinflammatory activity of NF-κB entirely intact [119]. In contrast, A20, a zinc finger containing cytoplasmic protein, suppresses the NF-κB activation pathway by deubiquitinization at the level of TRAF-6 [120]. It should be noted that the development of tolerance by many cell systems, when exposed to repeated TLR stimulation (often with endotoxin), is thought to be generated through modulation of these signalling controls rather than simple down-regulation of steady-state TLR expression [121–124].
Unsurprisingly, microbial pathogens have developed a multitude of strategies to exploit these signalling systems (Fig. 2). The bacterial species S. typhimurium and Yersinia utilize their type III effector proteins to suppress MAPK and NF-κB activation [125]. However, viruses appear to target the TLR signalling pathway even more directly. While the Vaccinia viral protein A52R resembles a signalling domain within TLRs, providing it with the capacity to subvert host proinflammatory responses through a direct interaction with IRAK2 and TRAF6, persistent intrahepatic hepatitis C infection may be facilitated by cleavage of TRIF [126,127]. Further down-stream Pox viruses also target the I-kappaB kinase complex to disrupt TLR signals to NF-κB and IRF3 [128]. Finally, viruses such as HIV can even directly utilize TLR signalling pathways to increase their intracellular expression [129].
Despite the clear advances in our understanding of TLR signalling, some fundamental questions still remain. Proximally, it appears that while some TLR-mediated effects require internalization of ligand–receptor complexes, others do not [38,130]. Down-stream, the processes linking the signal transduction cascade to transcriptional control elements also remain poorly defined. Although a number of signalling proteins such as RIP2, TAK1, and ECSIT have been identified, their detailed biology has yet to be determined [131–133]. Finally, the relative volume of signalling traffic through different adapter proteins remains unclear. Although all TLRs can operate through MyD88, the use of microarray expression profiling has suggested that following LPS stimulation of murine macrophages only 21·5% of LPS-responsive genes (n = 1055) are dependent on this adapter [100,134]. In addition, other important immune responses, such as the up-regulation of co-stimulatory receptors (CD40, CD80 and CD86), can operate in the absence of MyD88 activity [135].
Complex influence of TLRs in down-stream cellular and immune biology
As well as modulating the innate proinflammatory response through the production of cytokines and chemokines, TLRs are now known to participate in a number of other innate immune processes such as phagocytosis, the production of matrix metalloproteinases, iron sequestration (through induction of lipocalin 2) and defensin production [9,136–138]. However, TLRs are also now known to be crucial in a wide range of other more fundamental cellular processes (Fig. 3). These include actin polymerization, angiogenesis and the induction of apoptosis [139–142]. These specialized roles for TLRs not only underpin their more general role in tissue repair following injury at sites such as the gut and myocardium, but have also revealed new facets to host innate defence [19,21,143,144]. Finally, TLRs may even determine broader pathophysiology, such as the adrenal response to sepsis [145]. Importantly, these wide-ranging TLR-mediated effects are highly co-ordinated. This is demonstrated particularly well by the co-ordinated cell activation, migration and apoptosis of neutrophils and macrophages [15,21,24,47,48,146].
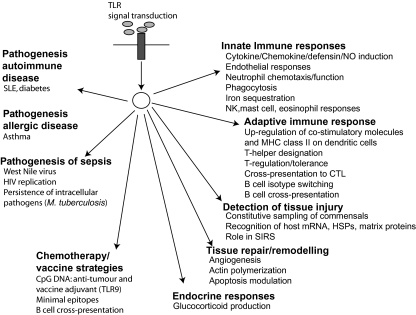
Fig. 3.
Summary of mammalian Toll-like receptor (TLR)-mediated biology. TLRs are now known to be pivotal in both immunological responses and more general cellular homeostasis. Their role in the pathogenesis of diverse disease processes may offer the prospect of new adjuvant immunological therapies in addition to improved vaccine design.
In addition to its role in primary innate responses, differential TLR ligation is now also known to drive the development of subsequent adaptive immunity [36,37,147]. Although TLRs are involved in diverse B cell activity, including isotype switching and T independent cross-priming, it is their role in T cell immunology that dominates [29,30,36]. Through their role in dendritic cell antigen capture, differentiation and migration to lymph nodes (as described above), TLRs designate the T helper response. While TLR4 and 9 are most associated with T helper 1 responses, TLR2 and TLR5 have been found to instigate T helper 2 responses. The adapter protein MyD88 appears to play a particularly complex role in this process. While it has been reported recently that the adaptive response to lipopolysaccharide or Mycobacterium tuberculosis may be entirely intact in MyD88-null mice, others have suggested a prominent role for MyD88 in directing both T helper 1 and T helper 2 responses, depending on which TLR is ligated [134,147–150]. TLRs also direct DC-mediated viral/tumour antigen cross-presentation to cytotoxic T cells (largely TLR3 dependent), and modulate peripheral T cell tolerance and regulation [32,81,151–153]. Most critically, it is the TLR-mediated induction of co-stimulatory molecules (CD80, CD86) on DCs and T cells that determines the crucial capacity for T cell responses to be pathogen-specific [33,36].
Complex roles in disease and therapy
TLRs are complicit in the pathogenesis of a multitude of diseases (Fig. 3). Not only is the absolute level of TLR expression altered in a diverse range of pathological states, but there also appears a direct mechanistic link between TLR activation and the development of conditions such as atherosclerosis [8,154–156]. Indeed, the unexpected link between atherogenesis and sepsis provided by TLRs has been particularly revealing. While a number of microbial products may trigger plaque development, the release of oxidized phospholipids from this process may feedback to down-regulate the inflammation associated with active infection [157,158]. It is unsurprising that while the TLR4 D299G polymorphism increases host susceptibility to sepsis, it decreases the risk of atherosclerotic disease [154,159]. The role of TLRs in sepsis is particularly complex. Although disruption of TLR–ligand interactions can blunt inflammatory injury, the net effect in loss of innate recognition is often harmful to the host [160–162]. Furthermore, while various TLR polymorphisms have been associated with susceptibility to certain diseases or infections, such reports have been inconsistent [163,164].
Therapeutic strategies for both inhibiting and augmenting TLR pathways are being developed [165–167]. It is now possible to achieve TLR activation by specific RNA transfection, thereby avoiding other toxic effects from exogenous ligands [168]. Most impressively, TLR signalling pathways have been utilized to improve vaccine design and efficacy through use of CpG-based adjuvants, minimal epitopes and the bypassing of T cell help [29,30,169]. However, the targeting of TLRs in any therapeutic strategy will not only risk disrupting beneficial host immunity or homeostatic functions, but may also provoke damaging autoimmune phenomena or interfere with the action of concurrent drug therapy [170–172]. In addition, the innate system may circumvent artificial TLR modulation by directing responses through TLR-independent routes [59].
Future investigation of TLR biology
There remain several key difficulties with respect to experimental interpretation in systems designed to investigate TLRs. First, the expression of TLRs either at mRNA or protein levels cannot always be extrapolated to a functional phenotype. For example, NK cells express TLR9 mRNA but may not respond to CpG DNA, while in eosinophils, responses to TLR7 ligands appear to dominate despite the constitutive expression of multiple TLR mRNAs 1, 4, 7, 9 and 10 [23,28]. There are also fundamental differences in TLR biology between different mammals, and even between different cell types from the same organism [13,42,85,173].
Furthermore, TLR expression is so ubiquitous, and their functions so wide-ranging, that isolation of specific receptor-function relationships is highly problematic. This is exemplified by difficulties encountered due to both impure TLR ligands (see above) and heterogeneity within ex-vivo cell populations [24]. The ubiquitous nature of TLR activity may subvert highly specific molecular techniques such as siRNA gene silencing [174]. Finally, many experimental models that have been used to interrogate TLR function rely on gene over-expression or ‘knockout’. It still remains unclear how to prevent the covert biological compensation inherent in such systems from obscuring a result. The application of gene array bioinformatics in combination with advanced mathematical modelling may offer new strategies to analyse the complex biology of TLRs [77,175,176].
Conclusion
Toll-like receptors constitute the best-described group of pattern-recognition receptors described. In mammals, their biology has come full circle: not only do they direct the co-ordinated response of innate and adaptive immunity to infective pathogens, but just as in Drosophilae they are also now known to be involved in more fundamental host processes. The recent stellar advances that have been made within the TLR field have revealed a multitude of new questions. These will have to be answered before these receptors can be exploited successfully and safely with adjuvant therapies and genetic manipulation.
Acknowledgments
P. A. H. is funded by the Intensive Care Society and by a clinical training fellowship awarded by the Medical Research Council, UK.
References
- 1.Hashimoto C, Hudson KL, Anderson KV. The toll gene of Drosophila, required for dorsal–ventral embryonic polarity, appears to encode a trans-membrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 2.Gay NJ, Keith FJ. Drosophila toll and IL-1 receptor. Nature. 1991;351:355–6. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi T, Mitcham JL, Dower SK, et al. Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor Toll, to human chromosome 4p14. Genomics. 1996;32:486–8. doi: 10.1006/geno.1996.0150. [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre B, Nicolas E, Michaut L, et al. The dorsoventral regulatory gene cassette spatzle/toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA., Jr The immune system evolved to discriminate infectious non-self from non-infectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 8.Muir A, Soong G, Sokol S, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–83. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 9.Hertz CJ, Wu Q, Porter EM, et al. Activation of toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171:6820–6. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 10.Guillot L, Le Goffic R, Bloch S, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 12.Hornef MW, Normark BH, Vandewalle A, et al. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–35. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Zhang G, Hayden MS, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Town T, Alexopoulou L, et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 15.Fan J, Frey RS, Malik AB. TLR4 signalling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–43. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte–endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels. J Immunol. 2002;169:2111–19. doi: 10.4049/jimmunol.169.4.2111. [DOI] [PubMed] [Google Scholar]
- 17.Andonegui G, Bonder CS, Green F, et al. Endothelium-derived toll-like receptor 4 is the key molecule in LPS-induced neutrophil sequestration in the lungs. J Clin Invest. 2003;111:1011–20. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega-Cava CF, Ishihara S, Rumi MA, et al. Strategic compartmentalization of toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–85. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 19.Chen LW, Egan L, Li ZW, et al. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischaemia-reperfusion. Nat Med. 2003;9:575–81. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 20.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Pull SL, Doherty JM, Mills JC, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2004;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 23.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 24.Sabroe I, Prince LR, Jones EC. Selective roles for toll-like receptor (TLR) 2 and TLR4 in the regulation of neutrophil activation and lifespan. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 25.Fan J, Malik AB. Toll-like receptor 4 signalling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–21. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 26.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–21. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCurdy JD, Olynych TJ, Maher LH, et al. Cutting edge: distinct toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–9. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 28.Nagase H, Okugawa S, Ota Y, et al. Expression and function of toll-like receptors in eosinophils: activation by toll-like receptor ligands. J Immunol. 2003;171:3977–82. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 29.Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 30.Heit A, Huster KM, Schmitz F, et al. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–7. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 31.Visintin A, Mazzoni A, Spitzer JH, et al. Regulation of toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 32.Caramalho I, Lopes-Carvalho T, Ostler D, et al. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komai-Koma M, Jones L, Ogg GS, et al. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–34. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokuno Y, Matsuguchi T, Takano M, et al. Expression of toll-like receptor 2 on gamma/delta T cells bearing invariant Vgamma6/Vgamma1 induced by E. coli infection in mice. J Immunol. 2000;165:931–40. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 35.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y-J. IPC: professional type I interferon-producing cells and plasmacytoid dendritic cell precursors. Ann Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 38.Latz EA, Visintin E, Lien E, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–43. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 39.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 40.Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signalling properties of toll-like receptors. J Biol Chem. 2004;279:19008–17. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- 41.Alexopolou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 42.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 43.Kariko K, Ni H, Capodici J, et al. mRNA is an endogenous ligand for toll-like receptor 3. J Biol Chem. 2004;279:12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 44.Underhill DM, Ozinsky A, Hajjar AM, et al. The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–15. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 45.Eaton-Bassiri A, Dillon SB, Cunningham M, et al. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun. 2004;72:7202–11. doi: 10.1128/IAI.72.12.7202-7211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funami K, Matsumoto M, Oshiumi H, et al. The cytoplasmic ‘linker region’ in toll-like receptor 3 controls receptor localization and signalling. Int Immunol. 2004;16:1143–54. doi: 10.1093/intimm/dxh115. [DOI] [PubMed] [Google Scholar]
- 47.Schilling JD, Martin SM, Hung CS, et al. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2003;100:4203–8. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato A, Iwasaki A. Induction of antiviral immunity requires toll-like receptor signal in both stromal and dendritic cell compartments. Proc Natl Acad Sci USA. 2004;101:16274–9. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renshaw M, Rockwell J, Engleman C, et al. Cutting edge: impaired toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 50.Ishida I, Kubo H, Suzuki S, et al. Hypoxia diminishes toll-like receptor expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–75. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- 51.Fan J, Kapus A, Marsden PA, et al. Regulation of toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–9. doi: 10.4049/jimmunol.168.10.5252. [DOI] [PubMed] [Google Scholar]
- 52.Monick MM, Yarovinsky TO, Powers LS, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells. J Biol Chem. 2003;278:53035–44. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- 53.Hopkins PA, Fraser JD, Pridmore AC, et al. Superantigen recognition by HLA class II on monocytes up-regulates toll-like receptor 4 and enhances proinflammatory responses to endotoxin. Blood. 2005 doi: 10.1182/blood-2004-07-2523. in press. [DOI] [PubMed] [Google Scholar]
- 54.Nilson N, Nonstad U, Khan N, et al. Lipopolysaccharide and double-stranded RNA up-regulate toll-like receptor 2 independently of myeloid differentiation factor 88. J Biol Chem. 2004;279:39727–35. doi: 10.1074/jbc.M405027200. [DOI] [PubMed] [Google Scholar]
- 55.Roger T, David J, Glauser MP, et al. MIF regulates innate immune responses through modulation of toll-like receptor 4. Nature. 2001;414:920–4. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 56.Triantafilou M, Miyake K, Golenbock DT, et al. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–11. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 57.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gantner BN, Simmons RM, Canavera SJ, et al. Collaborative induction of inflammatory responses by Dectin-1 and toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–45. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 60.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 61.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 62.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson GB, Brunn GJ, Kodaira Y, et al. Receptor-mediated monitoring of tissue well-being via detection of soluble heparin sulphate by toll-like receptor 4. J Immunol. 2002;168:5233–9. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 64.Biragyn A, Ruffini P, Leifer C, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 65.Ohashi K, Burkart V, Flohe S, et al. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor 4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 66.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (hsp70) preparation is responsible for the induction of tumour necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–9. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 67.Johnson BJ, Le TT, Dobbin CA, et al. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J Biol Chem. 2005;280:4037–47. doi: 10.1074/jbc.M411569200. [DOI] [PubMed] [Google Scholar]
- 68.Hirschfeld M, Ma Y, Weis JH, et al. Cutting edge: repurification of lipopolysaccharide eliminates signalling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 69.Yang RB, Mark MR, Gray A, et al. Toll-like receptor 2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–8. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 70.Hashimoto M, Asai Y, Ogawa T. Separation and structural analysis of lipoprotein in a lipopolysaccharide preparation from Porphyromonas gingivalis. Int Immunol. 2004;16:1431–7. doi: 10.1093/intimm/dxh146. [DOI] [PubMed] [Google Scholar]
- 71.Darveau RP, Pham TT, Lemley K, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–51. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Werts C, Tapping RI, Mathison JC, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–52. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 73.Mandell L, Moran AP, Cocchiarela A, et al. Intact Gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446–56. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epelman S, Stack D, Bell C, et al. Different domains of Pseudomonas aeruginosa exoenzyme S activate distinct TLRs. J Immunol. 2004;173:2031–40. doi: 10.4049/jimmunol.173.3.2031. [DOI] [PubMed] [Google Scholar]
- 75.Henneke P, Takeuchi O, van Strijp JA, et al. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–76. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- 76.Nau GJ, Schlesinger A, Richmond JF, et al. Cumulative toll-like receptor activation in human macrophages treated with whole bacteria. J Immunol. 2003;170:5203–9. doi: 10.4049/jimmunol.170.10.5203. [DOI] [PubMed] [Google Scholar]
- 77.McCaffrey RL, Fawcett P, O'Riordan M, et al. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci USA. 2004;101:11386–91. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellocchio S, Moretti S, Perruccio K, et al. TLRs govern neutrophil activity in aspergillosis. J Immunol. 2004;173:7406–15. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- 79.Eisenbarth SC, Piggot DA, Huleatt JW, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiss DS, Raupach B, Takeda K, et al. Toll-like receptors are temporally involved in host defence. J Immunol. 2004;172:4463–9. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y, Huang C-T, Huang X, et al. Persistent toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 82.Heikenwalde M, Polymenidou M, Junt T, et al. Lymphoid follicle destruction and immunosuppession after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10:187–92. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 83.Pai RK, Pennini ME, Tobian AA, et al. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect Immun. 2004;72:6603–14. doi: 10.1128/IAI.72.11.6603-6614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weber ANR, Tauszig-Delamasure S, Hoffman JA. Binding of the Drosophila cytokine Spatzle to toll is direct and establishes signalling. Nat Immunol. 2003;4:794–9. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 85.Koski GK, Kariko K, Xu S, et al. Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J Immunol. 2004;172:3989–93. doi: 10.4049/jimmunol.172.7.3989. [DOI] [PubMed] [Google Scholar]
- 86.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeuchi O, Kaufmann A, Grote K, et al. Cutting edge: preferentially the R-stereoisomer of the mycoplasma lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signalling pathway. J Immunol. 2000;164:554–7. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 88.Coats SR, Reife RA, Bainbridge BW, et al. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect Immun. 2003;71:6799–807. doi: 10.1128/IAI.71.12.6799-6807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poltorak A, Ricciardi-Castagnoli P, Citterio S, et al. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–7. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cornelie S, Hoebeke J, Schacht AM, et al. Direct evidence that toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine-phosphate-guanine motif recognition. J Biol Chem. 2004;279:15124–9. doi: 10.1074/jbc.M313406200. [DOI] [PubMed] [Google Scholar]
- 91.Iwaki D, Mitsuazawa H, Murakami S. The extracellular toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J Biol Chem. 2002;277:24315–20. doi: 10.1074/jbc.M107057200. [DOI] [PubMed] [Google Scholar]
- 92.Kerkmann M, Costa LT, Richter C, et al. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high IFN-a induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem. 2005;280:8086–93. doi: 10.1074/jbc.M410868200. [DOI] [PubMed] [Google Scholar]
- 93.Bell JK, Mullen GED, Leifer CA, et al. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–33. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 94.Kawasaki K, Ernst RK, Miller SI. 3-O-deacylation of lipid A by PagL, a Pho/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signalling through toll-like receptor 4. J Biol Chem. 2004;279:20044–8. doi: 10.1074/jbc.M401275200. [DOI] [PubMed] [Google Scholar]
- 95.Hajjar Am Ernst RK, Tsai JH, et al. Human toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–9. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 96.Ernst RK, Yi EC, Guo L, et al. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–5. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 97.Sing A, Rost D, Tvardovskaia N, et al. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for IL-10-mediated immunosuppression. J Exp Med. 2002;196:1017–24. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Netea MG, Warris A, Van der Meer JW, et al. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor 4 mediated signal transduction. J Infect Dis. 2003;188:320–6. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 99.Jude BA, Pobezinskaya Y, Bishop J, et al. Subversion of the innate immune system by a retrovirus. Nat Immunol. 2003;4:573–8. doi: 10.1038/ni926. [DOI] [PubMed] [Google Scholar]
- 100.Barton GM, Medzhotov R. Toll-like receptor signalling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 101.Akira S, Takeda K. Toll-like receptor signalling. Nature Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 102.Muzio M, Ni J, Feng P, et al. IRAK (Pelle) family members IRAK-2 and MyD88 as proximal mediators of IL-1 signalling. Science. 1997;278:1612–15. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto M, Sato S, Hemmi H, et al. Role of adapter TRIF in the MyD88-independent toll-like receptor signalling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 104.Hoebe KX, Georgel P, et al. Identification of lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 105.Kawai T, Sato S, Ishii KJ, et al. Interferon-alpha induction through toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–8. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 106.Honda K, Yanai H, Mizutani T, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in toll-like receptor signalling. Proc Natl Acad Sci USA. 2004;101:15416–21. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor 4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 108.Horng T, Barton GM, Flavell RA, et al. The adapter molecule TIRAP provides signalling specificity for toll-like receptors. Nature. 2002;420:329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the toll-like receptor 4-mediated MyD88-independent pathway. Nat Immunol. 2003;4:1144–50. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 110.Meylan E, Burns K, Hofmann K, et al. RIP1 is an essential mediator of toll-like receptor 3 induced NF-κB activation. Nat Immunol. 2004;5:503–7. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 111.Toshchakov V, Jones BW, Perera PY, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1 alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–8. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 112.Serbina NV, Kuziel W, Flavell R, et al. Sequential MyD88-independent and dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 113.Kawai T, Takeuchi O, Fujita T, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–94. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 114.Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–9. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 115.Zhang G, Gosh S. Negative regulation of toll-like receptor-mediated signalling by Tollip. J Biol Chem. 2002;277:7059–65. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- 116.Burns K, Janssens S, Brissoni B, et al. Inhibition of interleukin 1 receptor/Toll-like receptor signalling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–8. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kobayashi K, Hernandez LD, Galan JE, et al. IRAK-M is a negative regulator of toll-like receptor signalling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 118.Wald D, Qin J, Zhao Z, et al. SIGGR, a negative regulator of toll-like receptor-interleukin 1 receptor signalling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 119.Gingras S, Parganas E, de Pauw A, et al. Re-examination of the role of suppressor of cytokine signalling 1 (SOCS1) in the regulation of toll-like receptor signalling. J Biol Chem. 2004;279:54702–7. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 120.Boone DL, Turer EE, Lee EG, et al. The ubiquitin-modifying enzyme A20 is required for termination of toll-like receptor responses. Nat Immunol. 2004;5:1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 121.Adib-Conquy M, Cavaillon JM. Gamma interferon and granulocyte/monocytes colony-stimulating factor prevent endotoxin tolerance in human monocytes by promoting interleukin-1 receptor-associated kinase expression and its association to MyD88 and not by modulating TLR4 expression. J Biol Chem. 2002;277:27927–34. doi: 10.1074/jbc.M200705200. [DOI] [PubMed] [Google Scholar]
- 122.Medvedev AE, Lentschat A, Wahl, et al. Dysregulation of LPS-induced toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–16. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- 123.Mizel SB, Snipes JA. Gram-negative flagellin-induced self tolerance is associated with a block in interleukin-1 receptor-associated kinase release from toll-like receptor 5. J Biol Chem. 2002;277:22414–20. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- 124.Hatao F, Muroi M, Hiki N, et al. Prolonged toll-like receptor stimulation leads to down-regulation of IRAK-4 protein. J Leuk Biol. 2004;76:904–8. doi: 10.1189/jlb.0504277. [DOI] [PubMed] [Google Scholar]
- 125.Espinosa A, Alfano JR. Disabling surveillance: bacterial type III secretion system effectors that suppress innate immunity. Cell Microbiol. 2004;6:1027–40. doi: 10.1111/j.1462-5822.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 126.Harte MT, Haga IR, Maloney G, et al. The poxvirus protein A52R targets toll-like receptor signalling complexes to suppress host defense. J Exp Med. 2003;197:343–51. doi: 10.1084/jem.20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the toll-like receptor 3 adapter protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992–7. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DiPerna G, Stack J, Bowie AG, et al. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibiting signalling to NF-kB by the tumour necrosis factor superfamily of receptors, and inhibits NF-kB and IRF signalling by toll-like receptors. J Biol Chem. 2004;279:36570–8. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- 129.Equils O, Schito ML, Karahashi H, et al. Toll-like receptor 2 (TLR2) and TLR9 signalling results in HIV long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J Immunol. 2003;170:5159–64. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 130.Detmers PA, Thieblemont N, Vasselon T, et al. Potential role of membrane internalisation and vesicle fusion in adhesion of neutrophils in response to lipopolysaccharide and TNF. J Immunol. 1996;157:5589–96. [PubMed] [Google Scholar]
- 131.Kopp E, Medzhitov R, Carothers J, et al. ECSIT is an evolutionarily conserved intermediate in the toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–71. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deng L, Wang C, Spencer E, et al. Activation of the IkB kinase complex by TRAF-6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 133.Chin AI, Dempsey PW, Bruhn K, et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–4. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 134.Bjorkbacka H, Fitzgerald KA, Huet F, et al. The induction of macrophage gene expression by LPS predominantly utilizes MyD88-independent signalling cascades. Physiol Genomics. 2004;19:319–30. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- 135.Kaisho T, Takeuchi O, Kawai T, et al. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J Immunol. 2001;166:5688–94. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 136.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–7. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 137.Gebbia JA, Coleman JL, Benach JL. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via toll-like receptor 2. J Infect Dis. 2004;189:113–19. doi: 10.1086/380414. [DOI] [PubMed] [Google Scholar]
- 138.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 139.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodelling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 140.Pinhal-Enfield G, Ramanathan M, Hasko G, et al. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7 and 9 and adenosine A (2A) receptors. Am J Pathol. 2003;163:711–21. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor 2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 142.Hsu L-C, Park JM, Zhang K, et al. The protein kinase PKR is required for macrophage apoptosis after activation of toll-like receptor 4. Nature. 2004;428:341–5. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 143.Shishido T, Nozaki N, Yamaguchi S, et al. Toll-like receptor-2 modulates ventricular remodelling after myocardial infarction. Circulation. 2003;108:2905–10. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 144.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–73. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 145.Bornstein SR, Zacharowski P, Schumann RR, et al. Impaired adrenal stress response in toll-like receptor 2 deficient mice. Proc Natl Acad Sci USA. 2004;101:16695–700. doi: 10.1073/pnas.0407550101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lucas M, Stuart LM, Savill J, et al. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–5. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 147.Fremond CM, Yeremeev V, Nicolle DM, et al. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–9. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schnare M, Barton GM, Hol AC, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 149.Redecke V, Hacker H, Datta SK, et al. Cutting edge: activation of toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–43. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 150.Didierlaurent A, Ferrero I, Otten LA, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of the Th-2-type response. J Immunol. 2004;172:6922–30. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 151.Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus induced type I interferon. Nat Immunol. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 152.Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 153.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 154.Cook DN, Pisetsky DS. Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–95. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 155.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signalling pathways. Nat Med. 2004;10:416–21. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 156.Michelsen KS, Wong MH, Shah PK, et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters placque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leinonen M, Saikku P. Evidence for infectious agents in cardiovascular disease and atherosclerosis. Lancet Infect Dis. 2002;2:11–17. doi: 10.1016/s1473-3099(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 158.Bochkov VN, Kadl A, Huber J, et al. Protective role of phospholipids oxidation products in endotoxin induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 159.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 160.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 161.Hawn TR, Verbon A, Lettinga KD, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signalling and is associated with susceptibility to legionnaires’ disease. J Exp Med. 2003;198:1563–72. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Medvedev AE, Lentschat A, Kuhns DB, et al. Distinct mutations in IRAK4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med. 2003;198:521–31. doi: 10.1084/jem.20030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Read RC, Pullin J, Gregory S, et al. A functional polymorphism of toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J Infect Dis. 2001;184:640–2. doi: 10.1086/322798. [DOI] [PubMed] [Google Scholar]
- 164.Raby BA, Klimecki WT, Laprise C, et al. Polymorphisms in toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Resp Crit Care Med. 2002;166:1449–56. doi: 10.1164/rccm.200207-634OC. [DOI] [PubMed] [Google Scholar]
- 165.Christ WJ, Asano O, Robidoux AL, et al. E5531, a pure endotoxin antagonist of high potency. Science. 1995;268:80–3. doi: 10.1126/science.7701344. [DOI] [PubMed] [Google Scholar]
- 166.Meng G, Rutz M, Schiemann M, et al. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock–like syndromes. J Clin Invest. 2004;113:1473–81. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bartfai T, Behrens MM, Gaidarova S, et al. A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc Natl Acad Sci USA. 2003;100:7971–6. doi: 10.1073/pnas.0932746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cisco RM, Abdel-Wahab Z, Dannull J, et al. Induction of human dendritic cell maturation using transfection with RNA encoding a dominant positive toll-like receptor 4. J Immunol. 2004;172:7162–8. doi: 10.4049/jimmunol.172.11.7162. [DOI] [PubMed] [Google Scholar]
- 169.Jackson DC, Lau YF, Le T, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci USA. 2004;101:15440–5. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leadbetter EA, Rifkin IR, Hohlbaum AM, et al. Chromatin-IgG complexes activate B cells by duel engagement of IgM and toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 171.Lang KS, Recher M, Junt T, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–45. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 172.Boekholdt SM, Agema WR, Peters RJ, et al. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation. 2003;107:2416–21. doi: 10.1161/01.CIR.0000068311.40161.28. [DOI] [PubMed] [Google Scholar]
- 173.Andreakos E, Sacre SM, Smith C, et al. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and non-myeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2003;4:1356–61. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- 174.Kariko K, Bhuyan P, Capodici J, et al. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signalling through toll-like receptor 3. J Immunol. 2004;172:6545–9. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 175.Goldstein B, Faeder JR, Hlavacek WS, et al. Mathematical and computational models of immune-receptor signalling. Nat Immunol. 2004;4:445–56. doi: 10.1038/nri1374. [DOI] [PubMed] [Google Scholar]
- 176.De Bivort B, Huang S, Bar-Yam Y. Dynamics of cellular level function and regulation derived from murine expression array data. Proc Natl Acad Sci USA. 2004;101:17687–92. doi: 10.1073/pnas.0406707102. [DOI] [PMC free article] [PubMed] [Google Scholar]