Abstract
IL-6/STAT-3 signals play key roles in inflammatory bowel disease (IBD). It is known that Lactobacillus casei strain Shirota (LcS) improves inflammatory disorders. This study aimed to elucidate the effect of LcS on murine chronic IBD and to clarify the mechanism. We focused the inhibitory effect of LcS on the production of IL-6 in lipopolysaccharide (LPS)-stimulated large intestinal lamina propria mononuclear cells (LI-LPMC) isolated from mice with chronic colitis and in RAW264·7 cells in vitro. We also determined in vivo the effect of LcS on murine chronic IBD models induced with dextran sodium sulphate and SAMP1/Yit mice. Finally, we examined the cellular determinants of LcS for the down-regulation of IL-6 secretion by LI-LPMC, RAW264·7 cells and peripheral blood mononuclear cells (PBMC) derived from patients with ulcerative colitis (UC). LcS, but not other strains of Lactobacillus, inhibited the production of IL-6 in LPS-stimulated LI-LPMC and RAW264·7 cells, down-regulating the nuclear translocation of NF-κB. The LcS-diet-improved murine chronic colitis is associated with the reduction of IL-6 synthesis by LI-LPMC. LcS also improved chronic ileitis in SAMP1/Yit mice. The release of IL-6 in vitro in LPS-stimulated LI-LPMC, RAW 264·7 cells and UC-PBMC was inhibited by a polysaccharide-peptidoglycan complex (PSPG) derived from LcS. This probiotic-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines such as IL-6 and IFN-γ production in LPMC. Therefore, LcS may be a useful probiotic for the treatment of human inflammatory bowel disease.
Keywords: experimental colitis, inflammatory bowel disease, Lactobacillus, IL-6, SAMP1/Yit, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) is a severe form of intestinal inflammation, the pathogenesis of which is not well understood. It is thought that the disease might be due to complex mucosal immune responses to antigens of resident enteric bacteria [1,2]. Since various IBD models in rodents reared under germ-free conditions are free of intestinal inflammation [3–5]. Interleukin (IL)-6 has been shown to be one of the major cytokines secreted by lamina propria cells, and Crohn's disease (CD) patients produced substantially more IL-6 than did control patients [6–8]. A recent study demonstrated that IL-6 plays a critical role in the development of Th1 cell-mediated chronic colitis [9,10]. We confirmed that IL-6-gene-disrupted mice were resistant to DSS-induced colitis [11]. IL-6 is one of the major cytokines which use signal transducer and activator of transcription (STAT)-3 for its intracellular signalling pathway [12,13]. Recently, we investigated that the expression of the phosphorylated STAT-3 was enhanced in the intestinal mucosa of patients with IBD, and murine IBD models [11]. We observed phospho-STAT-3 in the nucleus of the intestinal laimina propria mononuclear cells (LI-LPMC). So, the secretion of IL-6 from LI-LPMC may be one of the mechanisms of spontaneous phosphorylation of STAT-3 in LI-LPMC during the pathogenesis of IBD. It is important to identify the kinds of stimuli that induce production of IL-6 in the pathogenesis of IBD. Lipopolysaccharide (LPS) is a well-known IL-6 inducer in host immunocytes [14,15]. LPS is a cell wall constituent of gram-negative enteric bacteria such as bacteroides [16]. Rath et al. [17] suggested that overgrowth of bacteroides induced severe intestinal inflammation in HLA-B27 transgenic rats. In contrast, Lange et al. [18] described that colitis was less severe in mice disrupted in the gene for toll-like receptor 4 (TLR4) than control mice. Thus, LPS signals are essential for the development of inflammatory bowel disease. Intestinal barrier function is disrupted in IBD models in rodents and in patients with IBD, and several antigens derived from intestinal microflora may be in contact with LI-LPMC [19]. One possibility is that bacterial products such as LPS interact directly with LI-LPMC, which in turn produce inflammatory cytokines such as IL-6. The results lead us to the hypothesis that LPS-signals trigger the production of IL-6 in LI-LPMC and initiate colito-genesis. Furthermore, removal of these signals from LI-LPMC may contribute to the improvement of intestinal inflammation.
Lactobacillus casei strain Shirota (LcS) was originally isolated from humans. It was reported that LcS is effective against murine models of inflammatory disorders such as collagen-induced arthritis, type I diabetes and systemic lupus [20–22]. However, the mechanism of its effect is still unknown. In the present study we focused on the activity of LcS in murine chronic inflammatory bowel disease in terms of the effect of LcS or LcS-derived cell components on the IL-6 signalling pathway.
Materials and methods
Animals
Female Balb/c mice (8-weeks-old) were purchased from Japan Clea laboratory (Tokyo Japan). Mice were maintained under specific-pathogen-free (SPF) conditions during the experiments. SAMP1/Yit mice were generated in our institute and maintained under SPF conditions during the experiment [23].
Preparation of cell derivatives of Lactobacillus casei strain Shirota (LcS)
Heat-killed LcS was prepared as previously described [24]. Heat-killed bacterial preparations from other strains of lactobacillus were also prepared (Lactobacillus rhamnosus ATCC 53103: Lr, Lactobacillus plantrarum ATCC 14917: Lp, Lactobacillus Johnsonii Y 50092: Lj) for the comparative analysis.
Intact cell wall (ICW) was prepared by the method of Sekine et [25]. The methods used for isolation of the polysaccharide-peptidoglycan (PSPG) were described previously [26]. The protoplast (PP) fraction was prepared using the procedure employed for the preparation of PSPG. In brief, the heat-killed cells were exhaustively digested with N-acetylmuramidase SG (Seikagaku corporation). The digest was then centrifuged at 10 000 × g for 45min. The precipitate was collected and washed in distilled water three times. The residue was lyophilized and used as the protoplast fraction.
Induction of chronic DSS colitis
Chronic DSS colitis was induced in Balb/c mice as previously described [27]. In brief, mice at 10 week of age were treated with 4% DSS (molecular mass, 40 kD; ICN Biomedicals, Aurora, OH, USA) dissolved in drinking water. Chronic colitis was induced by four administration cycles; each cycle consisted of a regimen of 4% DSS for 7 days followed by drinking water without DSS for the next 10 days.
In vitro IL-6 inhibition assay
LI-LPMC were prepared from DSS colitis-induced Balb/c mice as previously described [28]. LI-LPMC, murine macrophage RAW264·7 cells and PBMC (2·0 × 105/96-well) isolated from a patient with UC were cultured in 10% FCS/10 mM Hepes/penicillin-streptmycin/RPMI medium (Complete RPMI) under 5% CO2 at 37 °C. LPS (O55:B5) was purchased from Sigma. To assess the effect of heat-killed LcS or LcS-derived cellular components on the synthesis of IL-6 in LPS-stimulated LI-LPMC, RAW264·7 cells and UC-PBMC, 1 µg/ml of LPS was added to cells with or without various doses of LcS or LcS-derived cellular components and the cells were cultured for 48 h. After the culture, the supernatants were collected and stored at −84°C until the IL-6 ELISA. Data represent the percent inhibition of IL-6 synthesis in the cultures stimulated with LPS plus various doses of LcS compared with those stimulated with LPS only. The inhibitory effects among several strains of lactobacillus on IL-6 production in LPS-stimulated LI-LPMC were also determined using the same method. In some experiments, purified murine mAb against TLR4/MD2 complex (kindly provided by Dr Miyake K. Department Immunol. Saga Medical School), IL-10 (BD PharMingen) or TGF-β (SIGMA) was added to the assay system.
Nuclear protein extraction and the determination of NF-κB amounts
Nuclear proteins were extracted from LPS-stimulated RAW264·7 cells that were treated with or without LcS (2·5 µg/ml) for 1 and 3 h. The amounts of nuclear-localized NF-κB were determined using a NF-κB ELISA kit (BD Biosciences Clontech).
ELISA
Murine anti-IL-6 mAbs (clone: MP5–20F3, MP5–32C11) and human anti-IL6 mAbs (clone: 5IL6, 7IL6) were purchased from BD PharMingen (LA, CA, USA) and PIERCE-Endogen (Woburn, MA, USA). ELISA was performed by the standard protocol as recommended by the manufacturer.
In vivo effect of LcS on murine chronic IBD
Heat-killed LcS-containing MF chow (0·05% w/w) was prepared as described previously [19–21]. Chronic DSS colitis was induced in 10-week-old female Balb/c mice fed LcS MF or control MF diet (n = 10 in each group) by the method described above. Fifteen-week-old female SAMP1/Yit mice were also fed for LcS MF or control MF (n = 10 in each group) diet until 25 weeks old.
Assessment of disease severity
A disease activity index that has been confirmed to reflect changes in the clinical status of mice with DSS colitis consisting of the sum of the following parameters indicating abnormalities rated from 0 to 4: change in body weight, stool consistency, and intestinal bleeding, was used [29]. In the case of SAMP1/Yit mice, disease activity was determined from both the histological scores and the amount of serum amyloid A (SAA) protein in SAMP1/Yit mice fed the LcS or control diet [29,30].
Flow cytometry
LI-LPMC were stained with mAbs against TCRβ (H57-597), CD4 (RM4-5), and CD69 (H1.2F3), all of purchased from BD PharMingen.
Cell culture and cytokine assay
LI-LPMC (2·0 × 106 cells) were cultured in 24-well tissue culture plates in complete RPMI under stimulation with LPS (O55:B5) or immobilized anti-TCR β mAb (H57-597, 10 µg/ml); anti-CD28 mAb (clone: 37·51, 1 µg/ml) was added subsequently. After 72 h of culture, supernatants were collected and stored at −84°C until the assays. Cytokine-specific ELISA was performed using the antibody combinations of anti-interferon (IFN)-γ (clone: XMG1.2, R4–6A2), anti-IL-6 and anti-IL-4 (clone: 11B11, BVD6–24G2), all from BD PharMingen.
Role of TLR2 or TLR4 in the inhibitory effect on IL6 production by LcS
To prove the role of TLR4 in the effect of LcS, macrophage cell line, I-13·35, isolated from TLR4 null mice was purchased from ATCC (CRL-2471) and examined for inhibitory effects of LcS as described above. Neutralizing mAb raised against human TLR2 was used to investigate the role of TLR2 (TL2·1, Santa Cruz Biotechnology, Inc) in the effect of LcS [31]. U937 were treated with PMA (20 nM, Sigma) to differentiate the macrophage-like adherent cell type [32]. These cells were incubated with neutralizing mAb TLR2 (10–1 µg/ml) or control murine IgG2a, and LcS (5 µg/ml). Finally, the cells were stimulated with 1 µg/ml of LPS. Effective dose of mAb TLR2 to inhibit TLR2 signal was determined by the inhibition of peptigoglycan (Sigma) -induced IL-6 production on PMA-activated U937 before this experiment. Then we observed peptidoglycan-induced IL-6 production was inhibited dose-dependently (about 50–20%) by the addition of the same doses of mAb TLR2 (10–2·5 µg/ml).
Statistics
All data were expressed as the mean ± SE and evaluated by the Tukey or Tuley-Kramer test for multiple comparisons. P-values of less than 0·05 were assumed to be statistically significant.
Results
Effect of LcS on IL-6 production in LPS-stimulated LI-LPMC and RAW264·7 cells
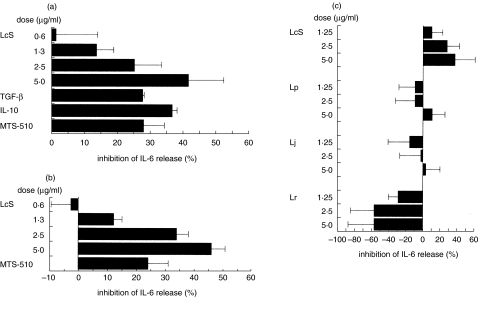
To clarify whether LcS regulates the production of IL-6 in LPS-stimulated LI-LPMC and RAW264·7 cells, various doses of heat-killed LcS were tested. LcS down-regulated the production of IL-6 in LPS-stimulated LI-LPMC isolated from the mice with chronic colitis and the effect was dose-dependent (Fig. 1a). The same effect of LcS could be observed in LPS-stimulated RAW264·7 cells (Fig. 1b). Addition of mAb against TLR4/MD2 inhibited the production of IL-6 in LPS-stimulated cells (Fig. 1a,b). Addition of IL-10 or TGF-β suppressed IL-6 production in LPS-stimulated LI-LPMC (Fig. 1a). Next, we compared the down-regulating activity of LcS among other strains of Lactobacillus. The down-regulation of IL-6 production in LPS-stimulated LI-LPMC was pronounced in LcS but not in other strains of Lactobacillus (Fig. 1c).
Fig. 1.
Inhibitory effects of heat-killed LcS on IL-6 synthesis in LPS-stimulated murine colonic lamina propria lymphocytes (a) LI-LPMC and (b) RAW264·7 cells. (c) Comparative study of the inhibitory effect among 4 strains of Lactobacillus on the production of IL-6 in LPS-stimulated LI-LPMC. (a, b) These cells were cultured in the presence or absence of both LPS (1 µg/ml) and various doses of LcS and the amounts of IL-6 in the culture supernatants were determined. (c) The inhibitory effects on IL-6 production among four strains of Lactobacillus (L. casei strain Shirota: LcS, L. rahmnosus: Lr, L. johnsonii: Lj, L. plantrum: Lp) were also determined in LPS-stimulated murine LI-LPMC isolated from colitis-afflicted mice. Data represent the percent inhibition of IL-6 synthesis in the cultures stimulated with LPS plus various doses of LcS compared with those stimulated with LPS only. Similar results were obtained from five independent experiments. Data are mean ± SD.
Effect of LcS in nuclear translocation of NF-κB in LPS-stimulated RAW264·7 cells
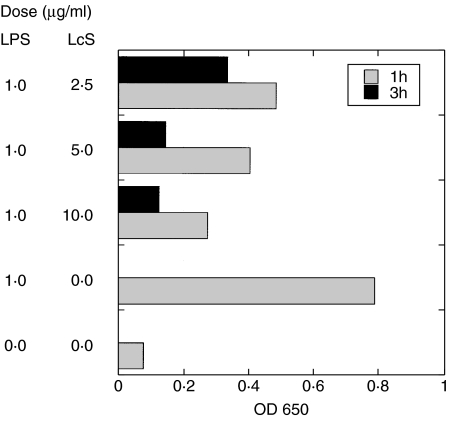
We examined the effect of LcS on NF-κB activity in LPS-stimulated RAW264·7 cells. RAW264·7 cells were stimulated with LPS in the presence or absence of LcS. One or three hours after culture, the amounts of NF-κB in nuclear extracts of the cells were determined. LcS inhibited the nuclear translocation of NF-κB in time and dose-dependent manners in LPS-stimulated RAW264·7 cells (Fig. 2).
Fig. 2.
The effects of heat-killed LcS on NF-κB activity in LPS-stimulated RAW264·7 cells. RAW264·7 cells were stimulated with 1 µg/ml of LPS in the presence or absence of heat-killed LcS for 1 h ( ) or 3 h (▪) and the amounts of nuclear-localized p65 NF-κB in the cells were determined. The amount of nuclear-localized NF-κB in LPS-stimulated RAW264·7 cells was reduced in the presence of LcS. Data were means of duplicate assays.
) or 3 h (▪) and the amounts of nuclear-localized p65 NF-κB in the cells were determined. The amount of nuclear-localized NF-κB in LPS-stimulated RAW264·7 cells was reduced in the presence of LcS. Data were means of duplicate assays.
In vivo effect of LcS on murine chronic colitis
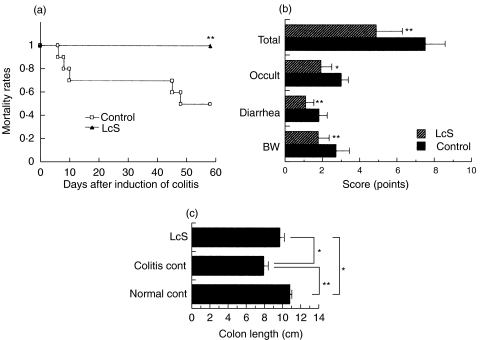
Next we examined the effect of LcS on murine chronic colitis in vivo. The mortality rate was markedly different between the LcS-treated group and control group (Fig. 3a). The scores of all the parameters in the disease activity index such as loss of body weight, diarrhoea and occult blood were reduced in Balb/c mice fed LcS compared with those fed the standard diet (Fig. 3b). Moreover, the colon was longer in colitis-afflicted mice fed LcS than those fed standard diet (Fig. 3c).
Fig. 3.
Effect of heat-killed LcS on (a) the mortality rate, (b) the disease activity index and (c) colon length in mice with chronic colitis. Balb/c mice were fed LcS-containing MF chow or control MF chow (n = 10 in each group) and chronic colitis was induced. Disease activity index and colon length were determined as described in the Materials and Methods. Similar results were obtained from two-independent experiments. Data are mean ± SD. Values (*P < 0·05, **P < 0·01) differ significantly among each experimental group.
Flow-cytometric analysis of LI-LPMC
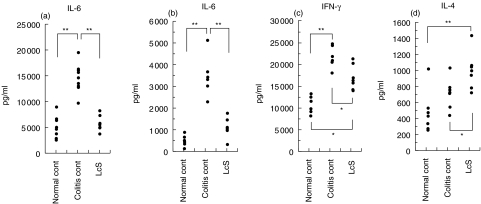
We compared the phenotypes of LI-LPMC among three groups of colitis-afflicted Balb/c mice fed the standard diet or LcS diet and colitis-free Balb/c mice. Total numbers of B220-positive B cells increased markedly in the mice fed the standard diet compared to those fed LcS (Table 1). The expression of CD69 in CD4+ TCRαβ LI-LPMC was higher in the mice fed the standard diet than those fed LcS (Table 1). Cytokine profiles in LPS-stimulated LI-LPMC indicated that the production of IL-6 was down-regulated in colitis-afflicted mice fed LcS compared to those fed the standard diet. IL-6 production in LPS-stimulated LI-LPMC decreased in colitis-afflicted mice fed LcS compared to those fed the standard diet (Fig. 4a). The profiles in TCR β/CD28-stimulated LI-LPMC indicated that the production of IL-6 and IFN-γ decreased and that of IL-4 increased in the mice fed LcS (Fig. 4b,c,d).
Table 1.
Flow-cytometric analysis of the phenotypes of LI-LPMC in colitis-free or colitis-afflicted Balb/c mice fed the control diet or LcS-containing diets
| Phenotype | ||||||
|---|---|---|---|---|---|---|
| CD4+TCRαβ | Estimated cell numbers (×106) | |||||
| CD4+TCRαβ | ||||||
| Exp. group | No of LI-LPMC (× 106) | TCRβ(%) | CD69 (%) | B220 (%) | TCR αβ T cells | B220 B cells |
| Normal cont (n = 6) | 6·95 ± 4·02 | 25·07 ± 10·47 | 5·43 ± 2·33 | 55·47 ± 16·51 | 0·63 ± 0·33 | 1·68 ± 1·25 |
| Colitis cont (n = 5) | 17.68 ± 4·37* | 16·50 ± 1·75 | 56·44 ± 8·79* | 68·14 ± 4·46 | 1·61 ± 0·34* | 5·81 ± 2·32* |
| LcS (n = 6) | 10·88 ± 2·56† | 20·48 ± 3·07 | 32·67 ± 8·76*† | 59·33 ± 6·67 | 0·95 ± 0·63 | 2·94 ± 0·79 |
†Values are means ±SE.
P < 0·05 compared with normal control.
P < 0·05 compared with colitis control.
Fig. 4.
Cytokine profiles in the culture supernatants of LPS (a) or TCRβ/CD28 (b,c,d) -stimulated LI-LPMC in colitis-afflicted Balb/c mice fed the control diet (colitis control) or heat-killed LcS-containing diets (LcS), or colitis-free Balb/c mice (normal control). Similar results were obtained from two-independent experiments. Values (*P < 0·05, **P < 0·01) differ significantly among each group.
In vivo effect of LcS on chronic ileitis
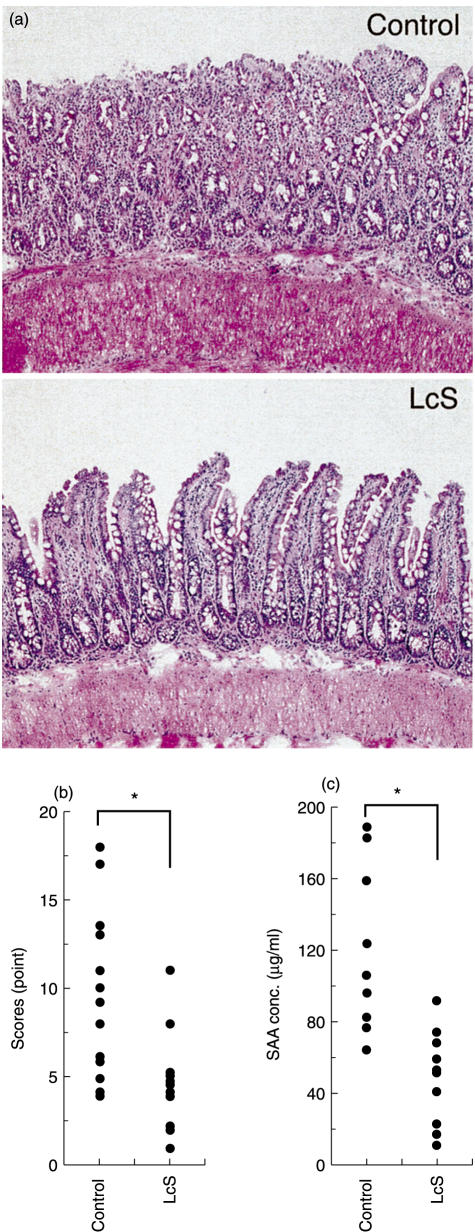
Next we examined the effect of LcS on chronic ileitis in SAMP1/Yit mice as a murine model of Crohn's disease. In the group of SAMP1/Yit mice fed the LcS diet, the histological scores improved compared with those for the mice fed the control diet (Fig. 5a,b). Moreover, the amount of SAA decreased in the group of SAMP1/Yit mice fed LcS (Fig. 5c).
Fig. 5.
Improved effect of LcS on a murine model of Crohn's disease. SAMP1/Yit mice were fed LcS MF chow or the control diet. Disease activities were determined from both histological scores (a, b) and the amount of serum Amyroid A protein (c). Values (*P < 0·05) differ significantly among each group.
Determination of cellular determinants of LcS that reduce the production of IL-6
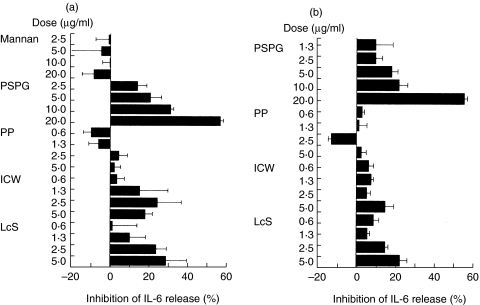
To clarify the cellular components in LcS that down-regulate the production of IL-6 in LPS-stimulated LI-LPMC and RAW264·7 cells, LcS-derived ICW or PP was isolated and examined for an effect on IL-6 production. ICW, but not PP, dose-dependently down-regulated the production of IL-6 in both LPS-stimulated LI-LPMC and RAW264·7 cells (Fig. 6a,b). Moreover, ICW-derived PSPG down-regulated IL-6 production.
Fig. 6.
Cellular determinants of LcS that reduce the production of IL-6 in LPS-stimulated LI-LPMC (a) or RAW264·7 cells (b). ICW and PP fractions were prepared from LcS as described in Materials and Methods. A PSPG fraction was also prepared from the ICW fraction. The inhibitory activity for IL-6 production in LPS-stimulated LI-LPMC and RAW264·7 cells by the cellular components was determined. Data represent the percent inhibition of IL-6 synthesis in the cultures stimulated with LPS plus LcS components compared with those stimulated with LPS only. Data are mean ± SD. Similar results were obtained from five independent experiments.
Inhibitory effect of LcS or PSPG on the production of IL-6 isolated from patients with ulcerative colitis
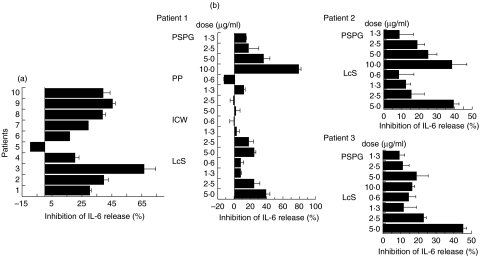
In LPS-stimulated PBMC derived from UC patients (n = 10), down-regulation of the production of IL-6 by LcS was observed in 9 cases (Fig. 7a). Addition of ICW or PSPG, but not PP, into the culture medium also dose-dependently inhibited the production of IL-6 in LPS-stimulated UC PBMC (Fig. 7b).
Fig. 7.
Inhibitory effect of LcS (a) or the cellular components of LcS (b) on the production of IL-6 in LPS-stimulated PBMC isolated from patients with ulcerative colitis. (a) PBMC isolated from 10 patients with UC were stimulated with LPS in the absence or presence of LcS (2·5 µg/ml). (b) Inhibitory effects of LcS or LcS-derived cellular components on the production of IL-6 in LPS-stimulated PBMC isolated from patients with ulcerative colitis. PBMC isolated from UC patients were stimulated with LPS in the absence or presence of various doses of the cell components. After 48 h culture, amounts of IL-6 in the culture supernatants were determined by sandwich ELISA. Data represent the percent inhibition of IL-6 synthesis in the cultures stimulated with LPS plus LcS components compared with those stimulated with LPS only. Data represent mean ± SD.
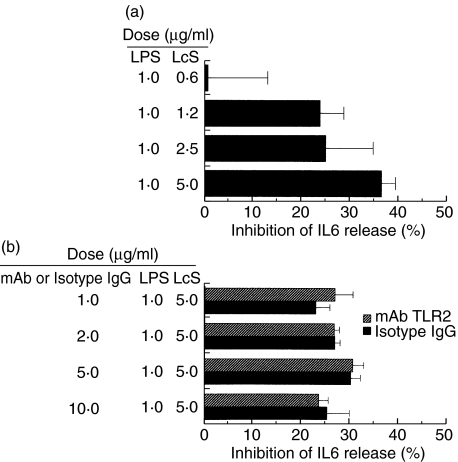
TLR2 or TLR4 signals were not necessary for the effect of LcS
Upon the stimulation with LPS of I-13·35 isolated from TLR4 null mice, the IL-6 response still remained (data not shown). On he addition of LcS to LPS-stimulated I-13·35, a dose-dependent inhibition of IL6 production was detected (Fig. 8a). Moreover, the addition of the neutralizing mAb TLR2 (10–1·25 µg/ml) had no influence on the inhibitory effect of LcS on IL-6 (Fig. 8b). The doses of sufficient to inhibit a peptidoglycan-induced IL-6 production in PMA-activated U937 cells.
Fig. 8.
Signalling through TLR2 (a) or TLR4 (b) did not influence the effect of LcS. (a) Macrophage cells which lacked TLR4 were cultured in the presence of LPS (1 µg/ml) and various doses of LcS. Then the amounts of IL-6 in the culture supernatants were determined. (b) Neutralizng mAb TLR2 was used to examine the role of TLR2 in the effect of LcS. Differentiated U937 cells were incubated with the neutralizing mAb TLR2 (10–1 µg/ml) or control murine IgG2a, and LcS (5 µg/ml). Finally the cells were stimulated with 1 µg/ml of LPS. The amount of IL-6 in the culture supernatants were determined. Data represent the percent inhibition of IL-6 synthesis in the cultures stimulated with LPS plus LcS compared with those stimulated with LPS only. Similar results were obtained from two independent experiments. Data are mean ± SD.
Discussion
In the present study, we examined the inhibitory effects of Lactobacillus casei strain Shirota (LcS) on the production of IL-6 in LPS-stimulated murine large intestinal lamina propria mononuclear cells and murine macrophage cell line RAW264·7 cells in vitro. We also investigated the effect of LcS on murine chronic IBD in vivo. Moreover, we analysed the active cellular determinants on LcS that inhibit the production of IL-6 in LPS-stimulated LI-LPMC and RAW264·7 cells. Finally, we examined the inhibitory effect on the production of IL-6 in peripheral blood mononuclear cells isolated from patients with ulcerative colitis.
IL-6 is important for host defence and over-production of IL-6 is thought to contribute to inflammatory disorders, including rheumatoid arthritis and inflammatory bowel disease [9,10]. We had described that DSS-induced colitis was less severe in IL-6 gene knockout mice while transgenic mice with a mutant CIS/SOCS3 gene, encoding a negative regulator for the IL6/STAT-3 signalling pathway, had increased susceptibility to DSS-induced colitis [10]. Sitaraman et al. [33] showed that IL-6 induced an intracellular [Ca2+] flux and degranulation in neutrophils. Taken together, these results suggested that the production of IL-6 by LI-LPMC is critical to the pathogenesis of IBD and preventing the secretion may be beneficial for the treatment of inflammatory bowel disease.
There are many reports that probiotic bacteria prevent murine IBD [34,35]. Moreover, VSL-3 has a preventive effect [36]. We also discovered that Lactobacillus- and Bifidobacterium-fermented milk prevented ileal inflammation in SAMP1/Yit, a murine model of CD, by down-regulating Th1-cytokine responses [29]. However, the mechanisms behind the effects of these probiotics remain obscure. The cell components of the bacteria used in fermented milk may alter host immune responses or the fermented products may alter the intestinal microflora, etc. Previously, Lactobacillus casei strain Shirota (LcS) improved murine immune disorders such as collagen-induced arthritis, murine lupus and murine type I diabetes [20–22]. Nanno et al. [37] described that intraperitoneal injection of LcS induced an increase in Mac-2-positive macrophages. It was reported that macrophages positive for Mac-2 induce the loss of ζ molecules in T cells and block antigen-triggered T cell proliferation [38]. Moreover, Takahashi et al. [39] reported that LcS was taken up by Peyer's patch M cells. Therefore, we hypothesized that cellular components of LcS modify host immune responses such as cytokine secretion and APC function in macrophages and prevent cytokine-dependent immunological disorders in rodents. To test this possibility, we analysed the inhibitory effect of heat-killed LcS on IL-6 secretion in LPS-stimulated LI-LPMC isolated from mice with chronic colitis and in RAW264·7 cells, and discovered a LcS dose-dependent effect with the down-regulation of the nuclear translocation of NF-κB in vitro. Next we examined the effect on murine chronic IBD of feeding mice with heat-killed LcS to exclude the influence of bio-products of LcS. Administration of heat-killed LcS to Balb/c mice with chronic colitis induced by DSS improved various disease parameters. Analysis of the immunological parameters also showed that the production of pro-inflammatory cytokines such as IL-6 and IFN-γ in LI-LPMC decreased in mice with chronic colitis fed LcS compared with mice fed standard diet. These results are important. It is known that IL-6 is a causative factor in the development of DSS-induced colitis [7]. In addition, we observed the improved effect of LcS in chronic ileitis in SAMP1/Yit mice and we had already described that the development of ileitis in this mice was also dependent on the activation of IL-6/Stat3 signalling pathways (unpublished observation).
The mechanisms underlying the inhibitory effect of LcS on IL-6 secretion in vitro and the prevention of colitis in vivo should be discussed. With blockage of TLR2 or TLR4, the inhibitory effect of LcS on IL6 secretion still remained. Therefore, These signalling pathways, were not necessary for this inhibitory pathway. It is possible that PSPG interacted with other surface receptor-like molecules present on macrophages, and this binding inhibited LPS-signalling and down-regulated the nuclear translocation of NF-κB and the production of cytokines such as IL-6, TNF-α, etc. The binding of a apathogenic bacteria to a human epithelial cell line induced ubiquinaization of IκB-α protein and then inhibited the NF-κB signalling pathway [40]. Therefore, the binding of LcS to LI-LPMC may have induced such an effect and altered immune responses on the cells. However, we could not confirm that the effect of LcS in vitro, here we described, is a proper mechanism of the improved effect of LcS on chronic IBD in vivo. Therefore, other possibilities might be involved in the improved effect of this probiotics on chronic IBD. Further analysis should clarify these problems.
In this paper, we described that a polysaccharide-peptidoglycan complex derived from Lactobacillus casei strain Shirota down-regulated the production of IL-6 in LPS-stimulated LI-LPMC, a murine macrophage cell line and PBMC derived from patients with ulcerative colitis in vitro. Moreover, this probiotics induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines such as IL-6 in LPMC. The safety of this probiotic strain has been demonstrated in a clinical trial [41]. Therefore, Lactobacillus casei strain Shirota may be a useful probiotic for the treatment of inflammatory bowel disease.
Acknowledgments
The authors are grateful to Dr Y. Umesaki for critical reading of the manuscript. We also thank Dr K. Miyake (Saga Medical College) for providing mAb against TLR4/MD2 complex (clone: MTS-510). We are indebted to the staff of the animal facility of Yakult Central Institute for their expertise in animal care.
References
- 1.Duchmann R, Kaiser I, Herhann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duchmann R, May E, Heike M, et al. T cell specifity and cross reactivity towards enterobacteria, Bacteroides, Bifidobacterium, and antigen from resident intestinal flora in humans. Gut. 1999;44:812–8. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadlack B, Merz H, Schorle H, et al. Ulcerative colitis-like disease in mice with disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 4.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross V, Andus T, Caesar I, Roth M, et al. Evidence for continuous stimulation of IL-6 production in Crohn's disease. Gastroenterology. 1992;102:514–9. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 7.Fuss IJ, Neurath M, Boirivant M, et al. Desperate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 8.Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumor necrosis factor-alpha, IL-6 and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Yoshizaki K, Kishimoto T, et al. IL-6 is required for the development of Th1 cell mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 10.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn's disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–81. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Farrell AM, Liu Y, Moore KW, et al. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and – independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–64. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 14.Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–50. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 15.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 16.Skidmore BJ, Morrison DC, Chiller JM, et al. Immunologic properties of bacteroides lipopolysaccharide (LPS): The unresponsiveness of C3H/HeJ mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975;142:1488–504. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rath HC, Ikeda JS, Linde HJ, et al. Varying cecal bacterial loads influences colitis and gastritis in HLA-B27 transgenic rats. Gastroenterology. 1999;116:310–9. doi: 10.1016/s0016-5085(99)70127-7. [DOI] [PubMed] [Google Scholar]
- 18.Lange S, Delbro DS, Jennische E, et al. The role of the Lps gene in experimental ulcerative colitis in mice. Apmis. 1996;104:823–33. doi: 10.1111/j.1699-0463.1996.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Lafuente A, Antolin M, Guarner F, et al. Incremination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol. 1997;272:G10–5. doi: 10.1152/ajpgi.1997.272.1.G10. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki T, Nagata Y, Kado S, et al. Prevention of onset in an insulin-dependent diabetes mellitus models, NOD mice, by oral feeding of Lactobacillus casei. Apmis. 1997;105:643–9. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 21.Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oal administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998;63:635–44. doi: 10.1016/s0024-3205(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 22.Mike A, Nagaoka N, Tagami Y, et al. Prevention of B220+ T cell expansion and prolongation of lifespan induced by Lactbacillus casei in MRL/lpr mice. Clin Exp Immunol. 1999;117:357–68. doi: 10.1046/j.1365-2249.1999.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato I, Yokokura T, Mutai M. Correlation between increase in Ia-bearing macrophages and induction of T-cell-dependent anti-tumor activity by Lactobacillus casei in mice. Cancer Immunol Immunother. 1998;26:215–21. doi: 10.1007/BF00199932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekine K, Toida T, Saito M, et al. A new morphologically characterized cell wall preparation (whole pepetidoglycan) from Bifidobacterium infantis with a higher efficacy on the regression of an established tumor in mice. Cancer Res. 1985;45:1300–7. [PubMed] [Google Scholar]
- 25.Nagaoka M, Muto M, Nomoto K, et al. Structure of polysaccharide-peptidoglycan complex from the cell wall of Lactobacillus casei YIT 9018. J Biochem. 1990;108:568–71. doi: 10.1093/oxfordjournals.jbchem.a123243. [DOI] [PubMed] [Google Scholar]
- 26.Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 27.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Laboratory Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 28.Matsumoto S, Watanabe N, Umesaki Y. Roles of chloroform-resistants variants in mouse models of experimental colitis. Microbial Ecol Health Dis. 2000;12:102–8. [Google Scholar]
- 29.Matsumoto S, Watanabe N, Imaoka A, et al. Preventive effects of Bifidobacterium- and Lactobacillus-fermented milk on the development of inflammatory bowel disease in senescence-accelerated mouse (SAM) P1/Yit strain mice. Digestion. 2001;64:92–9. doi: 10.1159/000048846. [DOI] [PubMed] [Google Scholar]
- 30.Takedatsu H, Mitsuyama K, Matsumoto S, et al. Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol. 2004;34:1561–9. doi: 10.1002/eji.200324680. [DOI] [PubMed] [Google Scholar]
- 31.Asai Y, Jinno T, Ogawa T. Oral treponemes and their outer membrane extracts activate human gingival epithelial cells through toll-like receptor 2. Infect Immun. 2003;71:717–25. doi: 10.1128/IAI.71.2.717-725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sordet O, Rebe C, Plenchette S, et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–53. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- 33.Sitaraman SV, Merlin D, Wang L, et al. Neutrophil-epithelial crosstalk at the intestinal luminal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–9. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin-10 gene-deficient mice. Gastroenterology. 1999;116:1107–14. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 35.O'Mahony L, Feeney M, O'Halloran S, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15:1219–25. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 36.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–91. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 37.Nanno M, Shimizu-Takeda T, Mike A, et al. Increased production of cytolytic macrophage progenitors by Lactobacillus casei in mice. J Leuk Biol. 1989;46:89–95. doi: 10.1002/jlb.46.2.89. [DOI] [PubMed] [Google Scholar]
- 38.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995;181:1881–9. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi M, Iwata S, Yamazaki N, et al. Phagocytosis of the lactic acid bacteria by M cells in the rabbit Peyer's patches. J Clin Electron Microscopy. 1991;24:5–6. [Google Scholar]
- 40.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-a ubiquitination. Science. 2000;289:1560–3. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 41.Aso Y, Akaza H, Kotake T, et al. Preventive effect of Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol. 1995;27:104–9. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]