Abstract
The immunodeficiency in Ataxia-telangiectasia (A-T) is characterised by low T and B cell counts, low levels of IgE, IgA and/or IgG2, and especially low levels of pneumococcal antibodies. The 23-valent pneumococcal polysaccharide vaccine (PPV23) has previously been shown not to be effective in A-T, but these patients are capable of making protective antibodies to other vaccines such as diphtheria and tetanus toxin, promising effect of the seven-valent pneumococcal conjugated vaccine (PCV7).
Nine A-T patients and 25 age and sex matched controls were vaccinated with both PCV7 and PPV23, and three A-T patients were vaccinated with PCV7 only. In the A-T patients, no significant increase in pneumococcal antibody levels were observed after the single PCV7, while the subsequent PPV23 vaccination resulted in a significant increase in antibody levels to the PPV23 mix, as well as to serotype 4, 14, 19F and to the geometric mean of serotype 4, 6B, 14, 18C, 19F, 23F which increased from median 0·2 (range 0·1–0·5) microg/mL to 0·6 (0·2–1·5) microg/mL (P = 0·014). Compared to the patients’ baseline levels, the vaccinations induced a 1·5- to 7-fold increase in antibodies to the six different serotypes tested. The increases in pneumococcal antibody titres were lower than those observed in the controls (9- to 34-fold increase). The results are valuable in planning the care of A-T patients, using PCV7 to trigger and PPV23 to booster the immune response and possibly prevent severe pneumococcal disease.
Keywords: ataxia-telangiectasia, conjugated pneumococcal vaccine, antibody response, mannose-binding lectin, diphtheria toxin
Introduction
Ataxia-telangiectasia (A-T) is an autosomal recessive disorder characterized by early onset progressive cerebellar ataxia, oculocutaneous telangiectasias, endocrine abnormalities, growth retardation and immunodeficiency. The responsible gene, ATM (A-T mutated), maps to chromosome 11q22.23 [1], spans ∼ 150 kb genomic DNA containing 66 exons and was identified in 1995 (MIM no. 208900) [2]. Malignancies or chronic lung failure with pulmonary infections cause death in early adulthood. Deficiencies in both humoral and cellular immunity are reported [3], often with a discrepancy between moderate clinical and more severe laboratory findings. We recently reported a group of 11 Norwegian A-T patients and found a correlation between IgG2 levels and an early truncating founder mutation, 3245delATCinsTGAT [4]. All our patients had extremely low levels of antibodies to Streptococcus pneumoniae despite recurrent respiratory infections, and there was a clear relationship between pneumococcal antibodies and IgG2 levels. Low IgG2 combined with low pneumococcal antibodies may explain the A-T patients’ increased susceptibility to respiratory infections [5]. Others have previously reported a low level of pneumococcal antibodies in A-T patients before and even after pneumococcal polysaccharide vaccine administration [6]. An antibody response in our A-T patients to diphtheria and tetanus vaccines and a partly successful response to Hib conjugate vaccine [4], indicated a possible effect of other conjugate vaccines such as the new 7-valent pneumococcal conjugated vaccine, PCV7 [7]. Here the pneumococcal polysaccharides are linked to a carrier protein derived from diphtheria toxin. In healthy infants the ordinary 23-valent vaccine (PPV23), after priming with PCV7, booster the IgG responses to the different serotypes in PCV7 [8], still the efficacy data are limited [9,10]. We wanted to test the antibody responses to the PCV7 followed by the PPV23. The PPV23 vaccine was administered to booster and to possibly broaden the pneumococcal serotype protection.
Materials and methods
Patients and controls
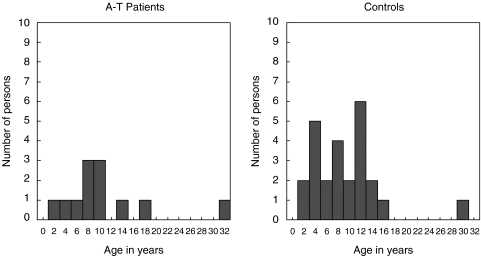
All living A-T patients in Norway (n = 13) were invited to participate in this study. The genetic and immunological phenotype of 10 of these patients has been described in detail elsewhere [4]. In addition, three newly diagnosed patients were also included (Table 1). Twelve patients (aged 2–32 years; 6 M; 6 F) consented to participate. Twenty-five individuals (13 M, 12 F) with no or minor heart disease served as sex and age matched controls (Fig. 1). Both patients and controls had followed the National children vaccination program. The exclusion criteria were: current infection, cancer/cancer treatment, corticosteroid treatment, previous adverse reactions to other vaccines including diphtheria, other vaccinations within 6 weeks before or 6 weeks after administration of the study vaccines.
Table 1.
ATM mutations, respiratory infectious problems, immunological results and pneumococcal vaccinations in the A-T patients
| Identity | ATM Mutations* paternal/maternal | ATM† | Respiratory infectious problems | MBL‡ (ng/ml) | Immunoglobulins§ | Pneumococcal vaccines |
|---|---|---|---|---|---|---|
| NOAT10 | 3245delATCinsTGAT(fs)/3245delATCinsTGAT(fs) | R/R | Otitis media | 327 L | Low IgA, IgG2, IgG4, IgE | PCV7 + PPV23 |
| NOAT 11 | 3245delATCinsTGAT(fs)/3245delATCinsTGAT(fs) | R/R | Pneumonia, interstitial lung disease | 722 | Low IgG2 | PCV7 + PPV23 |
| NOAT16 | 3245delATCinsTGAT(fs)/3245delATCinsTGAT(fs) | R/R | Pneumonia | 132 L | Low IgG2, IgG3, IgG4, IgE | PCV7 + PPV23 |
| NOAT17 | 6890A > C(ms)/3245delATCinsTGAT(fs) | R/a | None | 158 L | High IgD.Low IgG2, IgE | PCV7 + PPV23 |
| NOAT18 | 3245delATCinsTGAT(fs)/4110delG(ss) | R/a | None | 3032 | High IgD.Low IgA, IgG2, IgE | PCV7 + PPV23 |
| NOAT4 | 8978delGAAAinsAT(fs)/7875TG > GC(ds) | a/a | None | ND | High IgM, IgD, IgG1.Low IgA, IgG2, IgG3, IgE | PCV7 + PPV23 |
| NOAT13 | 8432delA(fs)/8432delA(fs) | a/a | None | 59 L | High IgG1.Low IgA, IgE | PCV7 + PPV2 |
| NOAT15 | 4110delG(ss)/2938delA(fs) | a/a | Chronic secretory otitis | 629 | Low IgG4 | PCV7 + PPV23 |
| NOAT20A | 5932G > T(ns)/2880delC(fs) | a/a | Otitis media | 78 L | High IgMLow IgA, IgG3 | PCV7 + PPV23 |
| NOAT20B | 5932G > T(ns)/2880delC(fs) | a/a | Pneumonia | 108 L | Low IgA, IgG2, IgG3, IgG4, IgE | PCV7 |
| NOAT21A | Not defined/not defined | a/a | None | 3910 | Low IgG2, IgE | PCV7 |
| NOAT21B | Not defined/not defined | a/a | None | 3136 | Low IgA, IgG4, IgE | PCV7 |
Mutations in ATM gene are designed according to recommended nomenclature [31–33] Abbreviations: Ins, insertion; del, deletion; fs, frame shift; ds, double substitution; ms, missense; ns, nonsense and ss, splice site mutation;
R/R, homozygous for the Norwegian founder mutation; R/a, compound heterozygous for the founder mutation; a/a, other mutations in ATM, founder mutation excluded.
MBL, Mannose binding lectin, Low MBL values are marked L; ND, Not done
Age specific reference values according to Supplement in reference [4].
Fig. 1.
Age distribution among A-T patients and controls.
The Norwegian Medicines Agency, the Regional Committee for Medical Research Ethics as well as the Norwegian Data Inspectorate approved this study. Oral and written information was given to patients, controls and their parents. Signed consent was obtained from each vaccinee or his/her parent.
Vaccination
The seven-valent pneumococcal conjugated vaccine (PCV7, Prevenar® Wyeth Lederle) was given as 0·5 ml injection in the deltoid muscle. Prevenar contains polysaccharides from seven serotypes (serotype 4 (2 µg), 6B (4 µg), 9V (2 µg), 14 (2 µg), 18C (2 µg), 19F (2 µg) and 23F (2 µg)) which are conjugated to a carrier protein (CRM197 from diphtheria toxin, about 20 µg). After 6–12 months, the patients received 0·5 ml of the 23-valent pneumococcal polysaccharide vaccine (PPV23, Pneumovax® Aventis Pasteur MSD) intramuscularly. Pneumovax contains polysaccharides from following 23 serotypes (25 µg of each): 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. All vaccinations were performed at our hospital by one trained person. Prior to each vaccination and six weeks after, a blood sample was collected. The serum samples were stored at −20°C until antibody testing analysis, and pre- and postimmunization samples were assayed simultaneously. The vaccinee or a parent answered a questionnaire concerning adverse reactions.
Immunology
IgG antibodies to individual pneumococcal serotypes 4, 6B, 14, 18C, 19F and 23F and also to a mix of PPV23, were measured with enzyme linked immunosorbent assay (ELISA) after CPS adsorption of sera (Statens Serum Institut, Denmark) [11,12]. For each serotype an IgG level > 1 µg/ml may be regarded as protective [13]. The antibody level to PPV23 was given in arbitrary units (U/ml) and a level below 2·5 U/ml was regarded as low. An in vitro toxin neutralization test was used for detection of diphtheria antibodies [14], and tetanus antitoxin was measured with ELISA [15]. Quantification of serum immunoglobulins, total IgG, IgA, IgM, IgE and IgG subclasses was performed by nephelometry (Dade Behring, Illinois, US), while IgD was measured by immunodiffusion (Behring), and age specific reference values were used [4]. Mannose-binding lectin (MBL) were quantified with ELISA (Antibodyshop/Statens Serum Institut, Copenhagen, Denmark). Low MBL levels were defined as < 400 ng/ml and extremely low < 100 ng/ml.
Statistics
The data was collected in a Microsoft Access 97 database. To meet legal requirement, the patient administrative data were coded when registered. SPSS 11·0 for Windows and SamplePower 2·0 was used for the statistical analyses. To compare two groups, t-tests were used, while one-way anova models were used to compare more than two groups. Bonferroni corrections were performed to accommodate multiple tests. To study the linear relationship between two continuous variables, Pearson coefficients and linear regression components were computed. Based on preliminary data, estimations of the statistical power done prior to the study assumed that an equal number of patients and control persons would be enough to show statistical differences between the two groups. Median values rather than mean values are reported since the different pneumococcal antibody levels measured seemed clustered within each group with a few high values influencing the mean values of the group.
Results
Prior to vaccinations
Prior to vaccination all nine patients and 10 of 25 controls had antibody levels to the PPV23 mix considered as low (<2·5 U/ml) with median 0·65 (range 0·2–3·6) U/ml in the A-T patients and 3·8 (0·4–109·4) U/ml in the controls (P = 0·045, independent samples t-test, equal variance not assumed) (Table 2). The geometric mean of pneumococcal antibody levels to the six tested serotypes was 0·2 (0·1–0·5) µg/ml in the A-T patients and 0·7 (0·2–9·8) µg/ml in the control group (P = 0·062, independent samples t-test, equal variance assumed). No correlation between baseline pneumococcal antibody level and age or gender was detected.
Table 2.
Median (range) titres of pneumococcal antibodies in patients with ataxia–telangiectasia and controls
| A-T patients | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pneumococcal serotype | Unit | Preimmunization n =12 | Postimmunization n = 9 | P-value | Fold increase‡ | Preimmunization n = 25 | Postimmunization n = 25 | P-value | Fold increase‡ |
| Serotype 4 | µg/ml | 0·1 (0·05–0·2) | 0·7 (0·1–4·6) | 0·014* | 7·0 | 0·3 (0·1–14·7) | 8·1 (2·0–48·4) | 0·001* | 27 |
| Serotype 6B | µg/ml | 0·2 (0·1–1·2) | 0·3 (0·1–1·0) | 0·506 | 1·5 | 0·7 (0·2–11·8) | 6·2 (0·3–190·3) | 0·001* | 8·9 |
| Serotype 14 | µg/ml | 0·3 (0·1–0·7) | 0·9 (0·1–13·0) | 0·037* | 3·0 | 0·65 (0·05–3·9) | 22 (3·4–92·7) | 0·001* | 33·9 |
| Serotype 18C | µg/ml | 0·1 (0·05–0·2) | 0·3 (0·1–3·4) | 0·059 | 3·0 | 0·8 (0·1–15·6) | 9·0 (1·5–20·1) | 0·001* | 11·3 |
| Serotype 19F | µg/ml | 0·25 (0·1–0·6) | 0·5 (0·2–1·2) | 0·009* | 2·0 | 1·2 (0·2–33·5) | 18·4 (0·4–75·8) | 0·001* | 15·3 |
| Serotype 23F | µg/ml | 0·2 (0·05–5·5) | 0·3 (0·1–7·9) | 0·430 | 1·5 | 0·9 (0·2–11·6) | 9·6 (0·9–183·8) | 0·001* | 10·7 |
| Geom.mean | µg/ml | 0·2 (0·1–0·5) | 0·6 (0·2–1·5) | 0·014* | 3 | 0·7 (0·2–9·8) | 10·3 (1·9–51·5) | 0·001* | 14·7 |
| PPV23 mix | U/ml | 0·65 (0·2–3·6) | 5·7 (1·0–28·3) | 0·001* | 8·8 | 3·8 (0·4–109·4) | 36·7 (8–292·6) | 0·001* | 9·7 |
Significant differences between pre- and postimmunization;
Fold increases are calculated from the median values.
After vaccinations
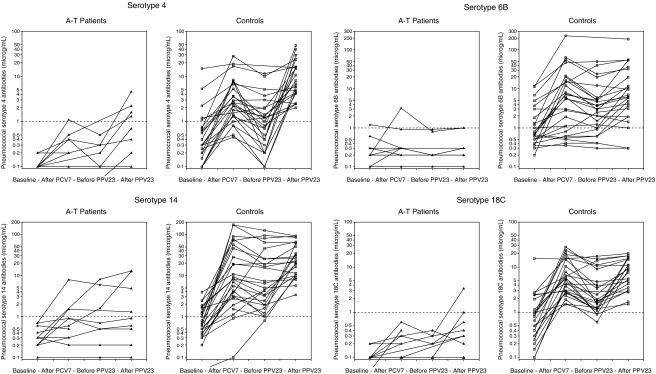
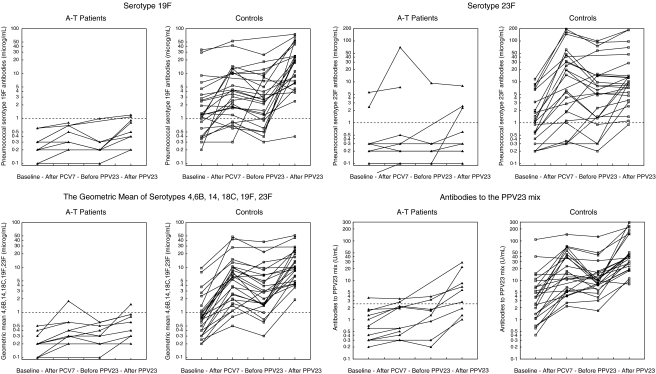
Nine A-T patients and all control persons were vaccinated with both PCV7 and PPV23, and three A-T patients were vaccinated with PCV7 only (Table 1). The interval between PCV7 and PPV23 was 9·4 (5·0–14·0) months in the A-T patients and 9·4 (5·1–16·5) months in the controls. The PCV7 vaccine followed by PPV23 induced an antibody response in the A-T patients. As can be seen in Table 2 the increases in serotype antibody titres were 1·5- to 7-fold and lower than the 9- to 34-fold increases observed in the control group (Table 2). In the A-T group no significant increase in pneumococcal antibody levels were observed after PCV7 (Fig. 2). However, after the subsequent PPV23 vaccination significant differences in antibody levels to the PPV23 mix as well as serotype 4, 14, 19F and the six serotypes’ geometric mean were obtained (Table 2). Variations between serotypes were observed, e.g. response to serotype 6B lowest in both groups. Also variation within the patient group were observed. One patient (NOAT11) did not show any response in five of the six serotypes tested. Another patient (NOAT17) showed low response to three serotypes, and three other patients (NOAT4, NOAT10, NOAT21B) showed low response to one serotype. All patients responded to serotype 19F. Defining the protective level to 1 µg/ml for each serotype, four patients reached the protective levels to serotype 4, two to serotype 6B, four to serotype 14, two to serotype 18C, two to serotype 19F and three to serotype 23F. Six of nine A-T patients who had received both PCV7 and PPV23 and all 25 controls had antibody levels to the PPV23 mix above 2·5 U/ml. In the control group an increase in levels of antibodies against serotype 6B, 14, 18C, and in geometric mean antibody levels, but not against PPV23 mix were observed after the PCV7 vaccine. Only the increase in antibodies to serotype 14 persisted in the period until the PPV23 vaccination (Fig. 2). After vaccination with PPV23 the level of antibodies against all six serotypes increased compared to the control group's baseline antibody levels (Table 2), and reached definite protective levels in almost all serotypes in all controls (Fig. 2). Large interindividual variations within the control group were also observed, but not influencing the overall significant increase in antibody levels after the vaccinations. No correlation between the pneumococcal antibody response and the baseline pneumococcal antibody levels was observed for the A-T patients or the controls. No correlation between the pneumococcal antibody response and age or gender was found.
Fig. 2.
Pneumococcal antibodies measured before and after immunization with PCV7 and PPV23 in patients and controls. Be aware that the y-axes are log transformed and may vary from serotype to serotype.
Study design and statistical power
With the sample size of 9 patients and 25 controls and the observed differences in pneumococcal antibodies before and after immunizations, the statistical power (0·05, 2-tailed) was 0·68 in serotype 4, 0·32 in serotype 6B, 0·81 in serotype 14, 0·97 in serotype 18C, 0·83 in serotype 19F, 0·36 in serotype 23F, 0·88 in the geometric mean of the six serotypes and 0·54 in the 23-mix antibodies. Adding the results of the three patients who only received one PCV7, the statistical power (0·05, 2-tailed) of our analyses increased to 0·82 in serotype 4, 0·41 in serotype 6B, 0·92 in serotype 14, 0·99 in serotype 18C, 0·96 in serotype 19F, 0·46 in serotype 23F, 0·96 in the geometric mean of the six serotypes and 0·69 in the 23-mix antibodies. Our analyses exhibited a high statistical power in the measurements of serotypes 4, 14, 18C, 19F and the geometric mean. The low statistical power in the analyses of the differences in serotype 6B and 23F was mainly due to the great interindividual variations observed in the control group reflected in the big range (Table 2) and large standard deviation of mean of these two serotypes (39·3 and 51·6 µg/ml).
Mannose-binding lectin and immunoglobulins
MBL levels and results of the immunoglobulin measurements in the patients are listed in Table 1. Four A-T patients and 2 controls had MBL values below 200 ng/ml (2 patients and 1 control had extremely low MBL < 100 ng/ml). The median MBL level for the A-T patients was 629 (59–3910) ng/ml, not significantly different from that of the control group, 1194 (51–4460) ng/ml. The four patients with the highest MBL levels had no respiratory infectious problems (Table 1). There was no correlation between MBL levels and baseline pneumococcal antibody levels or antibody response in the two groups, and there was no correlation between MBL levels and IgG2 levels. Eight A-T patients had IgG2 deficiency with values below 0·65 g/l, and seven had IgA below 0·15 g/l (four in combination with low IgG2). Those who were double homozygous with the Norwegian founder mutation had even lower levels of IgG2 (mean ± SD) (0·16 ± 0·11, n = 3) than the other A-T patients (0·73 ± 0·51, n = 9). A positive linear relationship existed between baseline pneumococcal antibodies and IgG2 levels (r = 0·79, P = 0·002) in the patient group, but not in the control group where IgG, IgG subclasses, IgA, IgM and IgD levels were within normal reference limits (data not shown). No correlation between IgG2 levels and pneumococcal antibody responses were observed within the two groups.
Antibodies to diphtheria and tetanus toxins
In the A-T patients, there was a significant difference between diphtheria toxin antibody levels before and after PCV7 was given; 0·32 (0·01–2·56) IU/ml versus 0·64 (0·32–10·0) IU/ml, P = 0·002. A similar increase in antibodies to tetanus toxin was not observed; 0·5 (<0·1–8·9) IU/ml versus 0·2 (<0·1–12·2) IU/ml, P = 0·18. In the control group, the baseline diphtheria toxin antibody level was 7·56 (0·64–10·24) IU/ml, and the tetanus toxin antibody level was 5·4 (0·2–20) IU/ml and no changes after vaccination were observed. A positive linear relationship was observed between diphtheria and tetanus antitoxins in the patient group (r = 0·83, P < 0·001) and among controls (r = 0·52, P = 0·002). The baseline antibody levels to diphtheria and tetanus were positively correlated to the pneumococcal vaccine response in the A-T patients. Four of five A-T patients with baseline diphtheria antibody levels < 0·1 IU/ml, and four out of five with baseline tetanus levels ≤0·1 IU/ml did not achieve protective antibody levels to the PPV23 mix. While five of the seven patients with the highest baseline diphtheria levels, 0·64 (0·16–2·56) IU/ml, had antibody levels 6·5 (2·85–19·75) U/ml to the PPV23 mix by the end of the study. And six of the seven A-T patients with baseline tetanus antibody at protective level were able to make antibody levels to the PPV23 mix beyond 2·5 IU/ml. In the control group the antibody levels to diphtheria or tetanus were not correlated to the pneumococcal vaccine response.
Adverse reactions
One A-T patient had diarrhoea for 24 h after the PCV7 vaccine, and irritability and itching after the PPV23 vaccine, while another patient had diarrhoea for one day. Six of the control persons reported local swelling, redness and pain at the injection site lasting for 1–3 days, and one control had short-lasting diarrhoea after the PCV7 vaccine. Fever (39–39·5°C) was observed in two controls the first day after the vaccines.
Discussion
Pneumococcal conjugate vaccine followed by pneumococcal polysaccaride vaccine induced an antibody response in the A-T patients. The increase in pneumococcal antibodies in the patients was up to tenfold lower than observed in the control group (Fig. 2 and Table 2), and variations between serotypes and individuals were observed. But the patients’ pneumococcal antibody levels to serotype 4, 14, 19F after the vaccinations were significantly higher than the baseline levels, showing signs of a booster effect of PPV23 after an initial PCV7 vaccination. The vaccinations induced a 1·5- to 7-fold increase in antibodies to the six serotypes tested (Table 2). This finding is different from the results obtained by administering only the PPV23 vaccine in A-T patients [6], and one PCV7 vaccine [16]. Others have recently documented a similareffect of two subsequent PCV7 vaccinations, inducing a 2·6- to 7·5-fold increase in the pneumococcal antibody titres of the same six serotypes [17]. The optimal pneumococcal vaccination regimen in A-T is not yet found, it may be one or more PCV7 vaccine followed by one PPV23.
In healthy infants the ordinary 23-valent vaccine (PPV23) after priming with PCV7, booster the IgG responses to the different serotypes in PCV7 [8]. But the efficacy data of such prime boost regimens are limited and somewhat disappointing [9,10]. We have characterized the respiratory infectious problems in our A-T patients before they received the pneumococcal vaccines (Table 1). Further studies will show the efficacy of these vaccines and the different vaccination regimens to prevent respiratory infections in A-T patients.
Calculations of the statistical power of the study shows that our study design was reliable for most of the serotypes tested to discover significant differences between the two groups. Great interindividual variations in postvaccination levels of serotype 6B and 23F were observed in the control group. In the patient group the definitive lowest responses were observed for these two serotypes. The 6B are known to be inducing a variable and often a lower response than the other serotypes. This is the reason why the PCV7 vaccine contains a double amount (4 µg) of 6B compared to the other serotypes.
Pneumococcal antibodies are normally of IgG2 and IgA isotypes [18], which could be the reason why the A-T patients with low IgG2 levels had lowest baseline levels of IgG pneumococcal antibodies. However we did not find that the patients with the lowest IgG2 levels before vaccination showed a lower increase in pneumococcal antibodies than the other A-T patients. Others have discussed a correlation between pneumococcal antibody levels and IgG2 deficiency, and between pneumococcal antibodies and MBL deficiency [19–21]. Such correlations were not found in our material. Low MBL levels may represent a separate additive effect of the immunodeficiency in these patients.
The antibody response to pneumococcal capsular polysaccaride has been called a T cell independent process (TI type 2) with a predominant B–B cell interaction [22]. Yet T cells and CD40 ligand contribute to regulation of this prosess [23,24]. Wild-type infections or nasopharyngeal carriages may induce pneumococcal antibodies, since pneumococcal vaccines induce more like a secondary immunological response [18]. We have previously shown that the A-T patients have low numbers of T-cells and very low levels of B-cells [4]. Few accessible B cells may result in inadequate B-B cell activation. Deficient V(D)J rearrangements and isotype switching, as have been documented by others in A-T patients [25,26] as well as few T cells, theoretically explains why A-T patients make low levels of pneumococcal antibodies. But still the exact mechanism remains to be described.
The PCV7 vaccine contains a carrier protein, CRM197, derived from diphtheria toxin and an effect of PCV7 on the amount of diphtheria antibodies was observed in the patient group. This booster effect may be due to the A-T patients’ low baseline levels of these antibodies. A low-grade booster effect of the diphtheria CRM197 conjugated protein may then be less apparent in the control group who had a higher baseline diphtheria antitoxin level. Conjugated vaccines in preschool (3–4-year olds) and adolescents (13–17-year olds) show that CRM197 induces a booster response to diphtheria and, similarly, that the tetanus toxin conjugates induce a response to tetanus. The responses correspond to those induced by diphtheria-tetanus vaccine booster doses [27]. However, in equivalent dosages, native CRM197 induces lower antibody levels than the diphtheria toxoid [28,29], which is used in the conventional diphtheria vaccines. Shelly et al. [30] found that low baseline antibody levels to diphtheria were associated with poor response to conjugated pneumococcal vaccine in adults. We also found that low baseline diphtheria levels were connected to low pneumococcal antibody response among the A-T patients, and that pre-existing protective antibody level to diphtheria and tetanus seemed to be an indicator of sufficient pneumococcal antibody response to these pneumococcal vaccines. A similar pattern was not detected in the control group.
Conclusion
PCV7 vaccine plus PPV23 vaccine induced a 3-fold increase in the geometric mean of the six serotypes (4, 6B, 14, 18C, 19F, 23F) in all A-T patients, and antibodies to the PPV23 mix above 2·5 U/ml in 6 out of 9 A-T patients. The PPV23 vaccine seems capable of boostering the antibody level of the different pneumococcal serotypes in PCV7. The PCV7 containing the diphtheria CRM197 conjugated protein, induced increase in diphtheria antibody levels in the A-T patients. Pre-existing protective antibody level to diphtheria and tetanus seemed to be an indicator of capability of making protective pneumococcal antibodies. The results are valuable in planning the care of these patients, using vaccinations with T cell-dependent immune response to circumvent the patients’ immunodeficiencies and possibly prevent pneumococcal disease.
Acknowledgments
This project has been financed with the aid of EXTRA funds from the Norwegian Foundation for Health and Rehabilitation. Thanks to Anne-Cathrine Kristoffersen, responsible for performing all the measurements of pneumococcal, diphtheria and tetanus antibodies. A special thanks to the participating families, patients and control persons, who made this study possible.
References
- 1.Gatti RA, Berkel I, Boder E, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22–23. Nature. 1988;336:577–80. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- 2.Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–53. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 3.Schubert R, Reichenbach J, Zielen S. Deficiencies in CD4+ and CD8+ T cell subsets in ataxia telangiectasia. Clin Exp Immunol. 2002;129:125–32. doi: 10.1046/j.1365-2249.2002.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stray-Pedersen A, Jónsson T, Heiberg A, et al. The impact of an early truncating founder ATM mutation on immunoglobulins, specific antibodies and lymphocyte populations in ataxia-telangiectasia patients and their parents. Clin Exp Immunol. 2004;137:178–86. doi: 10.1111/j.1365-2249.2004.02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhooge IJ, van Kempen MJ, Sanders LA, Rijkers GT. Deficient IgA and IgG2 anti-pneumococcal antibody levels and response to vaccination in otitis prone children. Int J Pediatr Otorhinolaryngol. 2002;64:133–41. doi: 10.1016/s0165-5876(02)00068-x. [DOI] [PubMed] [Google Scholar]
- 6.Sanal O, Ersoy F, Yel L, et al. Impaired IgG antibody production to pneumococcal polysaccharides in patients with ataxia-telangiectasia. J Clin Immunol. 1999;19:326–34. doi: 10.1023/a:1020599810261. [DOI] [PubMed] [Google Scholar]
- 7.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity og heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Choo S, Seymour L, Morris R, et al. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a Haemophilus influenzae type b conjugate vaccine in United Kingdom infants. Pediatr Infect Dis J. 2000;19:854–62. doi: 10.1097/00006454-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Veenhoven RH, Bogaert D, Schilder AG, et al. Nasopharyngeal pneumococcal carriage after combined pneumococcal conjugate and polysaccharide vaccination in children with a history of recurrent acute otitis media. Clin Infect Dis. 2004;39:911–9. doi: 10.1086/422651. [DOI] [PubMed] [Google Scholar]
- 10.Veenhoven RH, Bogaert D, Uiterwaal CS, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet. 2003;361:2189–95. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 11.Aaberge IS, Steinsvik TE, Groeng EC, Leikvold RB, Lovik M. Human antibody response to a pneumococcal vaccine in SCID-PBL-hu mice and simultaneously vaccinated human cell donors. Clin Exp Immunol. 1996;105:12–7. doi: 10.1046/j.1365-2249.1996.d01-728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielen S, Broker M, Strnad N, et al. Simple determination of polysaccharide specific antibodies by means of chemically modified ELISA plates. J Immunol Meth. 2004;193:1–7. doi: 10.1016/0022-1759(96)00033-6. [DOI] [PubMed] [Google Scholar]
- 13.Nordoy T, Husebekk A, Aaberge IS, et al. Humoral immunity to viral and bacterial antigens in lymphoma patients 4–10 years after high-dose therapy with ABMT. Serological responses to revaccinations according to EBMT guidelines. Bone Marrow Transplant. 2001;28:681–7. doi: 10.1038/sj.bmt.1703228. [DOI] [PubMed] [Google Scholar]
- 14.Skogen VPA, Koroleva VN, Danilova E, Halvorsen DS, Maksimova N, Sjursen H. Detection of diphtheria antitoxin by four different methods. Clin Microbiol Infect. 1999;5:628–33. doi: 10.1111/j.1469-0691.1999.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 15.Simonsen O, Schou C, Heron I. Modification of the ELISA for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1987;15:143–57. doi: 10.1016/0092-1157(87)90037-0. [DOI] [PubMed] [Google Scholar]
- 16.Sanal O, Ersoy F, Tezcan I, et al. Antibody response to a seven-valent pneumococcal conjugated vaccine in patients with ataxia-telangiectasia. J Clin Immunol. 2004;24:411–7. doi: 10.1023/B:JOCI.0000029109.15355.ba. [DOI] [PubMed] [Google Scholar]
- 17.Schubert R, Reichenbach J, Rose M, Zielen S. Immunogenicity of the seven valent pneumococcal conjugate vaccine in patients with ataxia-telangiectasia. Pediatr Infect Dis J. 2004;23:269–70. doi: 10.1097/01.inf.0000115737.35353.55. [DOI] [PubMed] [Google Scholar]
- 18.Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, Goldblatt D. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur J Immunol. 2000;30:1214–23. doi: 10.1002/(SICI)1521-4141(200004)30:4<1214::AID-IMMU1214>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Knox K, Segal S, et al. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 2002;359:1569–73. doi: 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- 20.Kronborg G, Weis N, Madsen HO, et al. Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J Infect Dis. 2002;185:1517–20. doi: 10.1086/340216. [DOI] [PubMed] [Google Scholar]
- 21.Kronborg G, Garred P. Mannose-binding lectin genotype as a risk factor for invasive pneumococcal infection. Lancet. 2002;360:1176. doi: 10.1016/S0140-6736(02)11223-2. [DOI] [PubMed] [Google Scholar]
- 22.Shinozaki K, Yasui K, Agematsu K. Direct B/B–cell interactions in immunoglobulin synthesis. Clin Exp Immunol. 2001;124:386–91. doi: 10.1046/j.1365-2249.2001.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect Immun. 2002;70:4083–91. doi: 10.1128/IAI.70.8.4083-4091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeurissen A, Wuyts M, Kasran A, et al. Essential role for CD40 ligand interactions in T lymphocyte-mediated modulation of the murine immune response to pneumococcal capsular polysaccharides. J Immunol. 2002;168:2773–81. doi: 10.4049/jimmunol.168.6.2773. [DOI] [PubMed] [Google Scholar]
- 25.Pan Q, Petit-Frere C, Lahdesmaki A, Gregorek H, Chrzanowska KH, Hammarstrom L. Alternative end joining during switch recombination in patients with Ataxia-Telangiectasia. Eur J Immunol. 2002;32:1300–8. doi: 10.1002/1521-4141(200205)32:5<1300::AID-IMMU1300>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Kinoshita K, Honjo T. Variable deletion and duplication at recombination junction ends: implication for staggered double-strand cleavage in class-switch recombination. Proc Natl Acad Sci USA. 2001;98:13860–5. doi: 10.1073/pnas.241524898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maple PAC, Jones CS, Wall EC, et al. Immunity to diphtheria and tetanus in England and Wales. Vaccine. 2000;19:167–73. doi: 10.1016/s0264-410x(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 28.Porro M, Saletti M, Nencioni L, Tagliaferri L, Marsili I. Immunogenic correlation between cross-reacting material (CRM197) produced by a mutant of Corynebacterium diphtheriae and diphtheria toxoid. J Infect Dis. 1980;142:716–24. doi: 10.1093/infdis/142.5.716. [DOI] [PubMed] [Google Scholar]
- 29.Gupta RK, Collier RJ, Rappuoli R, Siber GR. Differences in the immunogenicity of native and formalinized cross reacting material (CRM197) of diphtheria toxin in mice and guinea pigs and their implications on the development and control of diphtheria vaccine based on CRMs. Vaccine. 1997;15:1341–3. doi: 10.1016/s0264-410x(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 30.Shelly MA, Pichichero ME, Treanor JJ. Low baseline antibody level to diphtheria is associated with poor response to conjugated pneumococcal vaccine in adults. Scand J Infect Dis. 2001;33:542–4. doi: 10.1080/00365540110026502. [DOI] [PubMed] [Google Scholar]
- 31.Antonarakis SE. Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum Mutat. 1998;11:1–3. doi: 10.1002/(SICI)1098-1004(1998)11:1<1::AID-HUMU1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Savitsky K, Sfez S, Tagle DA, et al. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet. 1995;4:2025–32. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 33.Platzer M, Rotman G, Bauer D, et al. Ataxia-telangiectasia locus: sequence analysis of 184 kb of human genomic DNA containing the entire ATM gene. Genome Res. 1997;7:592–605. doi: 10.1101/gr.7.6.592. [DOI] [PubMed] [Google Scholar]