Abstract
Leptin produced by adipocytes increases Th1-dependent immunostimulation and autoimmune diseases. Lactobacilli are known to promote or suppress Th1 responses according to the isolates. We have investigated whether the sensitivity of Suriss Jim Lambert (SJL) mice to Th1-dependent immune diseases, when compared with C57BL/6 mice, may be modulated by selected lactobacilli able to decrease leptin release by adipocytes. White adipocytes were isolated from both C57BL/6 and SJL mice and incubated with bacterial extracts from new CBA4P and TPA3P isolates of Lactobacillus acidophilus and L. rhamnosus 9595 (LR), or with conditioned media (CM) from lactobacillus-treated macrophages. Immunomodulation induced by supernatants of treated adipocytes was determined by metabolic activity of syngenic splenic lymphocytes. Leptin produced by adipocytes, tumour necrosis factor (TNF)-α and interleukin (IL)-1β by macrophages, and IFN-γ and IL-4 by lymphocytes were quantified by enzyme-linked immunosorbent assay (ELISA) tests. Results revealed that supernatants from CBA4P- and LR-treated adipocytes decreased the metabolic activity of lymphocytes from SJL mice, whereas adipocytes incubated with CM from CBA4P-treated macrophages showed no stimulation of lymphocytes. Such effects correlated with leptin levels. Lower levels of leptin were produced by adipocytes from SJL mice in the presence of CBA4P and LR extracts. Lymphocytes from SJL mice produced low levels of IFN-γ when incubated with supernatants from CBA4P-treated cells. Such immunosuppressive effects were dependent on levels of TNF-α and IL-1β produced by lactobacillus-treated macrophages. Taken together, these results suggest that the CBA4P isolate reduces levels of leptin in SJL mice, leading to lower IFN-γ production. Therefore, the CBA4P isolate of L. acidophilus is a promising new probiotic strain for the control of Th1 inflammatory diseases.
Keywords: adipocytes, cytokines, lactobacillus, leptin, macrophages, SJL mice
Introduction
Lactobacilli are non-pathogenic Gram-positive lactic acid bacteria found in the normal gut microflora of mammals and humans. Some strains of lactobacilli are known to modulate mucosal immune functions when administrated orally [1–3]. Some of them promote Th1 responses through the release of proinflammatory cytokines such as interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-12 and IL-18, while others are immunosuppressive [4–6]. It has been shown that the attenuation or potentiation of autoimmune diseases by lactobacilli was strain-dependent and correlated with a specific lactobacillus-induced cytokine profile [7]. The SJL mouse strain, in contrast with C57BL/6 mice, is highly susceptible to experimental colitis because of a genetically programmed elevated lipopolysaccharide (LPS)-induced IL-12 response by macrophages. Such a genetic trait may explain the dominant Th1 development of autoantigen-specific T cells in this mouse strain [8,9]. A recent study indicated that leptin, a pleiotropic hormone produced by the adipose tissue, was required to induce autoimmune diseases such as experimental autoimmune encephalomyelitis in SJL mice [10]. In addition, leptin signaling deficiency decreased both humoral and cellular immune responses and experimental arthritis [11].
Leptin is an adipocytokine regulating multiple homeostatic functions such as food intake, reproductive functions and basal metabolism [12]. Leptin also modulates T cell-mediated inflammation, enhances proliferation and IL-2 production of naive T cells and promotes the Th1 phenotype by increasing interferon (IFN)-γ and inhibiting IL-4 production from CD4 cells [13]. Leptin production by adipocytes is known to be under control of proinflammatory cytokines IL-1β and TNF-α, but their precise role in the leptin secretion remains unclear [14–17]. In C57BL/6 mice, administration of TNF-α or IL-1 rapidly increased leptin expression in fat [18]. The potent involvement of leptin in the induction of intestinal inflammatory diseases such as Crohn's disease is suggested by abnormalities of fat in the mesentery, including adipose tissue hypertrophy and fat wrapping [19,20]. Furthermore, the inhibition of leptin production in retroperitoneal and mesenteric adipose tissues, has been shown to markedly reduce colonic inflammation [21,22]. This suggests that leptin, under the control of genetic factors, may contribute to intestinal inflammation processes.
The protective role of lactobacilli in intestinal inflammatory diseases has been suggested recently but the efficiency of lactobacillus strains to decrease the inflammation has not been demonstrated clearly. We have recently isolated novel Lactobacillus acidophilus type 2 strains, the CBA4P and TPA3P isolates, which suppress CD4+ cells and stimulate B cells in modulating pro- or anti-inflammatory cytokines according to mouse strains (manuscript submitted). Consequently, the levels of proinflammatory cytokines resulting from inflammatory diseases may play a crucial role in the modulation of leptin secretion and could, in turn, worsen the inflammatory process. We thus hypothesized that the sensitivity of SJL mice to Th1-dependent immune diseases may be modulated by selected lactobacillus strains in decreasing leptin release by adipocytes in the presence of proinflammatory macrophagic cytokines.
We report here that one novel L. acidophilus isolate decreased both leptin production and leptin-mediated immunostimulation by SJL adipocytes, leading to a lower Th1 response. The last effect is mediated by decreases of lactobacillus-induced IL-1β and TNF-α by macrophages.
Materials and methods
Mice
Female SJL and C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Before and during experiments, the animals were housed in a sterile atmosphere (Forma Scientific, Marietta, OH, USA). Female mice between 8 and 12 weeks of age were used in all experiments. Mice were killed by CO2 anoxia and the spleen, parametrial fat pads and peritoneal macrophages were collected.
Isolation of lactobacilli and preparation of crude extracts of lactobacilli cell walls
The CBA4P and TPA3P isolates of L. acidophilus type 2 were obtained from avian colon and caecum, respectively, and their biological properties were characterized (manuscript submitted). Lactobacillus isolates differ from the L. acidophilus type 2 reference strain by specific metabolic characteristics, such as galactose, lactose and cellobiose for the CBA4P isolate and lactose, trehalose and d-mannose for the TPA3P isolate. Both lactobacilli isolates were acid pH tolerant and grew at pH between 1 and 4. The CBA4P isolate was the most resistant to low pH levels. The L. rhamnosus 9595 was purchased from the American Type Culture Collection (USA) and used as lactobacillus control in the experiments.
Lactobacillus strains were grown anaerobically in MRS (Man, Rogosa and Sharpe) medium for 24 h. Lactobacilli were passaged twice before being used and the concentration was adjusted to 109 cfu/ml (corresponding to an optical density of 0·96 at 597 nm) in 10 ml of phosphate buffered saline (PBS). Lactobacilli were sonicated 15 min at 120 kW and centrifuged at 2000 g for 10 min. Supernatants containing crude cell wall extracts were sterilized using 0·45 µm filter and kept at −20°C.
Cells
Peritoneal macrophages (106) were obtained by peritoneal washings using RPMI-1640 (Gibco Laboratories, Grand Island, NY, USA) and enriched by adherence to plastic. Peritoneal exudate cells (106) were allowed to adhere for 2 h and then non-adherent cells were washed away. Spleens were teased apart using a 70 µm cell strainer (Falcon Scientific Co., Montréal, Québec, Canada) into RPMI-1640 medium supplemented with l-glutamine (2 mM), antibiotics [penicillin (100 U/ml), streptomycin (100 mg/ml)], 2-mercaptoethanol (ME 10−5 M) (Gibco Laboratories) and 20% fetal calf serum (FCS) (HyClone, Professional Diagnostic, Edmonton, Alta, Canada).
Total lymphocytes were enriched by a passage on a Lymphoprep gradient (Cedarlane, Ontario, Canada). The cell suspension was then washed and resuspended in RPMI-1640 at a final concentration of 106 cells/ml. All cell suspensions were counted electronically using a Coulter Counter (Coulter Electronics, Hialeah, FL, USA). Peritoneal macrophages and splenic lymphoid cells were cultured in RPMI-1640 medium supplemented with l-glutamine, antibiotics, 2-mercaptoethanol and 20% FCS.
Gonadal fat pads were minced into 1–2 mm pieces in medium 199 (Gibco Laboratories). Adipocytes were dissociated by a collagenase treatment (10 mg/ml; Boehringer Mannheim, Laval, Canada) for 45 min in a 37°C shaking water bath (120 rpm). The suspension was then filtered through a nylon mesh screen and washed three times with medium 199 to remove any residual collagenase. Adipocytes were identified morphologically and counted using a haemacytometer, adjusted to 106 cells/ml and cultured in medium 199 supplemented with l-glutamine (2 m M), antibiotics [penicillin (100 U/ml), streptomycin (100 mg/ml)] and 20% FCS.
Treatment of macrophages or adipocytes with lactobacillus extracts
Peritoneal macrophages were incubated with lactobacilli extracts (25 µl) and LPS from Escherichia coli 026:B6 (0·5 µg/ml) or peptidoglycan (PEP) from Staphylococcus aureus (10 µg/ml) (Sigma-Aldrich, Oakville, Ontario, Canada) as positive controls in a 96-well microtitre plate at a concentration of 1 × 106 cells/ml. The plates were then incubated for 24 h at 37°C in a 5% CO2 atmosphere. Adipocyte suspensions were incubated with lactobacilli extracts (25 µl) or conditioned media (CM) from lactobacilli-treated macrophages for 24 h, according to the experiments. Supernatants from adipocytes were collected and stored at −80°C. The syngeneic lymphocytes were then incubated with adipocyte supernatants for 24 h and their metabolic activities were recorded. In some experiments, supernatants from lymphocytes were collected and stored at −80°C for cytokine evaluation. All experiments were conducted in triplicate.
Metabolic activity of lymphocytes
The metabolic activity of lymphocytes was evaluated with a colourimetric cell proliferation assay (Promega, Madison, WI, USA). Cell activity was determined by conversion of the tetrazolium (MTS) into a soluble formazan product by dehydrogenases found in active cells. Lymphocytes were seeded at 105 per well (in 100 µl volume) in flat-bottomed microtitre plates in RPMI-1640 containing 20% FCS, Mercapto ethanol (ME) and antibiotics. Cells were incubated with adipocyte supernatants (100 µl) for 24 h at 37°C with 5% CO2. After 20 h of incubation, 3- (4,5-dimethylthiazol-2-y1)-5-(3-carboxymethoxyphenyl)-2- (4-sulphophenyl)-2H tetrazolium (MTS) and phenazine methosulphate (PMS) (Promega, Madison, WI, USA) were added and the cells were incubated further for up to 24 h. The optical density was then recorded at 490 nm using an enzyme-linked immunosorbent assay (ELISA) reader for viable cells (Molecular Devices, Sunnyvale, CA, USA). In some experiments, supernatants from adipocytes were incubated previously with 1 µg polyclonal antileptin antibodies for 30 min at 37°C (Sigma Chemicals). The appropriate dilution for optimal neutralization was determined by ELISA using a recombinant mouse leptin.
Cytokine assays
Levels of TNF-α and IL-1β in CM from macrophages, leptin in adipocyte supernatants and IL-4 and INF-γ in lymphocyte supernatants were quantified by ELISA. Levels of TNF-α, INF-γ and IL-4 were evaluated using OptEIA kits (Pharmingen, Toronto, Ontario, Canada), IL-1β content was measured using a CytoSet kit (BioSource, Nivelles, Belgium) and levels of leptin were determined with the Quantikine M kit (R&D, Hornby, Ontario, Canada) according to protocols provided by the manufacturer. In some experiments, levels of leptin were also quantified following neutralization of IL-1β and TNF-α in macrophage supernatants with specific monoclonal antibodies (Peprotech Canada, Ottawa, Ontario, Canada). The ND50 of the biological activity of anti-IL-1β was 50 pg/ml and 0·25 ng/ml for anti-TNF-α. The optimal concentrations required for neutralization (150 ng/ml for anti-IL-1β and 0·1 µg/ml for anti-TNF-α) were determined experimentally.
Statistical analysis
OD values from metabolic activity and cytokine ELISA tests of treated cells were compared to untreated cells using Student's t-test. Values of P ≤ 0·05 were considered significant.
Results
Lymphocyte activity induced by lactobacilli-treated adipocyte supernatants
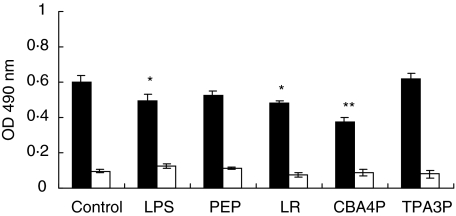
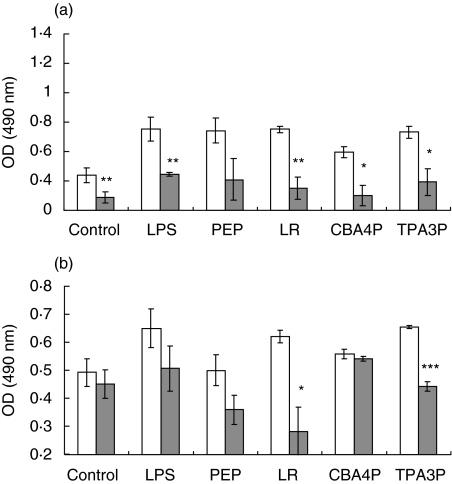
To investigate if the lactobacillus isolates may induce the production of immunomodulating factors by adipocytes, the parametrial adipocytes from SJL and C57BL/6 mice were isolated and incubated with bacterial extracts from the CBA4P and TPA3P isolates, L. rhamnosus (LR), LPS and PEP for 24 h. Supernatants from treated adipocytes were added to syngenic splenic lymphocytes for 24 h. Metabolic activity of the lymphocytes was evaluated by the mitochondrial dehydrogenase activity (MTS/PMS test). As shown in Fig. 1, supernatants from LR- and CBA4P-treated adipocytes decreased the metabolic activity of lymphocytes from SJL mice (P ≤ 0·05 to P ≤ 0·001) but not those of lymphocyte from C57BL/6 mice. Supernatant from LPS-treated adipocytes slightly decreased the activity of SJL-derived lymphocytes (P ≤ 0·05), while LPS- and PEP-treated adipocyte supernatants from C57BL/6 mice did not induce any lymphocyte stimulation. However, the metabolic activity of untreated and treated lymphocytes from SJL mice remained higher than that from C57BL/6 mice (P ≤ 0·05).
Fig. 1.
Metabolic activity of lymphocytes from SJL (▪) and C57BL/6 (□) mice induced by adipocyte supernatants treated with lipopolysaccharide (LPS), peptidoglycan (PEP) and lactobacillus extracts. Adipocytes were treated for 24 h and supernatants were added to syngenic lymphocytes for 24 h. Metabolic activity was measured using the mitochondrial dehydrogenase activity (MTS/PMS) test. Experiments were conducted in triplicate. These results are representative of three similar experiments. *P ≤ 0·05; **P ≤ 0·001.
Leptin release from lactobacillus isolates-treated adipocytes of SJL and C57BL/6 mice
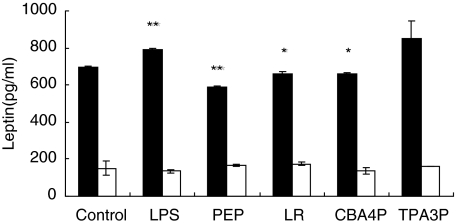
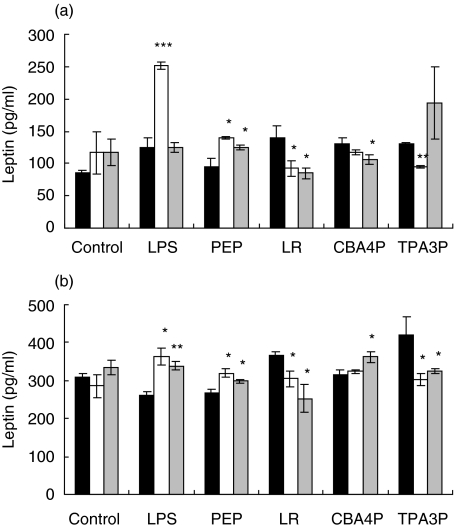
It is postulated that the decrease in lymphocyte activity induced by supernatants from CBA4P- and LR-treated adipocytes is related to a decrease in leptin production. To verify this hypothesis, leptin levels in supernatants from the lactobacilli-, LPS- and PEP-treated adipocytes from both mouse strains were assayed with an ELISA test. As shown in Fig. 2, only CBA4P, LR and PEP decreased leptin levels in SJL-derived adipocytes (P ≤ 0·05 to P ≤ 0·001), while LPS increased leptin release (P ≤ 0·01). The TPA3P isolate had no effect on leptin release. In contrast, low levels of leptin were released by both untreated and treated adipocytes from C57BL/6 mice.
Fig. 2.
Leptin release from adipocytes incubated with lipopolysaccharide (LPS), peptidoglycan (PEP) and lactobacilli from SJL (▪) and C57BL/6 (□) mice. Adipocytes were treated for 24 h with bacterial extracts and leptin levels were quantified using an enzyme-linked immunosorbent assay (ELISA) test. Experiments were conducted in triplicate. These results are representative of two similar experiments. *P ≤ 0·05; **P ≤ 0·01.
Effects of CM from lactobacillus-treated macrophages of SJL and C57BL/6 mice on leptin released by adipocytes
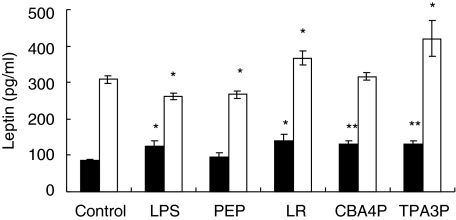
Leptin production by adipocytes may be under the control of lactobacillus-induced macrophagic proinflammatory cytokines. This hypothesis has been verified in evaluating levels of leptin release by adipocytes incubated with CM from macrophages treated with the different lactobacilli, LPS and PEP. As shown in Fig. 3, leptin production increased slightly in adipocytes of SJL mice treated with CM from LR-, CBA4P-, TPA3P- or LPS-treated macrophages (P ≤ 0·05 to P ≤ 0·01). Leptin secretion increased in adipocytes incubated with CM from TPA3P- and LR-treated macrophages of C57BL/6 mice (P ≤ 0·001; P ≤ 0·05). In contrast, LPS- and PEP-treated macrophage CM decreased leptin levels by adipocytes from C57BL/6 mice. Also, the basal level of leptin production by adipocytes incubated with CM from untreated macrophages was higher in cells from C57BL/6 than from SJL mice. Interestingly, the basal level of leptin produced by SJL or C57BL/6 adipocytes, respectively, decreased or increased when incubated with CM from macrophages (in comparison with results shown in Fig. 2).
Fig. 3.
Leptin released from adipocytes incubated with CM from lipopolysaccharide (LPS)-, peptidoglycan (PEP)- and lactobacilli- treated macrophages from SJL (▪) and C57BL/6 (□) mice. Macrophages were treated for 24 h with bacterial extracts and adipocytes were incubated with macrophage supernatants for 24 h. Leptin levels were quantified using an enzyme-linked immunosorbent assay (ELISA) test. Experiments were conducted in triplicate. These results are representative of two similar experiments. *P ≤ 0·05; **P ≤ 0·01.
Effects of CM from lactobacillus-treated macrophages on adipocytes-dependent activation of lymphocytes
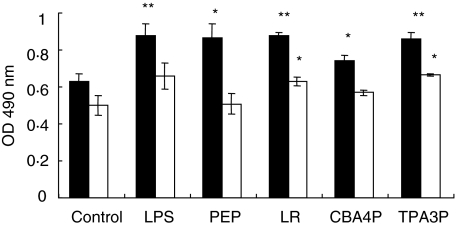
To evaluate the effects of leptin produced by adipocytes incubated with CM from lactobacilli-treated macrophages on metabolic activity of lymphocytes, splenic lymphocytes from SJL and C57BL/6 mice were cultured in the presence of supernatants from adipocytes incubated previously with CM from CBA4P-, TPA3P-, LR-, LPS- and PEP-treated macrophages and then their metabolic activity was evaluated, as described above. As shown in Fig. 4, supernatant from adipocytes of SJL mice incubated with CM from CBA4P-treated macrophages induced a lower activity of lymphocytes than other bacterial extracts (P ≤ 0·05). However, SJL-derived adipocytes incubated with CM from the lactobacillus extracts-, LPS- and PEP-treated macrophages stimulated lymphoid cell activity (P ≤ 0·05 to P ≤ 0·01). Adipocytes stimulated with CM from LR- and TPA3P-treated macrophages induced comparable lymphocyte stimulation levels to those from LPS- and PEP-treated macrophages. Lymphocytes from C57BL/6 mice were stimulated only by supernatants from adipocytes incubated CM from LR- and TPA3P-treated C57BL/6 macrophages (P ≤ 0·05) but not by the CBA4P isolate.
Fig. 4.
Metabolic activity of lymphocytes from SJL (▪) and C57BL/6 (□) mice induced by adipocyte supernatants incubated with conditioned media (CM) from lipopolysaccharide (LPS)-, peptidoglycan (PEP)- and lactobacilli-treated macrophages. Macrophages were exposed for 24 h to bacterial extracts and adipocytes were incubated with macrophage supernatants for 24 h. Metabolic activity of lymphocytes treated with adipocyte supernatants was determined by the mitochondrial dehydrogenase activity (MTS/PMS) test. Experiments were conducted in triplicate. These results are representative of three similar experiments. *P ≤ 0·05; **P ≤ 0·01.
Modulation of IFN-γ production from lymphoid cells by supernatants from lactobacillus-treated adipocytes from SJL and C57BL/6 mice
The stimulation of lymphocytes by leptin may favour the activation of Th1 rather than Th2 lymphocytes. To verify if lactobacilli-treated adipocytes may alter the Th1/Th2 imbalance, IFN-γ and IL-4 produced by splenic lymphocytes from both mouse strains treated with CM from lactobacilli-, LPS- and PEP-treated macrophages from both mouse strains were quantified. Results shown in Table 1 indicate that CBA4P isolate weakly increased IFN-γ production by lymphocytes from both mouse strains (P ≤ 0·05). LR and TPA3P isolates increased the IFN-γ production by lymphoid cells isolated from C57BL/6 and SJL mice (P ≤ 0·05–0·001). However, IFN-γ levels remained higher in lymphocytes from SJL mice than those from C57BL/6 mice. No IL-4 was detected in all treated lymphoid cells.
Table 1.
Interferon (IFN)-γ and interleukin (IL)-4 release by SJL and C57BL/6 lymphocytes in presence of supernatants from adipocytes incubated with conditioned media (CM) from lactobacillus extract-, peptidoglycan (PEP)- and lipopolysaccharide (LPS)-treated macrophages.
| Mouse strain | Treatment1 | IFN-γ (pg/ml) | IL-4 (pg/ml) |
|---|---|---|---|
| SJL | Control | 115 ± 11·0 | <7·8 |
| LPS | 349 ± 8·4** | <7·8 | |
| PEP | 232 ± 5·2** | <7·8 | |
| L. rhamnosus (LR) | 307 ± 53·0** | <7·8 | |
| CBA4P | 171 ± 9·9* | <7·8 | |
| TPA3P | 240 ± 14·0** | <7·8 | |
| C57BL/6 | Control | <31·3 | <7·8 |
| LPS | <31·3 | <7·8 | |
| PEP | 254 ± 9*** | <7·8 | |
| L. rhamnosus (LR) | 169 ± 19*** | <7·8 | |
| CBA4P | 96 ± 47* | <7·8 | |
| TPA3P | 120 ± 62* | <7·8 |
Peritoneal macrophages were treated with lactobacilli extracts for 24 h at 37°C and the supernatants were added to syngeneic adipocytes for 24 h. Splenic lymphocytes were incubated with the adipocyte supernatants. Cytokine levels in lymphocyte supernatants were quantified by enzyme-linked immunosorbent assay (ELISA) tests. Experiments were conducted in triplicate. These results are representative of two similar experiments.
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
Neutralization of leptin in supernatants from lactobacilli-treated adipocytes from SJL and C57BL/6 mice
To verify the role of leptin in lymphocyte activity, lactobacilli-treated adipocyte supernatants were incubated with antileptin antibodies. The neutralization of leptin in supernatants from adipocytes of SJL mice incubated with CM from PEP-, LPS- and all lactobacillus-treated macrophage supernatants decreased the lymphocyte proliferation (P ≤ 0·05 to P ≤ 0·01) (Fig. 5a). However, leptin neutralization decreased C57BL/6-derived lymphocyte stimulation by supernatants from adipocytes incubated only with CM from LR- and TPA3P-treated macrophages (P ≤ 0·05 to P ≤ 0·001) (Fig. 5b). No decrease in metabolic activity of C57BL/6 lymphocytes was observed in antileptin-treated CM from CBA4P-treated macrophages.
Fig. 5.
Metabolic activity of lymphocytes from SJL (a) and C57BL/6 (b) mice induced by untreated (□) or antileptin antibody-treated ( ) adipocyte supernatants previously incubated with conditioned media (CM) from lipopolysaccharide (LPS)-, peptidoglycan (PEP)- and lactobacillus extracts-treated macrophages. Lymphocytes were incubated with antileptin-treated supernatants for 24 h and metabolic activity was measured by the mitochondrial dehydrogenase activity (MTS/PMS) test. Experiments were conducted in triplicate. These results are representative of two similar experiments. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
) adipocyte supernatants previously incubated with conditioned media (CM) from lipopolysaccharide (LPS)-, peptidoglycan (PEP)- and lactobacillus extracts-treated macrophages. Lymphocytes were incubated with antileptin-treated supernatants for 24 h and metabolic activity was measured by the mitochondrial dehydrogenase activity (MTS/PMS) test. Experiments were conducted in triplicate. These results are representative of two similar experiments. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
TNF-α and IL-1β release by lactobacillus extracts-treated macrophages from SJL and C57BL/6 mice
It is known that TNF-α and IL-1β play a major role in the modulation of leptin release by adipocytes [18]. The decrease of leptin production by adipocytes incubated with CM from CBA4P isolate-treated macrophages may result from a decrease of proinflammatory cytokines induced by the cell wall extracts. To verify this hypothesis, peritoneal macrophages from SJL and C57BL/6 mice were stimulated with the various lactobacillus extracts, LPS and PEP, for 24 h. IL-1β and TNF-α levels were then quantified by ELISA tests. As shown in Table 2, TNF-α reached similar levels in supernatants from LR-, TPA3P-, CBA4P- and LPS-treated macrophages from SJL mice whereas PEP led to a higher TNF-α release (P ≤ 0·01 to P ≤ 0·001). PEP was also the strongest inducer of macrophagic IL-1β (P ≤ 0·001), while CBA4P and LPS induced intermediate levels of IL-1β (P ≤ 0·01; P ≤ 0·001), and LR induced lower levels of IL-1β by macrophages from SJL mice (P ≤ 0·001; P ≤ 0·01). In lactobacillus-treated macrophages from C57BL/6 mice, however, the CBA4P isolate induced a low level of TNF-α (P ≤ 0·01) while other treatments, especially PEP and TPA3P, increased TNF-α production (P ≤ 0·001). CBA4P-, TPA3P-, LR- and LPS-treated macrophages stimulated low IL-1β levels (P ≤ 0·01–0·001) while IL-1β was highly produced by PEP-treated macrophages (P ≤ 0·001).
Table 2.
Tumour necrosis factor (TNF)-α and interleukin (IL)-1β release by SJL and C57BL/6 macrophages treated with various lactobacillus extracts and lipopolysaccharide (LPS) and peptidoglycan (PEP).
| Mouse strain | Treatment 1 | TNF-α (pg/ml) | IL-1β (pg/ml) |
|---|---|---|---|
| SJL | Control | 144·0 ± 18·0 | <7·8 |
| LPS | 323·4 ± 20·1** | 76·9 ± 3·8*** | |
| PEP | 659·3 ± 46·6*** | 268·1 ± 18·7*** | |
| L. rhamnosus (LR) | 375·4 ± 13·8** | 28·9 ± 4·3** | |
| CBA4P | 366·2 ± 16·4** | 71·4 ± 5·3** | |
| TPA3P | 323·9 ± 2** | 33·7 ± 0·4*** | |
| C57BL/6 | Control | 156·1 ± 1·2 | <7·8 |
| LPS | 605·7 ± 4·9*** | 67·4 ± 1·7*** | |
| PEP | 538·3 ± 9·2*** | 272·2 ± 16·9*** | |
| L. rhamnosus (LR) | 430·6 ± 46*** | 38·5 ± 2·0*** | |
| CBA4P | 341·4 ± 39·2** | 26·9 ± 2·1** | |
| TPA3P | 599·8 ± 40·9*** | 28·6 ± 0·4*** |
Peritoneal macrophages were treated with lactobacilli extracts for 24 h at 37°C. Cytokine levels in macrophage supernatants were quantified by enzyme-linked immunosorbent assay (ELISA) tests. Experiments were conducted in triplicate. These results are representative of two similar experiments.
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
To verify whether the TNF-α and IL-1β released from lactobacillus-treated macrophages are involved in leptin production by adipocytes, supernatants from lactobacillus extracts-, LPS- and PEP-treated macrophages were incubated with anti-TNF-α and anti-IL-1β antibodies and deposited on syngeneic adipocytes. Leptin levels in adipocyte supernatants were then determined by an ELISA test. As shown in Fig. 6a, the neutralization of TNF-α and IL-1β in CM from control macrophages from SJL mice had no effect on leptin production because these cells were unstimulated and produced low or no levels of TNF-α and IL-1β. TNF-α neutralization in CBA4P-treated macrophage supernatants decreased leptin secretion (P ≤ 0·05), while neutralization of IL-1β in TPA3P-treated macrophage supernatants slightly decreased leptin secretion by adipocytes from SJL mice (P ≤ 0·01) Neutralization of both TNF-α and IL-1β in LR-treated macrophage supernatants decreased leptin secretion by adipocytes. However, neutralization of both cytokines in PEP-treated macrophage supernatants increased the leptin produced by adipocytes (P ≤ 0·05), while neutralization of IL-1β in LPS-treated macrophage CM increased the leptin levels (P ≤ 0·001). In C57BL/6 mice, inhibition of TNF-α activity in CBA4P-treated macrophage supernatant increased slightly leptin production by adipocytes (P ≤ 0·05) (Fig. 6b). Cytokine neutralization with both antibodies decreased leptin production (P ≤ 0·05) in LR- and TPA3P-treated macrophage supernatants while neutralization of TNF-α and IL-1β in LPS- and PEP-treated macrophage CM increased leptin production (P ≤ 0·05 to P ≤ 0·01).
Fig. 6.
Effects of untreated (▪) and anti-interleukin (IL)-1β (□) and anti-tumour necrosis factor (TNF)-α ( ) antibody-treated CM from bacterial extracts-stimulated macrophages in the leptin released by adipocytes from SJL (a) and C57BL/6 (b) mice. Adipocytes were incubated with various bacterial extracts-treated macrophage supernatants, treated previously with monoclonal IL-1β and TNF-α, for 24 h. Leptin levels from adipocyte supernatants were evaluated by an enzyme-linked immunosorbent assay (ELISA) test. Experiments were conducted in triplicate. These results are representative of two similar experiments. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
) antibody-treated CM from bacterial extracts-stimulated macrophages in the leptin released by adipocytes from SJL (a) and C57BL/6 (b) mice. Adipocytes were incubated with various bacterial extracts-treated macrophage supernatants, treated previously with monoclonal IL-1β and TNF-α, for 24 h. Leptin levels from adipocyte supernatants were evaluated by an enzyme-linked immunosorbent assay (ELISA) test. Experiments were conducted in triplicate. These results are representative of two similar experiments. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
Discussion
In this study, we have reported that the L. acidophilus CBA4P isolate is more potent to decrease the leptin-dependent immunostimulating activity of adipocytes from SJL mice than the TPA3P isolate or L. rhamnosus 9595 reference strain. The lactobacillus-mediated decrease of leptin is modulated by lower levels of macrophagic IL-1β and TNF-α in SJL mice. Therefore, the CBA4P lactobacillus isolate could contribute to control Th1-mediated inflammation diseases by decreasing the adipocytic leptin-induced IFN-γ. We have shown here that lactobacillus strains decreased the metabolic activity of lymphocytes. Supernatants from L. acidophilus CBA4P- and TPA3P- and L. rhamnosus-treated adipocytes from SJL mice led to a slight but significant decrease in lymphocyte activation. This decrease was due, at least, to the decrease of leptin by lactobacilli-treated adipocytes as leptin was reported to promote the activation of naive T cells [9]. The lower metabolic activity of lymphocytes from C57BL/6 may thus reflect no or low levels of leptin produced by adipocytes. Effectively, leptin was highly produced by SJL adipocytes rather than by cells from C57BL/6 mice when incubated directly with lactobacillus extracts. The role of leptin in the lymphocyte stimulation was confirmed by the use of antileptin antibodies. Lymphocyte activity decreased after neutralization of leptin in supernatants from adipocytes treated with CBA4P, TPA3P and L. rhamnosus.
On the other hand, CM from untreated macrophages or lactobacillus-treated macrophages increased leptin production by adipocytes from C57BL/6 mice while it decreased in cells from SJL mice. CM from CBA4P-treated macrophages of SJL mice favoured low leptin production by adipocytes. However, CM from TPA3P- and L. rhamnosus-treated macrophages increased the leptin production by adipocytes from both mouse strains. This suggests that lactobacilli-induced macrophagic cytokines may modulate the release of leptin according to both bacterial isolate and mouse strains [23]. Maassen et al. [24] have shown previously that each lactobacillus strain induced specific cytokine profiles in macrophages. Roles of IL-1β and TNF-α in the control of leptin production by adipocytes has already been demonstrated [16,18]. Our results indicate that lactobacilli induced lower productions of TNF-α in both mouse strains but TPA3P and CBA4P isolates were the weakest inducers in SJL and C57BL/6, respectively. CBA4P induced significant production levels of IL-1β, while TPA3P- and L. rhamnosus-treated macrophage supernatants contained smaller amounts. However, lactic acid bacteria are known to be good inducers of proinflammatory cytokines such as IL-6, IL-12 and TNF-α[25,26], while our isolates and L. rhamnosus are proved to be weak inducers.
Low levels in leptin production by adipocytes were related to low levels of macrophagic inflammatory cytokines induced by lactobacilli. Indeed, levels of leptin produced by SJL adipocytes when incubated with treated macrophage supernatants were lower than those from adipocytes incubated directly with the lactobacilli. These observations may be explained by the effects of TNF-α and IL-1β on the production of leptin by adipocytes [16,18]. Effectively, low increases in leptin release by adipocytes incubated with CM from the LPS-, LR-, CBA4P- and TPA3P-treated macrophages could reflect low levels of TNF-α and IL-1β. The role of TNF-α and IL-1β in leptin release by adipocytes from SJL was confirmed by the decrease of leptin in the presence of anti-cytokine antibodies. Low leptin production induced by the CBA4P-treated macrophage supernatants from SJL mice may thus reflect low levels of macrophagic TNF-α and/or IL-1β.
However, lactobacilli-treated supernatants from macrophages of C57BL/6 mice increased leptin production by C57BL/6 adipocytes. This effect may be due to higher levels of proinflammatory cytokines. Unfortunately, similar levels of TNF-α and IL-1β were produced by macrophages from C57BL/6 or SJL mice, suggesting that leptin produced by adipocytes from C57BL/6 mice incubated with CM from L. rhamnosus-treated macrophages are under control of other macrophagic cytokines or reflects a mouse strain-dependent sensitivity of adipocytes to these cytokines. The role of proinflammatory cytokines on leptin release still remains unclear, but recent studies have shown a mechanism controlled in a dose-dependent manner. It was proposed that low doses of TNF-α increased leptin production while high levels showed inhibitory effects [27–29]. In our work, PEP, which induced higher levels of macrophagic IL-1β and TNF-α decreased leptin production, supporting the inhibition of leptin release by adipocytes by high cytokine levels. In addition, neutralization of these cytokines with antibodies increased leptin release by adipocytes, suggesting that low levels or the absence of inflammatory cytokines may favour production of leptin.
Our findings provide in vitro evidence that our lactobacillus isolates could reduce Th1-mediated mucosal inflammation by reducing leptin release. According to our results, we speculate that the heightened susceptibility of SJL mice to autoimmune diseases might be the result of high levels of leptin production by their adipocytes. However, the L. acidophilus CBA4P rather than TPA3P isolates may be useful in decreasing leptin production, lymphocyte stimulation and IFN-γ production by lymphocytes from SJL mice. The inhibition of leptin may thus be a new way by which some lactobacilli strains may reduce inflammatory diseases.
Further work is in progress to evaluate the in vivo effects of our lactobacillus isolates on leptin secretion and Th1-dependent inflammatory diseases in SJL mice.
Acknowledgments
C. Bleau was supported by a fellowship from the ‘Fond de la recherche sur la nature et la technologie’ from the Quebec government.
References
- 1.Pestka JJ, Ha CL, Warner RW, Lee JH, Ustunol Z. Effects of ingestion of yogurts containing Bifidobacterium and Lactobacillus acidophilus on spleen and Peyer's patch lymphocyte populations in the mouse. J Food Prot. 2001;64:392–5. doi: 10.4315/0362-028x-64.3.392. [DOI] [PubMed] [Google Scholar]
- 2.Perdigon G, Alvarez S, Medina M, Vintini E, Roux E. Influence of the oral administration of lactic acid bacteria on IgA producing cells associated to bronchus. Int J Immunother Pharmacol. 1999;12:97–102. [PubMed] [Google Scholar]
- 3.Vitini E, Alvarez S, Medina M, Medici M, De Budeguer MV, Perdigon G. Gut mucosal immunostimulation by lactic acid bacteria. Biocell. 2000;24:223–32. [PubMed] [Google Scholar]
- 4.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and Streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:3965–70. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while Gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solis-Pereyra B, Aattouri N, Lemonnier D. Role of food in the stimulation of cytokine production. Am J Clin Nutr. 1997;66:S521–5. doi: 10.1093/ajcn/66.2.421S. [DOI] [PubMed] [Google Scholar]
- 7.Maassen CB, Van Holten-Neelen JC, Balk F, et al. Orally administered Lactobacillus strains differentially affect the direction and efficacy of the immune response. Vet Q. 1998;20:S81–3. [PubMed] [Google Scholar]
- 8.Alleva DG, Johnson EB, Wilson J, Beller DI, Conlon PJ. SJL and NOD macrophages are uniquely characterized by genetically programmed, elevated expression of the IL-12 (p40) gene, suggesting a conserved pathway for the induction of organ-specific autoimmunity. J Leuk Biol. 2001;69:440–8. [PubMed] [Google Scholar]
- 9.Bouma G, Kaushiva A, Strober W. Experimental murine colitis is regulated by two genetic loci, including one on chromosome 11 that regulates IL-12 responses. Gastroenterology. 2002;123:54–65. doi: 10.1053/gast.2002.34752. [DOI] [PubMed] [Google Scholar]
- 10.Matarese G, Di Giacomo A, Sanna V, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–16. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 11.Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–82. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 12.Friedman JM, Haalas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 13.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T cell immune response and reverses starvation-induced immunosupression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 14.Finck BN, Johnson RW. Tumor necrosis factor (TNF)-alpha induces leptin production through the p55 TNF receptor. Am J Physiol. 2000;278:R537–43. doi: 10.1152/ajpregu.2000.278.2.R537. [DOI] [PubMed] [Google Scholar]
- 15.Grunfeld C, Zhao C, Fuller J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product in hamsters: a role for leptin in the anorexia of infection. J Clin Invest. 1996;97:2152–7. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawcett RL, Waechter AS, Williams LB, et al. Tumor necrosis factor-alpha inhibits leptin production in subcutaneous and omental adipocytes from morbidly obese humans. J Clin Endocrinol Metab. 2000;85:530–5. doi: 10.1210/jcem.85.2.6359. [DOI] [PubMed] [Google Scholar]
- 17.Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Penicaud L, Casteilla L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw. 2000;11:634–9. [PubMed] [Google Scholar]
- 18.Sarraf P, Frederich RC, Turner EM, et al. Multiples cytokines and acute inflammation raise mouse leptin levels: potentiel role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pond CM. Long-term changes in adipose tissue in human disease. Proc Nutr Soc. 2001;60:365–74. doi: 10.1079/pns200198. [DOI] [PubMed] [Google Scholar]
- 20.Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 21.Barbier M, Attoub S, Joubert M, et al. Proinflammatory role of leptin in experimental colitis in rats benefit of cholecystokinin-B antagonist and beta3-agonist. Life Sci. 2001;69:567–80. doi: 10.1016/s0024-3205(01)01148-1. [DOI] [PubMed] [Google Scholar]
- 22.Villafuerte BC, Fine JB, Bai Y, Zhao W, Fleming S, Digirolamo M. Expressions of leptin and insulin-like growth factor-I are highly correlated and region-specific in adipose tissue of growing rats. Obes Res. 2000;8:646–55. doi: 10.1038/oby.2000.83. [DOI] [PubMed] [Google Scholar]
- 23.Sewter CP, Digby JE, Blows F, Prins J, O'Rahilly S. Regulation of tumor necrosis factor-alpha release from human adipose tissue in vitro. J Endocrinol. 1999;163:33–8. doi: 10.1677/joe.0.1630033. [DOI] [PubMed] [Google Scholar]
- 24.Maassen CB, Van Holten-Neelen JC, Balk F, et al. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine. 2000;22:2613–23. doi: 10.1016/s0264-410x(99)00378-3. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen M, Vuopio-Varlika J, Varlika K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced ba lactic acid bacteria. Infect Immun. 1996;64:5403–5. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessle C, Hanson LA, Wold AE. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–82. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang HH, Kumar S, Barnett AH, Eggo MC. Tumour necrosis factor-alpha exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol. 2000;159:79–88. doi: 10.1016/s0303-7207(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 28.Patel NG, Holder JC, Smith SA, Kumar S, Eggo MC. Differential regulation of lipogenesis and leptin production by independent signaling pathways and rosiglitazone during human adipocyte differentiation. Diabetes. 2003;52:43–50. doi: 10.2337/diabetes.52.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking Il−1 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002;3:59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- 30.Fantuzzi G, Lehr HA, Dinarello CA, Siegmund B. Leptin deficient (ob/ob) mice are resistant to experimental colitis: role of Th1 cytokines. FASEB J. 2001;15:A1067. [Google Scholar]
- 31.Powrie F, Leach MW, Mause S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 32.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 33.Sitaraman S, Liu X, Charrier L, et al. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004;18:696–8. doi: 10.1096/fj.03-0422fje. [DOI] [PubMed] [Google Scholar]
- 34.Madsen KL, Doyle JS, Tavernini MM, Jewell LD, Rennie RP, Fedorak RN. Antibiotic therapy attenuates colitis in interleukin-10 gene-deficient mice. Gastroenterology. 2000;118:1094–105. doi: 10.1016/s0016-5085(00)70362-3. [DOI] [PubMed] [Google Scholar]
- 35.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:S5–11. [PubMed] [Google Scholar]
- 36.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in IL-10 gene-deficient mice. Gastroenterology. 1999;116:1107–14. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 37.Schultz M, Sartor RB. Probiotics and inflammatory bowel diseases. Am J Gastroenterol. 2000;95:S19–21. doi: 10.1016/s0002-9270(99)00812-6. [DOI] [PubMed] [Google Scholar]