Abstract
One prominent feature of patients with the autosomal recessive disease ataxia telangiectasia (AT) is somatic growth retardation. Due to their essential roles in development we examined levels of insulin-like growth factor-I (IGF-I) as well as its main binding protein (IGFBP-3) in a group of AT patients. Growth status of 19 patients was assessed by body mass index (BMI) and nutritional protocols. As suspected, BMI was low in AT patients despite adequate nutrition. Serum levels of IGF-I were found to be below the 3rd percentile in 9 (56%) out of 16 patients and of IGFBP-3 in 13 (81%) out of 16 patients. Our observations demonstrate that IGF-I and IGFBP-3 levels reflect the impaired growth status in patients with AT.
Keywords: Ataxia telangiectasia (AT), IGF-I, IGFBP-3, body mass index (BMI)
Introduction
Ataxia telangiectasia (AT) is an autosomal recessive genetic disorder that is characterized by neurodegeneration with progressive ataxia, variable immunodeficiency and an increased predisposition to cancer. In addition, etiopathology of AT involves endocrinological abnormalities, such as insulin resistance, glucose intolerance and growth retardation [1]. Patients exhibit deficiencies in somatic growth and weight gain starting in early childhood and leading to progressive dystrophy. Accordingly, Atm deficient mice exhibit lower weight gain and are smaller than their wild-type counterparts [2].
The ataxia-telangiectasia mutated (ATM) gene product plays an important role in cell cycle checkpoint control in response to double strand DNA breaks. However, the broad spectrum of clinical abnormalities in patients has raised the possibility that ATM may also be involved in other cellular processes. Participation of the ATM protein in insulin signalling through phosphorylation of eIF-4E-binding protein 1 has been postulated [1]. Furthermore, Peretz et al. [3] described that expression of the Insulin-like growth factor-I (IGF-I) receptor is ATM dependent in a pathway regulating radiation response. In this regard, disturbed growth regulation is an important aspect in AT and might contribute to the progression of neurodegeneration and immunodeficiency.
Circulating Insulin-like growth factor-I (IGF-I) and its main binding protein 3 (IGFBP-3) are key factors in the regulation of growth and weight gain, and changes in their serum levels have been shown to be a potential marker for growth hormone failure [4]. This prompted us to investigate levels of IGF-I and IGFBP-3 in a cohort of AT patients.
Patients and methods
Nineteen patients with AT (median age 15 years, range 4–24 years; male : female 11 : 8) were studied. Diagnosis was established in accordance to WHO recommendations (OMIM no. 208900). All patients showed increased levels of alpha-fetoprotein and atypical G2 arrest. Mutation data were available in almost all of the cases and all of them were found truncated. All patients included in the study exhibit a severe AT phenotype. Additionally, we analysed data derived from two siblings with a milder phenotype (age: patient 1, 24 years; patient 2, 26 years). Whole blood was collected from patients for analysis of IGF-I and IGFBP-3 levels. To assess the growth status of our group of patients, weight and height were recorded to calculate the body mass index (BMI) and nutritional protocols were established in 8 patients [5]. Patients were classified in three different age groups: (A) before adolescence (≤12 years of age, n = 8) (B) adolescence (>12–18 years of age, n = 9) and (C) adulthood (>18 years of age, n = 3) to reflect progress of deregulated growth and to minimize bias effects of pubertal stage. A chemiluminescence immunoassay was used for quantitative determination of IGF-I serum levels (Nicholsen Advantage, Bad Nauheim, Germany). IGFBP-3 was analysed by radio-immunoassay (Mediagnost, Reutlingen, Germany). Study protocols were approved by the ethic committees of the University Bonn, Germany. Signed consent was obtained from all parents, and all aspects of Good Clinical Practice were followed. Pearson correlation coefficient was calculated using SPSS (SPSS Inc, Chicago, USA)
Results
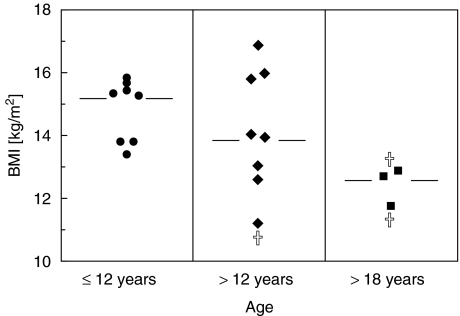
Median BMI of AT patients before adolescence (A) was 15·3 kg/m2 (range 13·42–15·86 kg/m2, n = 8), which is equivalent to the 50th percentile of normal growth (Fig. 1). Around adolescence (B), median BMI drops below the line of the 3rd percentile (median 13·99, range 11·24–16·88 kg/m2, n = 8) showing arrest of weight gain and growth in patients development. After adolescence (C) the BMI median further decreased (median 12·70 kg/m2, range 11·73–12·90 kg/m2, n = 3). However in this age group only a small number of patients was analysed, so that results might not be representative of this age group. In age groups A and B caloric intake was adequate (data not shown). Two of three patients in age group C had a lower nutritional intake, one of them despite use of a feeding tube.
Fig. 1.
Growth status of AT patients. Growth status of 19 patients with AT was assessed by body mass index (BMI). Patients were grouped as ≤ 12 years of age; > 12−18 years of age; and > 18 years of age. Results are shown as single values and median. † died during observation.
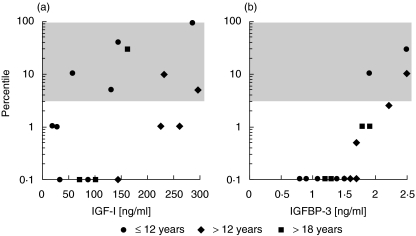
Due to their age-dependence, and to compare male and female levels of IGF-I and IGFBP-3 data were presented as percentiles (Nicholsen Advantage, Bad Nauheim, Germany, Mediagnost, Reutlingen, Germany). Reference intervals for healthy children and adults were defined as protein levels between the 3rd the 97th percentile. Both growth parameters are decreased in AT patients (Fig. 2). IGF-I levels were below the 3rd percentile in 9 (56%) of 16 patients. IGFBP-3 levels were below the 3rd percentile in 13 (81%) of 16 patients. Although low concentrations of IGF-I and IGFBP-3 levels were found in serum even in group A and no significant correlation between age, or BMI and levels of either IGF-I (Age: r = − 0·14; BMI r = 0·62) or IGFBP-3 (Age: r = − 0·19; r = , BMI: 0·124) could be detected, number of AT patients with IGF-I and IGFBP-3 levels below the 3rd percentile tends to increase with age (Fig. 2). These results were found for IGF-I as well as for IGFBP-3 (IGF-I: (A) 50% (4/8), (B) 66·6% (3/5), (C) 66·6% (2/3); IGFBP-3: (A) 75% (6/8), 83% (5/6), 100% (3/3)).
Fig. 2.
Growth factors of AT patients. (a) IGF-I and (b) IGFBP-3 levels of 16 patients with AT presented as percentiles. Shaded area shows the reference intervals for healthy children and adults defined as protein levels between the 3rd the 97th percentile (Nicholsen Advantage, Bad Nauheim, Germany; Mediagnost, Reutlingen, Germany).
In contrast to these findings, two siblings with a milder clinical phenotype did not show such dramatic changes neither in growth status (BMI: patient 1: 16·4 kg/m2; patient 2: 17·6 kg/m2) nor in growth factor parameters (IGF-I: patient 1, 60th percentile; patient 2: 50th percentile; IGFBP-3: patient 1, 7·5th percentile; patient 1, 20th percentile.
Discussion
AT patients show endocrinological abnormalities such as hypogonadism, insulin resistance and growth retardation. A potential role of the ATM protein in insulin signalling and IGF-I receptor expression has been described [1,3]. We could demonstrate that levels of IGF-I as well as of IGFBP-3 are decreased in patient's serum and reflect their impaired growth status. Furthermore, number of AT patients with IGF-I and IGFBP-3 levels below the 3rd percentile accumulates with increasing age. It is not clear whether IGF-I and IGFBP-3 deficiency is a cause or a consequence of stunted growth and impaired nutritional status in AT. Other disease associated factors such as pulmonary complications, hypogonadism, thymic dysplasia as well as neurological severity might also contribute to growth retardation in AT [7]. This is underlined by our findings that AT patients with a milder clinical phenotype showed less dramatic changes in growth status and growth factor parameters compared to the truncated AT phenotype.
Our results are in contrast to an earlier study that found increased IGF-I levels and unchanged IGFBP-3 levels in serum of 12 AT patients [8]. These discrepancies might be due the fact of a milder phenotype of patients included in this study. Furthermore, data in their study were not presented in terms of age-dependence, which is a crucial factor in growth hormone measurement [9].
Growth hormone (GH) supplementation could by used to induce weight gain and growth and may have a beneficial influence on neurodegeneration and/or immunodeficiency. However, keeping in mind the sensitivity of AT patients to lymphoma or leukaemia the use of GH-supplementation may be a threat for the patients. IGF-I and IGFBP-3 modulate cell growth and survival, and they are thought to be important in tumour development. It has been shown that circulating levels of IGF-I are associated with common cancers such as prostate cancer and premenopausal breast cancer [10]. In this regard, GH supplementation might influence the development of lymphoma or leukaemia in AT patients. On the other side our findings of low IGF-I levels in AT could reflect a protective effect to inhibit tumour growth. To test this hypothesis and to prove the effect of GH supplementation the Atm knockout mouse would be an excellent model. Further studies on growth hormones are required to potentially improve impaired growth status in patients with AT.
References
- 1.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–8. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 2.Barlow C, Hirotsune S, Paylor R, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–71. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 3.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci USA. 2001;98:1676–81. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strasburger CJ, Bidlingmaier M, Wu Z, Morrison KM. Normal values of insulin-like growth factor I and their clinical utility in adults. Horm Res. 2001;55:100–5. doi: 10.1159/000063484. [DOI] [PubMed] [Google Scholar]
- 5.Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. Perzentile für den Body-mass-Index für das kindes-und Jugendalter unter Heranziehung verschiedener deutscher Stichproben Monatsschr. Kinderheilkd. 2001;149:807–18. [Google Scholar]
- 6.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 7.Geenen V. The thymic insulin-like growth factor axis: involvement in physiology and disease. Horm Metab Res. 2003;35:656–63. doi: 10.1055/s-2004-814161. [DOI] [PubMed] [Google Scholar]
- 8.Busiguina S, Fer andez AM, Barrios V, Clark R, Tolbert DL, Berciano J, Torres-Aleman I. Neurodegeneration is associated to changes in serum insulin-like growth factors. Neurobiol Dis. 2000;7:657–65. doi: 10.1006/nbdi.2000.0311. [DOI] [PubMed] [Google Scholar]
- 9.Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regulation. 1994;4:11–9. [PubMed] [Google Scholar]
- 10.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protain-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004 Apr 24;363(9418):1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]