Abstract
Our study investigated the immunomodulatory activities of human plasma-derived serum immunoglobulin (Ig)A. Previous findings seem contradictory indicating either pro- or anti-inflammatory activities. We used serum IgA purified from large plasma pools and studied the modulation of the release of cytokines and chemokines from resting and lipopolysaccharide (LPS, endotoxin)-stimulated human adherent monocytes and human peripheral blood mononuclear cells (PBMC). Our results indicate that IgA down-modulates the release of the pro-inflammatory chemokines monocyte chemoattractant protein (MCP) 1, macrophage inflammatory protein (MIP) 1α and MIP1β from LPS-stimulated PBMC and the release of MCP1, MIP1α and MIP1β from LPS-stimulated monocytes. Furthermore, we confirmed previous reports that plasma-derived serum IgA down-modulates the release of the pro-inflammatory cytokines, interleukin (IL)-6 and tumour necrosis factor (TNF)-α, from LPS-stimulated monocytes and PBMC, and up-regulates the release of IL-1 receptor antagonist (IL-1RA) from resting and LPS-stimulated monocytes and resting PBMC. This IgA-mediated up-regulation of IL-1RA is independent of the simultaneous up-regulation of IL-1β release, as shown by blocking the biological activity of IL-1β with a neutralizing antibody. On the other hand, we also found an IgA-induced pro-inflammatory activity, namely IgA-mediated up-regutation of the release of pro-inflammatory IL-1β as well as down-regulation of the anti-inflammatory cytokines IL-10 and IL-12p40 from LPS-stimulated monocytes and PBMC and a down-regulation of transforming growth factor (TGF)-β from resting and LPS-stimulated PBMC. We conclude that human serum IgA has both an anti-inflammatory and a pro-inflammatory capacity and this dual capacity might contribute to the feedback mechanisms maintaining a balance between pro-inflammatory and anti-inflammatory activities.
Keywords: serum IgA, pro-inflammatory cytokines, anti-inflammatory cytokines, human monocytes, human PBMC
Introduction
Immunoglobulin (Ig) A is the most abundant immunoglobulin in mucosal tissue and second most abundant Ig in the circulation. More IgA is produced in humans than all other types of immunoglobulins together (66 mg/kg/day) [1,2].
There are two IgA subclasses, which occur in monomeric or polymeric forms. Serum IgA is primarily produced by plasma cells in the bone marrow, lymph nodes and spleen and occurs predominantly as a monomeric IgA class 1. Secretory IgA is synthesized locally and occurs in a polymeric, mostly dimeric, form with similar levels of IgA subclasses 1 and 2 [1,3–7].
The main role of secretory IgA, as well documented, is to inhibit bacterial attachment and neutralize viruses in mucosal tissue. In addition IgA, but not IgG, is translocated across epithelial tissue and can neutralize viruses intracellularly. This indicates that IgA is the first line of defence in the mucosal compartment [3,8]. Secretory IgA is generally considered to be a noninflammatory antibody because it does not trigger inflammatory processes when it binds to antigens [2,9,10], although contrary results have also been published [11].
Recent studies have shown that intact native human IgA when complexed with antigen fails to activate complement by either the classical or the alternative pathway [12,13] and even inhibits complement activation by IgM and IgG antibodies [13,14]. On the other hand under certain circumstances complement factors and receptors seem to play a part in IgA-mediated triggering of cellular effector functions [15,16].
In humans, the induction of IgA-mediated cellular effector functions requires interaction with specific Fc receptors (FcαR) on the cell surface, five of which have been identified in humans [17]. The most important receptor, FcαRI (CD89), is constitutively expressed on myeloid cells including monocytes, neutrophils, eosinophils, macrophages, as well as monocyte-derived dendritic cells and Kupffer cells and can be up-regulated by certain cytokines [18–23]. Activation of cells via FcαRI is a key factor in immunological defence because it mediates cytokine release, degranulation, respiratory burst and phagocytosis. The receptor, a heterogeneously glycosylated protein, binds serum and secretory IgA, in both monomeric and polymeric forms, and both IgA subclasses [5,21]. Signalling is mediated through an associated γ subunit [24]. Although all IgAs bind to FcαRI, the response seems to be influenced by the type because serum IgA triggers phagocytosis and respiratory burst in PMN but secretory IgA does not. This supports the concept of secretory IgA as a noninflammatory immunoglobulin on the one hand and also the reports of the pro-inflammatory capacity of the serum IgA on the other. The role of serum IgA is, however, still not fully understood and contradictory reports have been published.
Unprimed monocytes have been shown to secrete pro-inflammatory interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-6, IL-8, prostaglandin E2 and leukotrienes C4, B4 following cross-linking of CD89 with IgA containing immune complexes or anti-CD89 monoclonal antibodies [2,25–27]. Other reports have, however, shown an anti-inflammatory effect of IgA on human monocytes; Fcα-mediated down-regulation of the pro-inflammatory cytokines IL-6 and TNF-α release from endotoxin-stimulated monocytes and furthermore a Fcα-mediated release of IL-1 receptor antagonist (IL-1RA) on resting as well as and on stimulated monocytes [28,29]. These contradictory reports may be due to different activation levels of the monocytes: a dual function of IgA has been shown for neutrophils; an inhibition of phagocytosis in resting and an enhancement in activated cells [30].
Against this background of apparently inconsistent reports we investigated the influence of purified plasma-derived serum IgA on resting and endotoxin-activated human adherent monocytes and human peripheral blood mononuclear cells (PBMC).
Materials and methods
Human plasma-derived serum IgA preparations
Human serum IgA was prepared from a large plasma pool (Cohn fractions II and III) [31] by ethanol precipitation and cation-exchange, followed by anion-exchange chromatography. Two different lots: numbers 582 and 593 were prepared. Lot 593 was further purified by re-chromatography on the anion-exchange resin using lot 582 as a starting material.
The molecular size distribution, determined by high-performance liquid chromatography (HPLC), revealed an IgA monomer content of > 80%. The IgA, IgG and IgM amount, further the amount of transferrin, albumin and α2-macroglobulin as well as the IgA subclass distribution was determined by quantitative ELISA.
To determine the endotoxin content in the IgA-products we used the kit Toxicolor (Medac, Hamburg, Germany), based on the chromogenic determination of Limulus Amoebocytes Lysate (LAL).
We compared commercially available polyvalent human serum IgG preparation for intravenous use (Endobulin, Baxter AG, Vienna, Austria), obtained from a large plasma pool (Cohn fractions II and III), with plasma-derived serum IgA. The preparation was stored lyophilized in aliquots at +4°C and dissolved immediately before use.
Preparation of human peripheral mononuclear cells and monocytes
Human PBMC were isolated from heparinized blood (8·9 IU of preservative-free heparin per millilitre) from healthy donors by Lymphoprep (Nycomed Pharma, Oslo, Norway) density gradient purification [32]. The cells from the interface were collected and washed three times in phosphate-buffered saline (PBS, Gibco-Invitrogen, UK). After the last washing step the cells were resuspended in RPMI 1640 medium, supplemented with 50 µg/ml gentamycine, 2 m m/l l-glutamine (all from Gibco-Invitrogen) and 10% of prescreened, heat-inactivated (30 min 56°C) human AB serum (ICN Biomedicals, Irvine, CA, USA). This medium is referred to as RPMI-AB. For the preparation of monocyte monolayers, PBMC were incubated in 24-well tissue culture plates (Falcon 3047; Becton Dickinson, Lincoln Park, NJ, USA) at a concentration of 1 × 106/ml/well in RPMI-AB medium. After incubation at 37°C, 5% CO2 and 95% relative humidity for 90 min, the nonadherent cells were removed and the adherent cell monolayers were washed two times in RPMI 1640.
Stimulation of peripheral mononuclear cells and monocytes
The induction of cytokine release was based on the method described by Wolf et al. [28]. The adherent monocytes were incubated overnight in RPMI-AB medium to reduce background nonspecific cytokine production and subsequently washed two times. The number of adherent cells, determined by gently scrapping cells from the plate with a cell scraper and counting with a Coulter Counter, averaged 2·6 ± 0·5 × 105 cells/well.
The monocytes were stimulated for 24 h with lipopolysaccharide (LPS) with or without serum IgA in RPMI 1640 supplemented with gentamycine and l-glutamine as described above and containing 1% human serum albumin for intravenous medication supplied by Baxter AG, Vienna, Austria (RPMI-HSA). IgA (or for comparison human IgG) was used at a final concentration of 10 mg/ml. We used LPS purified from Escherichia coli, strain 0111:B4, sterilized by γ irradiation (no. L-4391, Sigma Chemical Co., St Louis, MO, USA), in a final concentration of 100 ng/ml. Unstimulated monocytes were cultured in medium RPMI-HSA alone.
In selected experiments IgA was used in a range of concentrations between 0·1 and 10 mg/ml. Kinetic experiments on cytokine release were done after 24, 48 and 72 h of incubation. The maximum cytokine secretion of LPS-stimulated cells was found for most cytokines after 24 h of incubation (data not shown). Therefore incubation was maintained for 24 h in all experiments. The cells supernatants were collected at the end of the incubation, centrifuged for 3 min at 9000 g to remove cellular material, then frozen in aliquots at −70°C. The cytokine release was measured by quantitative cytokine ELISAs using commercially available kits as described below.
LPS prestimulation experiments
The adherent monocyte cultures were prestimulated with LPS (100 ng/ml) for 3 h. The LPS was then removed from the culture by extensive washing with RPMI medium. Subsequently IgA (10 mg/ml) or RPMI-HSA alone (control) was added to the system and the cells cultured for a further 21 h. After a total incubation time of 24 h the supernatants were collected and cytokine release determined by ELISA.
IL-1β neutralization assay
An anti-human IL-1β monoclonal antibody tested for its ability to neutralize the biological activity of human IL-1β (clone: 8516, R & D Systems, Mineapolis, MN, USA) was added to the monocyte cultures at the same time point as LPS and IgA. Two concentrations of the antibody were used: 3·0 and 1·0 µg/ml. The cultures were cultivated for 24 h as described above.
PBMC stimulation experiments
The human mononuclear cells were isolated from human peripheral blood by density gradient centrifugation as described above, and immediately afterwards stimulated with LPS (100 ng/ml) with or without IgA (10 mg/ml) in RPMI-HSA medium in 24-well culture plates at a final concentration of 1 × 106/ml/well. After stimulation for 24 h the cell supernatants for cytokine measurement were collected as described above. Unstimulated PBMC were cultured in medium RPMI-HSA alone.
Measurement of the cytokine release
The cytokine release was measure by quantitative cytokine ELISAs using commercially available kits: IL-1RA Quantikine Immunoassay, IL-12p40 Quantikine Immunoassay, MCP1 Quantikine Immunoassay, MIP1α DuoSet ELISA, MIP1β DuoSet ELISA, TGF-β DuoSet ELISA (all from R & D Systems, Mineapolis, MN, USA); IL-1β-OptEIA Set, TNF-α-OptEIA Set, IL-10-OptEIA Set, IL-6-OptEIA Set (all from BD Pharmingen, San Diego, CA, USA). The manufacturers’ instructions for the assays were followed, and the results expressed in pg/ml and calculated from a standard curve obtained by linear regression analysis of the log transformation of the optical density on the y-axis versus the log of the standards concentrations on the x-axis.
Statistical analysis
In all experiments we used at least three donors. Each representative result was obtained from triplicate cultures from a single donor. Results are expressed as means ± standard deviation (SD) and were compared using unpaired two-tailed Student's t-test. A difference between two groups was considered to be significant if P < 0·05.
Intra-assay variation
We validated our assays for intra-assay variation. Twelve replicate samples were analysed in all ELISA systems and the coefficient of intra-assay variation was calculated using the following equation:
The calculated intra-assay variations were as follows:
IL-1RA immunoassay: %CV = 6·9;
IL-12p40 immunoassay: %CV = 5·2;
MCP1 immunoassay: %CV = 7·0;
MIP1α immunoassay: %CV = 3·3;
MIP1β immunoassay: %CV = 6·1;
TGF-β immunoassay: %CV = 8·5;
IL-1β-OptEIA %CV = 7·0; TNF-α-OptEIA %CV = 4·6;
IL-10-OptEIA%CV = 3·4; IL-6-OptEIA %CV = 5·4.
Results
Characterization of serum IgA preparations
The final IgA products (two different lots of IgA from the same plasma pool) contained ≥ 90% IgA, <1·9% IgG and <0·5% IgM (Table 1). The IgA subclass distribution corresponded to that found in serum (IgA1, 73 and 74%; IgA2, 27 and 26%). Both IgA preparations contain traces (<0·05%) of transferrin, albumin and α2-macroglobulin, as determined by quantitative ELISA. The molecular size distribution, determined by HPLC, revealed an IgA monomer content of >80%.
Table 1.
Characterization of Plasma-derived IgA
| IgA preparation no. 582 (% of IgA) | IgA preparation no. 593 (% of IgA) | |
|---|---|---|
| IgA1 | 73 | 74 |
| IgA2 | 27 | 26 |
| IgG | 1·9 | 1·6 |
| IgM | 0·4 | 0·2 |
| Transferrin | <0·05 | <0·05 |
| Albumin | <0·05 | <0·05 |
| α2-macroglobulin | <0·05 | <0·05 |
| Aggregate | 3·8 | 5·7 |
| Oligo/dimer | 10·1 | 13·8 |
| Monomer | 84·5 | 80·2 |
| Endotoxin LAL: | <0·2 EU/mg IgA | <0·5 EU/mg IgA |
Endotoxin LAL:HSA:<0·3 EU/ml.
Endotoxin LAL:IgG (Endobulin):<0·2 EU/mg.
Modulation of TNF-α, IL-6, IL-1β and IL-1RA by serum IgA
Monocytes release a range of pro-inflammatory cytokines when stimulated with LPS from Gram-negative bacteria [33–35]. We found that human serum IgA, but not human serum IgG, significantly down-regulated the release of the pro-inflammatory cytokines IL-6 and TNF-α from LPS-stimulated monocytes and PBMC in a dose-dependent manner. At the same time IgA up-regulated the release of TNF-α in resting monocytes and PBMC (Fig. 1).
Fig. 1.
Modulation of TNF-α and IL-6 by serum IgA (10 mg/ml). The results are arithmetic means from triplicate cultures ± SD (n = 3) stimulated for 24 h (representative results from one blood donor). Monocytes and PBMC were stimulated with LPS alone ( ), or with LPS in the presence of serum IgA Lot 582 (
), or with LPS in the presence of serum IgA Lot 582 ( ). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone Lot 582 (
). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone Lot 582 ( ). Control cells were cultured in medium (Med) alone (□). Statistically significant differences between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
). Control cells were cultured in medium (Med) alone (□). Statistically significant differences between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
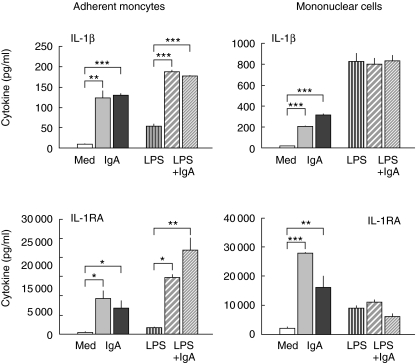
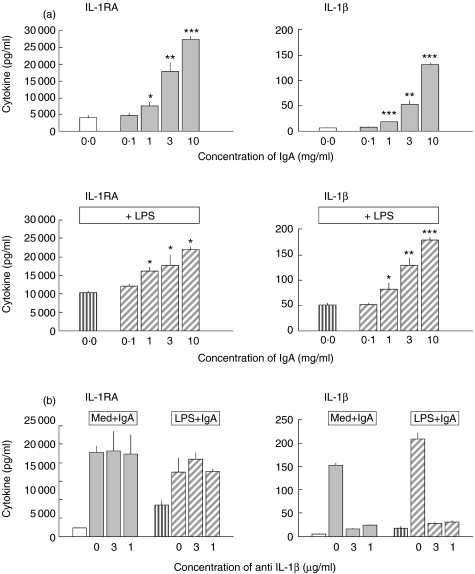
Furthermore in resting monocytes and PBMC, IgA induced IL-1RA production in a dose-dependent manner (Figs 2 and 3a). A significant IgA-mediated enhancement could also be shown in LPS-activated monocytes, but IgA showed no influence on IL-1RA production in LPS-stimulated PBMC. Moreover, parallel to IL-1RA release IgA induced a dose-dependent induction of IL-1β release. To exclude the possibility that IgA-induced up-regulation of IL-1RA is the consequence of this IgA-induced up-regulation of IL-1β rather than a direct FcαR-mediated effect we neutralized the IL-1β activity by a monoclonal antibody. We found that the up-regulation of IL-1RA release by serum IgA occurs independently of IL-1β release in resting as well as LPS-stimulated monocytes (Fig. 3b).
Fig. 2.
Modulation of IL-1β and IL-1receptor antagonist by serum IgA (10 mg/ml). The results are arithmetic means from triplicate cultures ± SD (n = 3) stimulated for 24 h (representative results from one blood donor). Monocytes and PBMC were stimulated with LPS alone ( , or with LPS in the presence of serum IgA; Lot 582 (
, or with LPS in the presence of serum IgA; Lot 582 ( ) or Lot 593 (
) or Lot 593 ( ). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone: Lot 582 (
). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone: Lot 582 ( ) or Lot 593 (▪). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
) or Lot 593 (▪). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
Fig. 3.
(a) Dose-dependent modulation of IL-1β and IL-1receptor antagonist by serum IgA. Monocytes were stimulated with LPS alone ( ), or with LPS in the presence of serum IgA no. 582 (
), or with LPS in the presence of serum IgA no. 582 ( ). To estimate the influence of IgA on resting monocytes the cells were incubated with IgA alone Lot 582 (
). To estimate the influence of IgA on resting monocytes the cells were incubated with IgA alone Lot 582 ( ). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001. (b) Up-regulation of IL-1RA by serum IgA (10 mg/ml) in resting and LPS-activated monocytes does not depend on IL-1β. Monocytes were stimulated with LPS alone (
). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001. (b) Up-regulation of IL-1RA by serum IgA (10 mg/ml) in resting and LPS-activated monocytes does not depend on IL-1β. Monocytes were stimulated with LPS alone ( ), or with LPS in the presence of serum IgA no. 582 (
), or with LPS in the presence of serum IgA no. 582 ( ). Resting monocytes were incubated with IgA alone Lot 582 (
). Resting monocytes were incubated with IgA alone Lot 582 ( ). Control cells were cultured in medium (Med) alone (□). Neutralization of the IL-1β activity by a monoclonal antibody (in concentrations 3 µg/ml and 1 µg/ml) shows that the IgA-mediated IL-1RA up-regulation is IL-1β independent.
). Control cells were cultured in medium (Med) alone (□). Neutralization of the IL-1β activity by a monoclonal antibody (in concentrations 3 µg/ml and 1 µg/ml) shows that the IgA-mediated IL-1RA up-regulation is IL-1β independent.
However, this effect seems not to be unique to IgA because human serum IgG also induced an enhancement of the release of both IL-1RA and IL-1β (data not shown).
Modulation of anti-inflammatory cytokines IL-10, IL-12p40 and transforming growth factor (TGF)-β by serum IgA
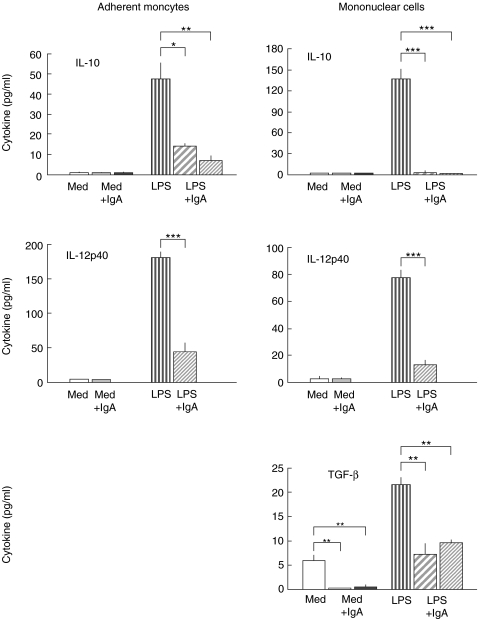
Human serum IgA (Fig. 4) and also serum IgG (data not shown) down-regulated the release of the anti-inflammatory cytokines IL-10 and IL-12p40 from LPS-activated monocytes and PBMC in a dose-dependent manner. IL-12p40 was recently proved to be an antagonist of pro-inflammatory IL-12p70 [36,37] and so regarded as an anti-inflammatory cytokine. Intact IL-12p70 release was under the detection limit of ELISA. IgA also down-regulated the release of anti-inflammatory TGF-β in LPS-stimulated and resting PBMC. In monocytes TGF-β release was under the detection limit of ELISA.
Fig. 4.
Modulation of IL-10, TGF-β and IL-12p40 by serum IgA (10 mg/ml). The results are arithmetic means from triplicate cultures ± SD (n = 3) stimulated for 24 h (representative results from one blood donor). Monocytes and PBMC were stimulated with LPS alone ( ), or with LPS in the presence of serum IgA: Lot 582 (
), or with LPS in the presence of serum IgA: Lot 582 ( ) or Lot 593 (
) or Lot 593 ( ). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone: Lot 582 (
). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone: Lot 582 ( ) or Lot 593 (▪). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
) or Lot 593 (▪). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
Soluble TNF receptors, I and II, have been shown to bind circulating TNF-α and inhibit its biological activity [38,39]. We evaluated the possible influence of serum IgA on the release of these soluble receptors but no modification by IgA could be found in our system (data not shown).
Modulation of the chemokines monocyte chemoattractant protein 1 (MCP1) and macrophage inflammatory protein 1 alpha (MIP1α) and beta (MIP1β) by IgA
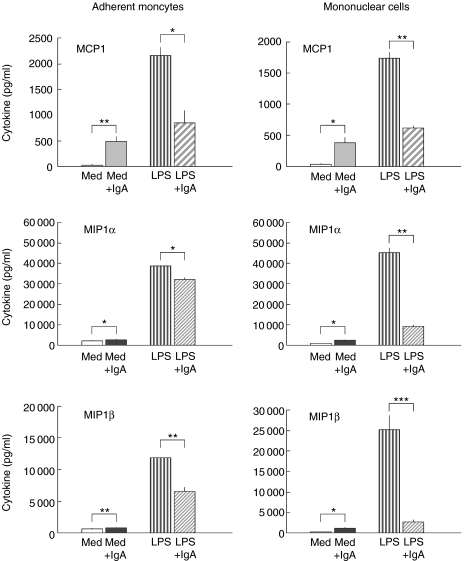
Serum IgA, but not serum IgG, decreased the release of the pro-inflammatory monocyte-derived chemokines, MIP1α, MIP1β and MCP1 in LPS-activated monocytes and PBMC (Fig. 5). Under resting conditions (without LPS-stimulation), however, IgA increased the chemokines release in both monocytes and PBMC.
Fig. 5.
Serum IgA (10 mg/ml) induced modulation of the release of chemokines: monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein 1 alpha (MIP1α) and beta (MIP1β). The results are arithmetic means from triplicate cultures ± SD (n = 3) stimulated for 24 h (representative results from one blood donor). Monocytes and PBMC were stimulated with LPS alone ( ), or with LPS in the presence of serum IgA: Lot 582 (
), or with LPS in the presence of serum IgA: Lot 582 ( ) or Lot 593 (
) or Lot 593 ( ). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone: Lot 582 (
). To estimate the influence of IgA on resting monocytes or on resting PBMC the cells were incubated with IgA alone: Lot 582 ( ) or Lot 593 (▪). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
) or Lot 593 (▪). Control cells were cultured in medium (Med) alone (□). Statistically significant difference between either IgA-treated versus control cultures or IgA + LPS-treated versus LPS alone denoted as: *P < 0·05, **P < 0·01, ***P < 0·001.
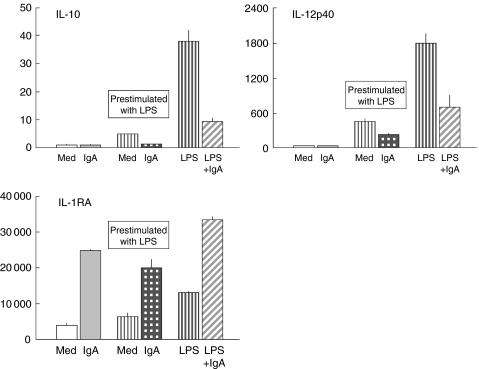
Prestimulation of monocytes with LPS and subsequent removal of unbound LPS before addition of IgA to the culture
The pretreatment for 3 h with LPS was much less potent in stimulating cytokine release from monocytes than continuous LPS-stimulation for 24 h, as might be expected (Fig. 6). Nevertheless serum IgA, when added to the prestimulated cultures after LPS had been removed, showed a similar pattern of IgA-mediated effects on the release of cytokines as had been found when cells were treated with LPS and IgA simultaneously for 24 h. These results exclude the possibility that the effects which we have found of the down-modulatory activities of IgA on LPS-stimulated cells were simply caused by neutralization of LPS by IgA antibodies. Such LPS neutralizing antibodies might be present in an IgA-preparation from a plasma pool.
Fig. 6.
Pre-stimulation of monocytes with LPS for 3 h ( with subsequent removal of unbound LPS before addition of IgA (10 mg/ml) for 21 h to the culture (
with subsequent removal of unbound LPS before addition of IgA (10 mg/ml) for 21 h to the culture ( ). Monocytes were then stimulated either for 24 h with LPS alone (
). Monocytes were then stimulated either for 24 h with LPS alone ( , or with LPS in the presence of serum IgA (
, or with LPS in the presence of serum IgA ( ). Resting monocytes were incubated with IgA alone Lot 582 (
). Resting monocytes were incubated with IgA alone Lot 582 ( ). Control cells were cultured in medium (Med) alone (□). Serum IgA, when added to the pre-stimulated cultures after LPS had been removed, showed a similar pattern of IgA-mediated effects on the release of cytokines to that of cells treated with LPS and IgA simultaneously for 24 h.
). Control cells were cultured in medium (Med) alone (□). Serum IgA, when added to the pre-stimulated cultures after LPS had been removed, showed a similar pattern of IgA-mediated effects on the release of cytokines to that of cells treated with LPS and IgA simultaneously for 24 h.
Discussion
Our study investigated the immunomodulatory activities of human plasma-derived serum IgA. Previous studies have produced apparently conflicting data indicating that IgA has either pro- or anti-inflammatory activities.
We found that human serum IgA has a dose-dependent modulating effect over a physiologically relevant concentration range on the release of a panel of monocyte-derived cytokines and chemokines.
Our results to some extent confirm the anti-inflammatory concept of serum IgA. We could show that IgA has a down-modulating effect on the release of the chemokines MCP1, MIP1α and MIP1β in LPS-activated monocytes as well as PBMC. These monocyte-derived chemokines are essential for an effective innate immune response and acute inflammation. They stimulate migration and cell activation of human neutrophils and basophils, and induce synthesis of pro-inflammatory cytokines such as IL-6, TNF-αand IL-1.
Furthermore, our findings concerning the IgA-induced down-modulation of the release of pro-inflammatory cytokines IL-6 and TNF-α from LPS-activated monocytes and PBMC, additionally confirm the anti-inflammatory concept of serum IgA. These mainly monocyte-derived cytokines play a major part in cellular interactions and multiple effector functions in both innate and adaptive immunity necessary for effective host defence. Regulatory mechanisms are necessary, however, because an exaggerated, self-amplifying release of these inflammation mediators may result in a variety of disorders including intravascular thrombosis and lethal septic shock [40]. Moreover, TNF-α and IL-6 are known to be involved in the pathogenesis of chronic inflammatory disorders, such as inflammatory bowel disease, rheumatoid arthritis or neonatal necrotizing enterocolitis [38,41–43].
We investigated IgA-induced modulation of the release of a panel of anti-inflammatory cytokines: IL1RA, IL-10, IL-12p40 and TGF-β. We found an IgA-induced dose-dependent up-regulation of the release of IL-1RA in resting as well as in activated monocytes and resting PBMC. This as well as down-modulation of LPS-induced IL-6 and TNF-α release by serum IgA confirms previous findings by Wolf et al. [29]. IL-1RA inhibits the pro-inflammatory activity of IL-1 through blocking the binding of IL-1 to its receptor. In contrast to findings by Wolf et al. [29] we found this up-regulation of IL-1RA, which would contribute to an anti-inflammatory capacity of IgA, was counteracted by parallel IgA-induced up-regulation of IL-1β release. IL-1β itself is known to induce IL-1RA synthesis in humans [44]. We could show, however, that the up-regulation of IL-1RA by serum IgA occurs independently of IL-1β release and is therefore truly IgA-dependent.
We found some difference in the effect of IgA on the release of IL-1RA and IL-1β between LPS-activated monocytes and activated PBMC. IgA induced dose-dependent up-regulation of the release of IL-1RA and IL-1β in resting as well as in activated monocytes and in resting but not in activated PBMC. This difference might be due to the interaction of monocytes and T- and B-specific cytokines present in activated PBMC cultures (but not in monocyte cultures) which could influence the release of these monokines. It is also possible that the expression of additional IgA-receptors described on activated B cells (FCα/µ) as well as on dividing T cells [45,46] could have an influence on the results in PBMC.
On the other hand, our findings of IgA-induced down-regulation of the LPS-induced release of anti-inflammatory cytokines IL-10, IL-12p40 and TGF-β seem to support the pro-inflammatory concept of serum IgA.
IL-12p40 was recently proved to be an antagonist of pro-inflammatory IL-12p70 [36,37]. Following a down-regulation of IL-12p40, an enhancement of cellular immunity can be expected; down-regulation of multifunctional IL-10, which inhibits activation of Th1 cells would lead to an increase in Th1 activity and innate immunity. Although contra-productive in an inflammatory environment, an increase in Th1 activity might be helpful in immunological disorders characterized by enhanced ratios of Th2 to Th1 cytokines. In patients with systemic lupus erythematosus (SLE) an increased production of Th2 cytokines, including IL-6 and IL-10, is considered to lead to up-regulated B-cell activation, which typifies SLE [47–52]. Treatment with anti-IL-10 antibodies has been shown to delay the onset of SLE in NZB/W mice [52]. Gene therapy regulating these two cytokines has been discussed as a possible future therapy for SLE [47]. The down-regulation of IL-10 and IL-6 by serum IgA shown in our study could offer a new therapeutical approach for SLE.
We hypothesize that the effects of serum IgA shown in our study were triggered by FcαR. This receptor is expressed on myeloid cells either alone or in complex with Fcγ chain [53]. Signalling is via the γ chain. This common activation motif for Fcα and Fcγ receptors might explain our finding of some effects of serum IgA similar to those caused by serum IgG. We found that human serum IgG, like serum IgA, enhanced the release of both IL-1RA and IL-1β in our system, confirming previous findings [54]. Also the down-regulation of anti-inflammatory cytokines IL-10 and IL-12p40 occurred with both IgA and IgG.
Nevertheless, the capacity to down-regulate the LPS-induced release of pro-inflammatory cytokines, IL-6 and TNF-α, and chemokines MCP1, MIP1α and MIP1β, appears specific for serum IgA. Serum IgG caused no such effects. This suggests that serum IgA has some specific regulatory capacity.
Our results suggest that IgA can function in a dual manner depending on the activation level of the cells. We found an IgA-induced enhancement of TNF-α and MCP1 production in monocytes and PBMC under resting conditions and a down-regulation when cells were LPS activated. A dual function of IgA has been shown in neutrophils, with phagocytosis inhibited in resting and enhanced in activated cells [30].
Contradictory reports of IgA-induced effects could be due to differences between the experimental settings, cell activation levels, and molecular forms of IgA used in earlier studies. Use of soluble monomeric IgA on the one hand, or IgA in a form that cross-links Fcα receptors on the cell surface on other hand as well as inconsistent activation levels influences results considerably. Under noninflammatory conditions different myeloid cell types have shown pro-inflammatory activities in experimental settings with multivalent cross-linking of IgA-receptors. In unprimed polymorphonuclear neutrophils each way of cross-linking the Fcα receptors, either by heat aggeregated IgA [55], or by IgA bound to a solid surface [56], or by cross-linking of anti-FcαR antibodies by a secondary antibody [57–59], has lead to respiratory burst and phagocytosis. IgA bound to aggarose was the most potent stimulus for eosinophil degranulation [60]. In unprimed monocytes and PBMC, the picture is similar. Again each way of multivalent crosslinking of Fcα receptors, either by polymeric IgA, or by monomeric IgA bound to the surface, or by cross-linking of anti-FcαR antibodies by a secondary antibody, has been shown to up-regulate the release of pro-inflammatory cytokines such as TNF-α, IL-6, IL-8, prostaglandin E2 and leukotrienes C4, B4[25,27,61,62]. Immune complexes of IgA formed by a secondary antibody added to serum IgA likewise dose-dependently increased TNF-α and IL-1β production by unprimed monocytes [62,63].
Monomeric IgA, which does not cross-link FcαR, has however, under resting conditions, shown no such effects, neither in monocytes nor neutrophils [61–63].
Our finding that IgA induces the release of pro-inflammatory IL-1β, TNF-α, MCP1, MIP1α and MIP1β from unprimed monocytes and PBMC under resting conditions, confirms this pro-inflammatory view of IgA. In the light of previous findings the up-regulation we found might be caused by the cross-linking of FcαRs by the ‘polymeric fraction’ of the serum IgA preparation. The IgA antibodies against LPS, which might be present in our serum IgA preparation and cause cross-linking of IgA in immune complexes, could contribute to this effect.
The contradictory view of IgA as an anti-inflammatory agent under pro-inflammatory conditions, however, is also known from the literature. Purified serum IgA has been shown to induce down-regulation of the release of pro-inflammatory cytokines IL-6 and TNF-α from monocytes activated by endotoxin [28]. The same investigators found that serum IgA induced an increase in IL-1RA production in resting and LPS-activated monocytes and a down-regulation of IL-1β secretion in activated PBMC [29]. Soluble serum IgA purified from large plasma pools was used in these studies but no molecular analysis of the IgA preparation was published.
To some extent our results obtained under inflammatory conditions (with LPS) confirm this anti-inflammatory concept of serum IgA. We found that IgA enhanced anti-inflammatory IL-1RA release from LPS-stimulated as well as resting cells. Furthermore serum IgA induced down-modulation of the pro-inflammatory cytokines, IL-6 and TNF-α, and pro-inflammatory chemokines, MIP1α, MIP1β and MCP1.
Our results together with those published previously indicate that for the down-regulation of cytokines release under pro-inflammatory conditions the ‘monomeric fraction’ of serum IgA is responsible. Stimulation with LPS rapidly enhances the expression of FcαR on human monocytes [64] and therefore enhances the capacity of monomeric IgA, which binds to FcαR with much lower affinity than polymeric forms [65], to exert its effects. This disparity in binding affinity might be the reason why the modus of IgA activities depends on two conditions: the molecular form of IgA and the activation level of the IgA-receptor-bearing cells. This might explain why serum IgA can apparently act differently under different activation levels. Although predominantly monomeric, it consists of mixed molecular forms that act differently under different activation conditions. The molecular forms of our serum IgA preparation correlates with that of serum IgA in the circulation. Serum IgA consists of approximately 80% monomeric IgA, 10% J-chain-containing dimeric IgA and, as was recently shown, 10% covalently linked IgA-CD89 complexes containing polymeric IgA [66]. This polymorphic molecular form of serum IgA might add another complexity to the role of IgA in immunity. Serum IgA antibodies may have different functional activities depending on their molecular form, and the activation level of cells bearing the IgA receptor. Further studies on the effects of the various molecular fractions of serum IgA will be necessary to clarify the picture.
In the light of our findings human serum IgA seems to posses anti-inflammatory as well as pro-inflammatory properties. These properties might not be contradictory but reflect a dual capacity of serum IgA that contributes to the complicated feedback mechanisms maintaining a balance between pro-inflammatory and anti-inflammatory activities in human body.
Acknowledgments
We wish to thank Josenato Ilas and Georg Koca for excellent technical assistance and Elise Langdon-Neuner for editing the manuscript.
References
- 1.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–96. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JG. IgA and the IgA Fc receptor. Trends Immunol. 2001;22:205–11. doi: 10.1016/s1471-4906(01)01873-7. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam BP, Raeder RH, Nedrud JG, Bucher DJ, Le J, Metzger DW. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J Immunol. 2001;166:226–31. doi: 10.4049/jimmunol.166.1.226. [DOI] [PubMed] [Google Scholar]
- 4.McGhee JR, Mestecky J, Elson CO, Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989;9:175–99. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- 5.Morton HC, Brandtzaeg P. CD89: the human myeloid IgA Fc receptor. Arch Immunol Ther Exp (Warsz) 2001;49:217–29. [PubMed] [Google Scholar]
- 6.Kutteh WH, Prince SJ, Mestecky J. Tissue origins of human polymeric and monomeric IgA. J Immunol. 1982;128:990–5. [PubMed] [Google Scholar]
- 7.Kett K, Brandtzaeg P, Radl J, Haaijman JJ. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631–5. [PubMed] [Google Scholar]
- 8.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–40. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 9.Honorio-Franca AC, Launay P, Carneiro-Sampaio MM, Monteiro RC. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J Leukoc Biol. 2001;69:289–96. [PubMed] [Google Scholar]
- 10.Heystek HC, Moulon C, Woltman AM, Garonne P, van Kooten C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J Immunol. 2002;168:102–7. doi: 10.4049/jimmunol.168.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Pleass RJ, Areschoug T, Lindahl G, Woof JM. Streptococcal IgA-binding proteins bind in the Calpha 2-Calpha 3 interdomain region and inhibit binding of IgA to human CD89. J Biol Chem. 2001;276:8197–204. doi: 10.1074/jbc.M009396200. [DOI] [PubMed] [Google Scholar]
- 12.Russell MW, Mansa B. Complement-fixing properties of human IgA antibodies. Alternative pathway complement activation by plastic-bound, but not specific antigen-bound, IgA. Scand J Immunol. 1989;30:175–83. doi: 10.1111/j.1365-3083.1989.tb01199.x. [DOI] [PubMed] [Google Scholar]
- 13.Russell MW, Sibley DA, Nikolova EB, Tomana M, Mestecky J. IgA antibody as a non-inflammatory regulator of immunity. Biochem Soc Trans. 1997;25:466–70. doi: 10.1042/bst0250466. [DOI] [PubMed] [Google Scholar]
- 14.Russell MW, Reinholdt J, Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–9. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 15.Janoff EN, Fasching C, Orenstein JM, Rubins JB, Opstad NL, Dalmasso AP. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Invest. 1999;104:1139–47. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Lachmann PJ. Neutrophil lactoferrin release induced by IgA immune complexes can be mediated either by Fc alpha receptors or by complement receptors through different pathways. J Immunol. 1996;156:2599–606. [PubMed] [Google Scholar]
- 17.Moura IC, Centelles MN, Arcos-Fajardo M, et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig) A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med. 2001;194:417–25. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Egmond M, van Garderen E, van Spriel AB, et al. FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–5. doi: 10.1038/76261. [DOI] [PubMed] [Google Scholar]
- 19.Mazengera RL, Kerr MA. The specificity of the IgA receptor purified from human neutrophils. Biochem J. 1990;272:159–65. doi: 10.1042/bj2720159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pleass RJ, Andrews PD, Kerr MA, Woof JM. Alternative splicing of the human IgA Fc receptor CD89 in neutrophils and eosinophils. Biochem J. 1996;318:771–7. doi: 10.1042/bj3180771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro RC, Cooper MD, Kubagawa H. Molecular heterogeneity of Fc alpha receptors detected by receptor- specific monoclonal antibodies. J Immunol. 1992;148:1764–70. [PubMed] [Google Scholar]
- 22.Geissmann F, Launay P, Pasquier B, et al. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol. 2001;166:346–52. doi: 10.4049/jimmunol.166.1.346. [DOI] [PubMed] [Google Scholar]
- 23.Patry C, Sibille Y, Lehuen A, Monteiro RC. Identification of Fc alpha receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J Immunol. 1996;156:4442–8. [PubMed] [Google Scholar]
- 24.Morton HC, van Egmond M, van de Winkel JG. Structure and function of human IgA Fc receptors (Fc alpha R) Crit Rev Immunol. 1996;16:423–40. [PubMed] [Google Scholar]
- 25.Foreback JL, Remick DG, Crockett-Torabi E, Ward PA. Cytokine responses of human blood monocytes stimulated with Igs. Inflammation. 1997;21:501–17. doi: 10.1023/a:1027359629838. [DOI] [PubMed] [Google Scholar]
- 26.Ferreri NR, Howland WC, Spiegelberg HL. Release of leukotrienes C4 and B4 and prostaglandin E2 from human monocytes stimulated with aggregated IgG, IgA, and IgE. J Immunol. 1986;136:4188–93. [PubMed] [Google Scholar]
- 27.Patry C, Herbelin A, Lehuen A, Bach JF, Monteiro RC. Fc alpha receptors mediate release of tumour necrosis factor-alpha and interleukin-6 by human monocytes following receptor aggregation. Immunology. 1995;86:1–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood. 1994;83:1278–88. [PubMed] [Google Scholar]
- 29.Wolf HM, Hauber I, Gulle H, et al. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89) -mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin Exp Immunol. 1996;105:537–43. doi: 10.1046/j.1365-2249.1996.d01-793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolova EB, Russell MW. Dual function of human IgA antibodies: inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J Leukoc Biol. 1995;57:875–82. doi: 10.1002/jlb.57.6.875. [DOI] [PubMed] [Google Scholar]
- 31.Cohn EJ, Strong LE, Hughes WI, Jr, Mulford DJ, Ashworth JN, Melin M, Taylor HL. Preparation and properties of serum and palsma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–75. doi: 10.1021/ja01207a034. [DOI] [PubMed] [Google Scholar]
- 32.Bøyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Laboratory Invest. 1968;21:77. [PubMed] [Google Scholar]
- 33.Heumann D, Gallay P, Barras C, et al. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–12. [PubMed] [Google Scholar]
- 34.Gessani S, Testa U, Varano B, et al. Enhanced production of LPS-induced cytokines during differentiation of human monocytes to macrophages. Role of LPS receptors. J Immunol. 1993;151:3758–66. [PubMed] [Google Scholar]
- 35.Liebler JM, Kunkel SL, Burdick MD, Standiford TJ, Rolfe MW, Strieter RM. Production of IL-8 and monocyte chemotactic peptide-1 by peripheral blood monocytes. Disparate responses to phytohemagglutinin and lipopolysaccharide. J Immunol. 1994;152:241–9. [PubMed] [Google Scholar]
- 36.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E (2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–9. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 37.Camoglio L, Juffermans NP, Peppelenbosch M, et al. Contrasting roles of IL-12p40 and IL-12p35 in the development of hapten-induced colitis. Eur J Immunol. 2002;32:261–9. doi: 10.1002/1521-4141(200201)32:1<261::AID-IMMU261>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.Arend WP, Dayer JM. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33:305–15. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 39.Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–60. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 40.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–4. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 41.Stokkers PC, Camoglio L, Van Deventer SJ. Tumor necrosis factor (TNF) in inflammatory bowel disease: gene polymorphisms, animal models, and potential for anti-TNF therapy. J Inflamm. 1995;47:97–103. [PubMed] [Google Scholar]
- 42.Matsuno H, Yudoh K, Katayama R, et al. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatology. 2002;41:329–37. doi: 10.1093/rheumatology/41.3.329. [DOI] [PubMed] [Google Scholar]
- 43.Mackay F, Browning JL, Lawton P, et al. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998;115:1464–75. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- 44.Bargetzi MJ, Lantz M, Smith CG, et al. Interleukin-1 beta induces interleukin-1 receptor antagonist and tumor necrosis factor binding protein in humans. Cancer Res. 1993;53:4010–3. [PubMed] [Google Scholar]
- 45.Millet I, Panaye G, Revillard JP. Expression of receptors for IgA on mitogen-stimulated human T lymphocytes. Eur J Immunol. 1988;18:621–6. doi: 10.1002/eji.1830180420. [DOI] [PubMed] [Google Scholar]
- 46.Millet I, Briere F, de Vries J, Revillard JP. Up-regulation of receptors for IgA on activated human B lymphocytes. Immunol Lett. 1988;19:153–7. doi: 10.1016/0165-2478(88)90135-6. [DOI] [PubMed] [Google Scholar]
- 47.Cross JT, Benton HP. The roles of interleukin-6 and interleukin-10 in B cell hyperactivity in systemic lupus erythematosus. Inflamm Res. 1999;48:255–61. doi: 10.1007/s000110050456. [DOI] [PubMed] [Google Scholar]
- 48.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest. 1994;94:585–91. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitani A, Hara M, Hirose T, et al. Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clin Exp Immunol. 1992;88:75–83. doi: 10.1111/j.1365-2249.1992.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miret C, Font J, Molina R, et al. Relationship of oncogenes (sFas, Bcl-2) and cytokines (IL-10, alfa-TNF) with the activity of systemic lupus erythematosus. Anticancer Res. 2001;21:3053–9. [PubMed] [Google Scholar]
- 51.Ronnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann Rheum Dis. 2003;62:37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–10. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Launay P, Patry C, Lehuen A, Pasquier B, Blank U, Monteiro RC. Alternative endocytic pathway for immunoglobulin A Fc receptors (CD89) depends on the lack of FcRgamma association and protects against degradation of bound ligand. J Biol Chem. 1999;274:7216–25. doi: 10.1074/jbc.274.11.7216. [DOI] [PubMed] [Google Scholar]
- 54.Arend WP, Leung DY. IgG induction of IL-1 receptor antagonist production by human monocytes. Immunol Rev. 1994;139:71–8. doi: 10.1111/j.1600-065x.1994.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 55.Gorter A, Hiemstra PS, Leijh PC, et al. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology. 1987;61:303–9. [PMC free article] [PubMed] [Google Scholar]
- 56.Shen L, Collins J. Monocyte superoxide secretion triggered by human IgA. Immunology. 1989;68:491–6. [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart WW, Mazengera RL, Shen L, Kerr MA. Unaggregated serum IgA binds to neutrophil Fc alpha R at physiological concentrations and is endocytosed but cross-linking is necessary to elicit a respiratory burst. J Leukoc Biol. 1994;56:481–7. doi: 10.1002/jlb.56.4.481. [DOI] [PubMed] [Google Scholar]
- 58.Shen L. A monoclonal antibody specific for immunoglobulin A receptor triggers polymorphonuclear neutrophil superoxide release. J Leukoc Biol. 1992;51:373–8. doi: 10.1002/jlb.51.4.373. [DOI] [PubMed] [Google Scholar]
- 59.Gorter A, Hiemstra PS, Leijh PC, van Es LA, Daha MR. Stimulation of human polymorphonuclear leukocytes by serum IgA or secretory IgA. Adv Exp Med Biol. 1987;216B:1325–31. [PubMed] [Google Scholar]
- 60.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–400. [PubMed] [Google Scholar]
- 61.Deviere J, Content J, Denys C, et al. Immunoglobulin A and interleukin 6 form a positive secretory feedback loop: a study of normal subjects and alcoholic cirrhotics. Gastroenterology. 1992;103:1296–301. doi: 10.1016/0016-5085(92)91519-a. [DOI] [PubMed] [Google Scholar]
- 62.Deviere J, Vaerman JP, Content J, et al. IgA triggers tumor necrosis factor alpha secretion by monocytes: a study in normal subjects and patients with alcoholic cirrhosis. Hepatology. 1991;13:670–5. [PubMed] [Google Scholar]
- 63.Polat GL, Laufer J, Fabian I, Passwell JH. Cross-linking of monocyte plasma membrane Fc alpha, Fc gamma or mannose receptors induces TNF production. Immunology. 1993;80:287–92. [PMC free article] [PubMed] [Google Scholar]
- 64.Shen L, Collins JE, Schoenborn MA, Maliszewski CR. Lipopolysaccharide and cytokine augmentation of human monocyte IgA receptor expression and function. J Immunol. 1994;152:4080–6. [PubMed] [Google Scholar]
- 65.Reterink TJ, van Zandbergen G, van Egmond M, et al. Size-dependent effect of IgA on the IgA Fc receptor (CD89) Eur J Immunol. 1997;27:2219–24. doi: 10.1002/eji.1830270915. [DOI] [PubMed] [Google Scholar]
- 66.van der Boog PJ, van Zandbergen G, de Fijter JW, et al. Fc alpha RI/CD89 circulates in human serum covalently linked to IgA in a polymeric state. J Immunol. 2002;168:1252–8. doi: 10.4049/jimmunol.168.3.1252. [DOI] [PubMed] [Google Scholar]