Abstract
X-linked agammaglobulinaemia (XLA) is an inherited immunodeficiency that is caused by a block in early B-cell differentiation. Whereas early B precursors in the bone marrow are present in substantial numbers, XLA-affected individuals have dramatically reduced numbers of circulating mature B cells, plasma cells and immunoglobulins of all isotypes. We report on a Japanese family with 3 XLA patients, in whom the serum immunoglobulin levels and number of B cells showed a significant difference among them in spite of harbouring the same splice donor site mutation in the BTK gene. We developed concise method for detection of this mutation, which is helpful for discovering the carrier. Patient 2 showed a significant serum immunoglobulin levels of all isotypes, including allergen-specific IgE. Expression of a normal and truncated size BTK gene was detected in patient 2′s peripheral blood mononuclear cells (PBMCs). Expression of BTK protein was also detected in some B cells. These results suggest that the leaky phenotype in patient 2 was caused in part by the expression of a normal BTK gene transcript. The increased frequency of infection with age expanded the number of B cells with normal BTK gene expression and produced the serum immunoglobulin, including IgE.
Keywords: XLA, BTK gene, leaky phenotype, splice mutation, IgE production
Introduction
X-linked agammaglobulinaemia (XLA) is a rare genetic disorder of B-cell maturation characterized by the absence of mature B cells, very low serum levels of all immunoglobulin isotypes, and a lack of specific antibody production. Mutations in the gene coding for a tyrosine kinase (BTK, Bruton tyrosine kinase) have been identified as responsible for XLA but the exact role of this kinase in B cell development has not yet been established [1–5].
It is known that there was wide variability in a clinical presentation, even among the members of one family who are likely to be carrying the same gene.
Phenotypic variation within a 3-generation family has been described previously [6], in which a 51-year-old man with recurrent sinusitis and sporadic pneumonia was confirmed to have a mutation in a premature stop codon in the BTK gene. Other factors, such as infection exposures, have been postulated as possible reasons for phenotypic variation.
We came across a Japanese family with 3 X-linked agammaglobulinaemia. patients, one of whom exhibited a leaky phenotype. The patient showed the significant serum level of IgG, IgM, IgA and IgE. The analysis of XLA in a large family is useful for studying the genotype/phenotype relationship and our PCR-based method of detecting the mutation is helpful for discovering carriers of a BTK gene mutation.
Materials and methods
All of the XLA patients were diagnosed as clinical features, immunological phenotype and BTK protein expression. Patient 1 was a 3-years-old boy who was introduced to our hospital since he suffered from recurrent pyoderma. After the beginning of immunoglobulin replacement therapy, no severe infection has been observed. Patients 2 and 3 are p55–2 and p55–1, respectively [7]. The XLA 1–3 patients have different mutations of the BTK gene in this family. BTK gene mutations in XLA 1 and 3 were 1235–1247deletion and 1885G to T, respectively [7]. Informed consent for gene analysis was obtained from the patients or their parents.
Specific IgE antibodies
Specific antibodies for house dust and Dermatophagoides were measured with a fluoroenzyme immunoassay by means of a Uni-Cap assay kit (Pharmacia, Uppsala, Sweden). A specific IgE level higher than 3·5 IU/ml was considered positive.
Amplification and electrophoresis of the BTK gene
Peripheral blood mononuclear cells (PBMCs) were separated using Ficoll-Paque (Amersham Bioscience, Uppsala, Sweden). RNA was prepared from PBMCs and cDNA was synthesized with MMTV reverse transcriptase. Genomic DNA from PBMCs was prepared using a Sepa Gene kit (Sanko Jyunyaku, Tokyo, Japan). PCR primers for genomic DNA are as previously described [8]. PCR consisted of 35 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. The amplified DNA fragment was electrophoresed using 2% agarose gel or 20% acrylamide gel [9].
For the concise detection of the IVS11 + 3G→T mutation we used mismatch primers, which were introduced artificially into the MseI site. The underlined nucleotide was a mismatched nucleotide.
Following PCR amplification, the PCR product was digested using MseI. DNA was electrophoresed using 4% agarose gel or 20% acrylamide gel [10].
RT-PCR primers for the detection of the expression of exon 11 are as follows.
Sequencing of the BTK gene
The PCR fragment was subcloned into a T-vector (Novagen, Madison, WI, USA) and sequenced using a dye primer or a dye terminator method with an autosequencer (Applied Biosystems, Indianapolis, IN, USA). For a dye primer sequencing five independent colonies were picked and sequenced.
Flow cytometric analysis of BTK expression in B cells
Intracellular BTK staining of B cells was performed as described previously [11]. The stained cells were analysed with by means of a flow cytometer (EPICS Cytomics FC500; Beckman Coulter KK, Tokyo, Japan). BTK protein expression was analysed in CD 19 positive B cells.
Results and discussion
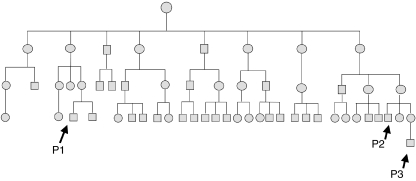
Patient 1 was introduced to our hospital because of recurrent skin infection. His family has at least 3 XLA patients (Fig. 1). As shown in Table 1, patient 1 showed an extremely low serum immunoglobulin level in IgG, IgM, IgA and IgE (Patient 1's initial Ig levels before immunoglobulin replacement were as follows; IgG 136 mg/dl, IgA under 5 mg/dl, IgM 10 mg/dl, IgE 2·5 IU/ml). However, patient 2 showed detectable IgG, IgM and IgA. In his PBMCs CD19-positive B cells were also detected. Interestingly, IgE and allergen-specific IgE for house dust and mites was detected. Typical XLA patients (XLA 1–3) have no serum IgE (Table 1). It is assumed that IgE was the critical marker for the detection of the leaky phenotype.
Fig. 1.
The family tree of a large XLA family. The spouses in all cases were omitted. P1-P3 represent XLA patients 1–3 in Table 1, respectively.
Table 1.
Immunological data of XLA patients with IVS11 + 3G→T and the other mutations of the BTK gene.
| Age (years) | IgG | IgM | IgA | IgE | CD3 | CD19 | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 3 | 422 | <5 | <5 | 2·6 | 86·6 | 0·4 |
| Patient2 | 29 | 480 | 63 | 151 | 269 | 85·2 | 2·1 |
| Patient 3† | 10 | 63 | <5 | <5 | |||
| XLA 1 | 31 | 495 | <5 | <5 | <0·3 | 88·9 | 0·1 |
| XLA 2 | 31 | 459 | <5 | <5 | <0·3 | 68·7 | 0·1 |
| XLA 3 | 20 | 381 | 6 | <5 | <0·3 | 89·0 | 0·1 |
The serum immunoglobulin level except patient 3 is measured just before the immunoglobulin infusion. Patient 3's data is at 4 years old.
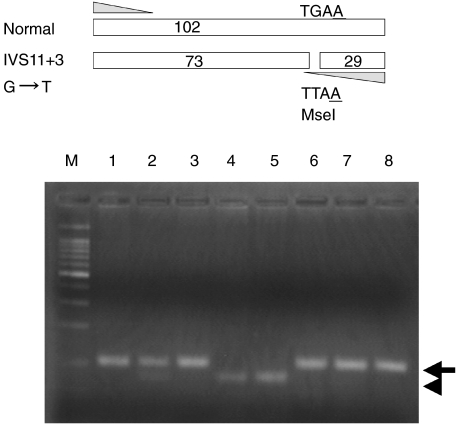
Genomic DNA of the BTK gene in patient 1 revealed IVS11 + 3G→T resulting in the exon 11 skip. For the concise detection of the IVS11 + 3G→T mutation we used mismatch primers, which were introduced artificially into the MseI site. The mutant-type allele was digested into 73-bp and 29-bp fragments using MseI (Fig. 2), which indicated that the mother had both normal and mutant allele.
Fig. 2.
The concise detection of the BTK gene mutation of IVS11 + 3G→T in this family. The upper panel shows the strategy for the detection of the mutation. An antisense strand mismatch primer, which introduced a MseI site de novo in the mutant sequence (underlined A), was used. The arrow indicates a band corresponding to normal DNA. The arrowhead indicates a MseI-digested band of a mutant DNA.M: size marker of DNA, lane 1: father of patient 1; lane 2: mother of patient 1; lane 3: elder brother of patient 1; lane 4: patient 1; lane 5: patient 2; lane 6–8: normal control.
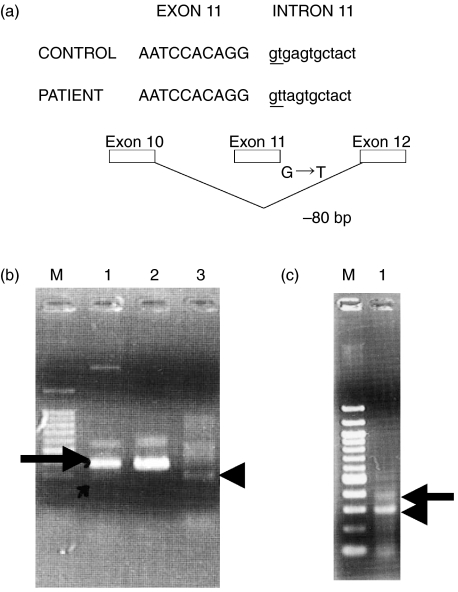
Expression of the BTK gene in patient 1 showed 80-bp-deleted transcripts and larger size transcripts compared with the normal size transcripts (Fig. 3a). Patient 1's mother showed weak 80-bp-truncated transcripts (Fig. 3b). On the other hand, patient 2 showed an 80 bp-truncated-band and normal size BTK gene transcripts (Fig. 3c).
Fig. 3.
The detection of normal size BTK gene expression in patient 2 but not in patient 1. (a) The abnormally smaller size PCR product in (b) and (c) was sequenced and found to be the result of a splicing abnormality caused by the mutation of intron 11. (b) The abnormally small size PCR product in the family of patient 1. The arrow indicates the normal size PCR product detected in the mother. The arrowhead indicates an abnormally small size PCR product. M: size marker; lane 1: mother of patient 1; lane 2: father of patient 1; lane 3: patient 1. (c) The abnormally small size and normal size PCR product in patient 2. The arrow indicates the normal size PCR product. The arrowhead indicates abnormally small size PCR product. M: DNA size marker; lane 1: patient 2.
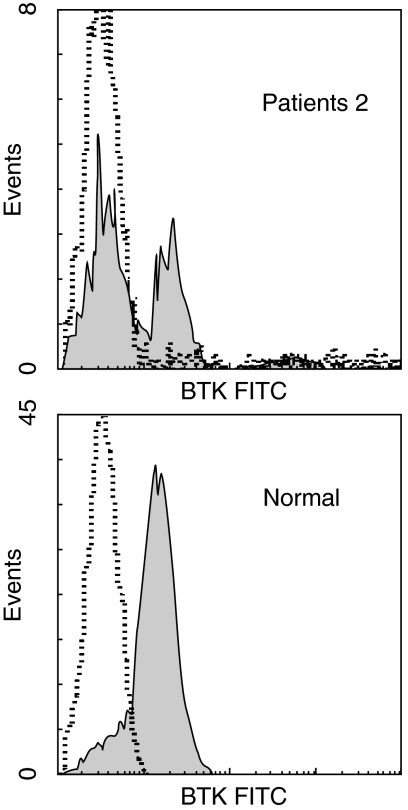
We speculated that a leaky phenotype in the patient was the result of the occurrence of normal BTK transcripts in some B cells having the BTK gene mutation causing the exon 11 skip, which might lead to the antigen-driven expansion of B cells with normal BTK protein. To further validate this point, we tried to demonstrate the presence of normal BTK protein in some B cells in the patient with a leaky phenotype. For this purpose, we used a flow cytometric analysis using a monoclonal anti-BTK antibody. Figure 4 shows that 31% of CD19 positive B cells expressed normal BTK protein in patient 2. These results suggest that patient 2's peripheral B cells consist of BTK protein negative and positive B cells. In the patient 2's monocyte BTK expression was not detected by a flow cytometry (data not shown).
Fig. 4.
Flow cytometric analysis of CD19 cell BTK expression in an normal donor and patient 2. The closed areas and dashed lines indicate the staining with anti-BTK and control IgG1 mouse antibodies in B cells, respectively.
It is assumed that B cells which have normal BTK transcripts proliferate due to antigen stimulation since it is considered that the leaky B cells have the normal response to the antigen and might differentiate into memory B cells [12]. The difference of the function between BTK protein negative and positive B cells in patient 2 remains to be elucidated.
The serum immunoglobulin levels of patient 2 are significantly different from his nephew and the other typical XLA patients. These results suggest that the leaky phenotype of XLA might depend on the age and frequency of infection. We developed gel analysis after enzyme digestion for the concise detection of IVS11 + 3G→T. The analysis of XLA in a large family is useful for studying the genotype/phenotype relationship and our PCR-based method of detecting the mutation is helpful for discovering the carrier.
| sense | 5′-GGAGGTTTCATTGTCAGAGAC-3′ |
| antisense | 5′-GTCCTCAGGGCCTTGGAATAGTAGCATT-3′ |
| sense | 5′-GTATGAGTGGTATTCCAAAC-3′ |
| antisense | 5′-GTCCTTTGGATCAATTTCCCAT-3′ |
Acknowledgments
The authors would like to thank Drs H. Kanegane and T. Miyawaki (Department of Paediatrics, Faculty of Medicine, Toyama Medical and Pharmaceutical University) for analysing the mutation of the BTK gene. This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Tsukada S, Saffran DC, Rawlings DJ, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 2.Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 3.Rawlings DJ, Saffran DC, Tsukada S, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient xid mice. Science. 1993;261:358. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 4.Holinski-Feder E, Weiss M, Brandau O, et al. Mutation screening of the BTK gene in 56 families with X-linked agammaglobulinemia (XLA): 47 unique mutations without correlation to clinical course. Pediatrics. 1998;101:276–84. doi: 10.1542/peds.101.2.276. [DOI] [PubMed] [Google Scholar]
- 5.Speletas M, Kanariou M, Kanakoudi-Tsakalidou F, et al. Analysis of Btk mutations in patients with X-linked agammaglobulinaemia (XLA) and determination of carrier status in normal female relatives: a nationwide study of Btk deficiency in Greece. Scand J Immunol. 2001;54:321–7. doi: 10.1046/j.1365-3083.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 6.Morwood K, Bourne H. Phenotypic variability: Clinical presentation between the 6 th and 60th year in a family with X-linked agammaglobulinemia. J Allergy Clin Immunol. 2004;113:783–5. doi: 10.1016/j.jaci.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Kanegane H, Futatani T, Wang Y, et al. Clinical and mutational characteristics of X-linked agammaglobulinemia and its carrier identified by flow cytometric assessment combined with genetic analysis. J Allergy Clin Immunol. 2001;108:1012–20. doi: 10.1067/mai.2001.120133. [DOI] [PubMed] [Google Scholar]
- 8.Ohta Y, Haire RN, Litman RT, et al. Genomic organization and structure of Bruton agammaglobulinemia tyrosine kinase: localization of mutations associated with varied clinical presentations and course in X chromosome-linked agammaglobulinemia. Proc Natl Acad Sci USA. 1994;91:9062–6. doi: 10.1073/pnas.91.19.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo N, Inoue R, Kasahara K, Fukao T, Kaneko H, Tashita H, Teramoto T. Reduced expression of the interferon-gamma messenger RNA in IgG2 deficiency. Scand J Immunol. 1997;45:227–30. doi: 10.1046/j.1365-3083.1997.d01-387.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasahara Y, Kaneko H, Fukao T, Terada T, Asano T, Kasahara K, Kondo N. Hyper-IgM syndrome with putative dominant negative mutation in activation-induced cytidine deaminase. J Allergy Clin Immunol. 2003;112:755–60. doi: 10.1016/s0091-6749(03)01860-8. [DOI] [PubMed] [Google Scholar]
- 11.Futatani T, Miyawaki T, Tsukada S, et al. Deficient expression of Buruton's tyrosine kinase in monocytes from X-linked agammaglobulinemia as evaluated by a flow cytometric analysis and its clinical application to carrier detection. Blood. 1998;91:595–602. [PubMed] [Google Scholar]
- 12.Nonoyama S, Tsukada S, Yamadori T, et al. Functional analysis of peripheral blood B cells in patients with X-linked agammaglobulinemia. J Immunol. 1998;161:3925–9. [PubMed] [Google Scholar]