Abstract
Respiratory syncytial virus infects almost all children by 2 years of age. Neutrophils are the predominant airway leucocytes in RSV bronchiolitis and they are activated in the presence of infection. However it is not clear whether RSV can directly signal to activate neutrophil cytotoxic function. To investigate this we have used a preparation of RSV washed using a new centrifugal diafiltration method to rapidly remove inflammatory molecules produced by the epithelial cells used to propagate the RSV stock. Human neutrophils were isolated from peripheral blood and activated with either the unwashed crude RSV preparations or the purified intact RSV. Neutrophils were also challenged with purified RSV G-glycoprotein. The effect of challenging human neutrophils with these preparations of intact RSV, or the RSV G-glycoprotein, was assessed by measuring the cell surface expression of CD11b and CD18b, the phagocytic oxidative burst, and intracellular release of calcium pools. Neutrophils challenged with the washed RSV exhibited significantly lower activation of surface marker expression (P < 0·001) and oxidative burst (P < 0·001) than those challenged with unwashed virus or with virus free supernatant. There was no increase in intracellular calcium release on exposure to the washed RSV. Purified G glycoprotein did not stimulate neutrophils, whilst the use of a blocking antibody to the F protein did not prevent unwashed RSV from activating cytotoxic responses. These results suggest that neutrophils have no innate signalling system that recognizes RSV but they are activated at sites of RSV infection as a result of the cytokines and inflammatory molecules released by virally infected cells.
Keywords: respiratory syncytial virus, neutrophil, integrin, free radical, intracellular calcium
Introduction
Respiratory syncytial virus (RSV) causes annual epidemics of respiratory disease affecting the whole population [1,2]. This virus infects many infants during their first winter, and it has long been recognized that RSV infection is responsible for the majority of cases of acute bronchiolitis and viral pneumonia in infancy. Its importance as a respiratory pathogen in the elderly has been increasingly recognized in recent years [3]. It is now recognized that the inflammatory process in the airways of infants with RSV bronchiolitis is dominated by an intense neutrophil influx in both the lower and upper airway [4,5] and that neutrophil products such as myeloperoxidase and neutrophil elastase are release into the airways [6]. Unbound neutrophil elastase is found in excess of its inhibitors in the airways of infants with acute bronchiolitis and accounts for the majority of protease activity present in the airways of infants with acute bronchiolitis [7] re-enforcing the suggestion that neutrophils play a major role in the causation of symptoms. It is still unclear whether these cells participate in clearance of virus though experimental studies have demonstrated that neutrophils damage respiratory epithelial cells infected with RSV [8].
The very high numbers of neutrophils observed in the airways of infants with acute bronchiolitis appears to be a consequence of both recruitment and prolonged survival within the infant airways. In vitro studies have indicated that RSV infection results in the release of high concentrations of IL-8 and of other pro-inflammatory molecules including IL-1, TNFα, RANTES, MIP-1α[9–12] and these finding reflect those from clinical studies involving infants with RSV infections [6,13,14] More recently it has been shown that there are factor(s) present within the infant airway which prolong neutrophil survival which will contribute to the observed neutrophilia [15].
While it is clear that activation of neutrophils is an important feature of the host response to RSV infection it is unclear whether the neutrophils are activated directly in response to virus or whether activation is mediated indirectly through the release of inflammatory cytokines by infected epithelial cells. It is well established that the cytotoxic function of these cells can be activated by lipopolysaccharide and peptidoglycan from gram-negative bacteria via specific receptor (e.g. TLR4). Toll receptors are part of the innate immune signalling responses to infection and have been implicated as important in the pathogenesis of RSV infection in an animal model [16]. Antibody bound bacterial activate neutrophils via specific Fc receptor [17] and this mechanism may also be relevant to the activation of cytotoxic immunity during RSV infection [18].
There have been studies assessing possible direct effects of RSV on neutrophils [19,20] but the purity of the viral preparations used for these studies was not demonstrated. Propagation of RSV in a cell line results in the release of inflammatory cytokines and pro inflammatory molecules in addition to the virus. Therefore immune responses attributed to the virus may be due to the activating properties of these other molecules. Traditional methods to purify RSV are cumbersome, time consuming and may results in large reduction to the replicative capacity of the virus. For example sucrose density sedimentation [21] takes many hours to complete, and requires the addition of a high concentration of magnesium sulphate [22] to stabilize the virus and prevent loss of replicative capacity. Furthermore to prevent interference in cellular assays the magnesium sulphate may need to be removed.
In order to avoid these problems and produce RSV free from contaminating cytokines and inflammatory products, a rapid centrifugal diafiltration unit was employed to wash RSV, separating the viral particle from the smaller inflammatory molecules. Having established this method, the potential for RSV to directly activate human neutrophils was examined. Peripherally circulating neutrophils donated by healthy adults were purified and then exposed to samples of unwashed and washed RSV and the activation of these cells measured with respect to cell surface expression of the integrins CD11b and CD18 and to intracellular signalling due to calcium release; and the cytotoxic superoxide free radical burst. Further experiments were performed in order to determine whether important RSV surface antigens played a role in activating neutrophils using purified RSV envelope G glycoprotein, and a neutralizing antibody to the F glycoprotein [23].
Materials and methods
RSV production
HeLa cells were grown to 80% confluence in T75 flasks, and infected with 1 × 104 pfu/ml of the A2 RSV strain. The cells were incubated at 37°C for 5 days in 2% FCS maintenance medium (DMEM + 2% heat inactivated FCS) until syncytia formation was observed. All of the culture medium was removed, and the cell layer was scraped into 5 ml of protein free Ham F12 medium (Sigma, UK) and immediately snap frozen in liquid nitrogen, thawed, and centrifuged at 640 g for 10 min to remove the intact cell membranes and organelles.
Diafiltration
Vivaspin-20 (Vivascience, Gloucester, UK) ultra-filtration tubes (20 ml capacity) with a polyethersulphone membrane containing a pore size of 1000 000 Daltons MWCO (1000 kD) were used. To minimize nonspecific protein binding and sticking of the viral particles the filter membrane was coated with 20 ml of 0·1% purified Casein (I-block™; Tropix Inc, Perkin Elmer, UK) prepared in sterile endotoxin free PBS (BioWhittaker, Workingham). 19 ml of Ham-F12 basal medium and 1 ml of RSV thawed stock preparation was added and the unit was then centrifuged at 1121 g (at 4°C for 40 min) until the volume of the retentate was less than the starting volume of the RSV aliquot (i.e. <1 ml). The membrane was then vigorously pipetted with 1 m of 4°C Ham-F12 basal medium to dislodge virus particles trapped on the membrane surface. The virus fractions were pooled, the total volume measured, and 4°C basal Ham F12 added to a volume of 20 ml. 1 ml aliquots of this 1/20 diluted washed stock were then snap frozen in liquid nitrogen and stored at −70°C. Samples of the unwashed RSV were also diluted 1/20 in basal Ham-F12. The filtrate fraction was also collected, frozen, and stored.
Plaque assay
HeLa cells were grown in 96 well plates to subconfluence (80% coverage) and infected with neat and 10 fold dilutions down to 10−8 pfu/ml of washed and unwashed RSV and equivalent dilutions of the filtrate sample. The cells were maintained in a 37°C CO2/air incubator for 48 h and then fixed in 50% acetone: 50% PBS and stained with a mouse monoclonal antibody pool against the major RSV antigens (Novocastra, UK). The presence of the viral infected cells was revealed by incubation with a secondary peroxidase labelled goat anti mouse antibody (Sigma, UK) and the insoluble substrate 4-chloronapthol substrate (Sigma-Aldrich, Poole, UK).
SDS-PAGE and silver staining of gels
Washed and unwashed RSV preparations and filtrate samples were separated on a discontinuous 10% PAGE minigel with a 3·7% stacking gel. The gels were fixed in methanol acetic acid, washed thoroughly in ultrapure water, activated in 0·034 m potassium dichromate/nitric acid solution, and then stained in 0·1% silver. Following development in 2·5% sodium carbonate the protein bands were detected sensitively in the different samples.
Measurement of IL-8 by ELISA
The concentration of human IL-8 in the washed and unwashed RSV preparations and in the filtrate samples was measured using a DuoSetTM sandwich ELISA (R & D, Oxford, UK), lower limit of sensitivity 62·5 pg/ml.
Neutrophil isolation
PMNs were isolated using Histopaque-1077 (Sigma-Aldrich, Poole, UK) and NH4Cl lysis [24] and were >95% viable and >95% pure. They were then resuspended at a density of 4 × 106 cells/ml in a serum free medium based upon Ham F12 containing 2% Albumax (Gibco BRL, Paisley, UK) with 3·7 m m CaCl2 and 4 m m glutamine stock. Aliquots of 1 × 106 PMNs were put into FACS tubes.
Neutrophil stimulants
For the neutrophil stimulation experiments 1 µm of the bacterial chemotactic agents fMLP was used as a positive control. Unwashed and washed RSV was used at the same concentration of 1 × 104 pfu/ml. The filtrate collected from the washing of the virus was also used as a control at an equivalent dilution to assess whether the inflammatory products released by the infected epithelium were able to activate the PMN in the absence of viral particles.
Measurement of cell surface expression of CD11b/CD18
PMNs were exposed to different dilutions of washed and unwashed RSV, fMLP and filtrate for 30 min in a gassed 37°C incubator. They were then washed and stained without fixation using direct mouse IgG-FITC conjugates to CD18 (Serotec, Kidlington, UK) and IgG-PE conjugates to CD11b (Serotec) at 4°C, and analysed by flow cytometry. All results were corrected for IgG isotype control and expressed as the Mean fluorescent intensity (MFI).
Measurement of intracellular calcium release
Purified PMNs at 2 × 106/ml were incubated in serum free Ham-F12 with 2 µm Indo-1 (Molecular Probes Inc; Cambridge, UK) at 37°C for 30 min, then left at 20°C in the dark for 15 min prior to analysis with the flow cytometer. One hundred µl of the cell suspension was added to 400 µl of 1 m m calcium chloride in a FACS tube, incubated for 2 min at 37°C and then run on the flow cytometer for 30 s prior to adding the different stimulants. Along with the addition of washed and unwashed RSV, the cells were treated with 0·1 µm Ionomycin as a positive control to activate the release of the total intracellular calcium stores the PMNs. The changes to the fluorescence of Indo-1 following its binding to calcium were measured at short wavelength 210 nm and long wavelength at 381 nm using a Herc laser at 325 nm.
Measurement of neutrophil oxidative burst
Purified PMNs at 1 × 105/ml were incubated in serum free Ham-F12 with 20 µm 2·7-Dichloro-dihydrofluorescein diacetate (DC-FDA) for 15 min at 37°C. They were then washed and incubated with 1 × 104 pfu/ml of washed RSV or unwashed RSV or 1 µm fMLP for 30 min at 37°C. A positive control using 20 µm t-butyl hyproperoxide was used to convert all of the intracellular DC-FDA to its oxidized fluorescent state. DC-FDA changes were measured at an excitation wavelength of 480 nm and fluorescent emission at 530 nm using an Argon Laser.
Neutrophil activation experiments with G-glycoprotein and F-glycoprotein blocking antibody
PMNs were isolated as described above and incubated with 1 × 104pfu/ml and 100 µg/ml of humanized mouse anti F-glycoprotein antibody (Palivizumab; gift from Abbot Laboratories; UK) or various concentrations of highly purified G-glycoprotein (gift from Dr E Blair, GlaxoWellcome) at 10–100 µg/ml or on its own. The effective blocking concentration of Palivizumab was previously determined by serial dilution. The PMNs were stained with antibodies for CD11b and CD18 as described above and the fluorescence measured by flow cytometry.
Detection of viable, apoptotic and necrotic cells
Levels of necrosis and apoptosis were quantified on morphological characteristics using fluorescent vital and nonvital DNA biding dyes. The vital and apoptotic cells were stained with Hoechst 33342 (Sigma Chemicals Ltd) while necrotic cells were stained with propidium iodide (Molecular Probes, Cambridge), both to a final concentration of 1 µm. After 5 min the cells were observed using a Leica DMIRB UV fluorescent inverted microscope using the UV filter to visualize both dyes simultaneously. The nuclei of the viable cells appear blue (Hoechst +ve) and were morphologically normal. Apoptotic cells with intact plasma membranes were also Hoechst +ve but displayed morphological characteristics of DNA condensation and nuclear fragmentation. Necrotic cells were bright orange/red stained. This direct staining method allows all dead cells to be counted whereas methods that require centrifugal harvesting of cells can selectively remove buoyant necrotic cells.
Statistical analysis of the results
The flow cytometry data was presented as MFI ± standard error (SE) of the distribution of the staining. Differences between specific groups were examined using the Students T-test with correction for samples of unequal variance. Differences were considered to be significant at the 5% level (P < 0·05).
Results
Effect of diafiltration on infectivity of RSV
The RSV plaque assay results demonstrated the presence of viable virus in the washed retentate sample although there was a significant reduction (10-fold loss from 107 to 106 pfu/ml) compared to the levels of unfiltered virus. The filtrate sample, even when used neat, contained no detectable infective virus in the immune plaque assay. The infectivity and toxicity of the washed and unwashed RSV samples were also examined using a Hoechst/Propidium iodide viability assay following addition of the different samples to the HeLa cell line. Unwashed RSV caused large amounts of epithelial necrosis that was not observed with the washed RSV preparation when used at equivalent infective titre.
Removal of cellular proteins by diafiltration
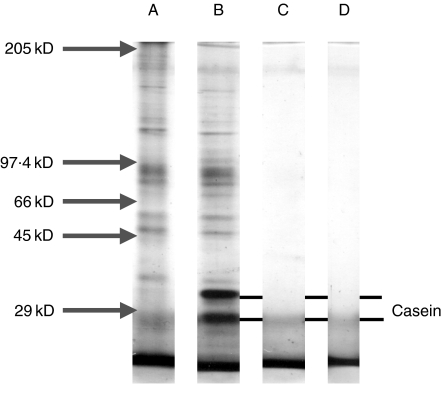
Following diafiltration the different samples of the RSV preparation, were separated by SDS-PAGE and then stained in silver (Fig. 1). The unwashed sample of RSV showed many protein bands across the molecular weight range of this gel (5–200 kD) (Fig. 1, lane A). The same bands were also observed with very little difference in the filtrate sample (Fig. 1, lane B). The only difference was the presence of two additional small molecular weight bands (∼20–30 kD), which were consistent with the reported molecular weight of the casein used to coat the filter (Fig. 1, lane B). However, only a few trace protein bands were detected in the washed sample at molecular weights of ∼42 kD and 97 kD (Fig. 1, lane C). The intense bands present at the lowest molecular weight positions i.e. <20 kD, were also observed in the blank lane and were due to silver reacting with ions in the migration front of the gel (Fig. 1, lane D).
Fig. 1.
10% SDS PAGE mini gel stained in silver and showing removal of proteins following centrifugal dia filtration of the RSV sample. The samples were separated by SDS-PAGE under denaturing dissociating conditions at the dilution equivalent to the original sample and show the following. Lane A: shows the sample of unwashed RSV; Lane B: shows the sample of filtrate from the RSV preparation and the line marks the presence of the casein in the filtrate; Lane C: shows the washed RSV sample; Lane D: shows a lane with no sample added but demonstrates the non specific silver staining with the ion front.
Removal of IL-8 following diafiltration
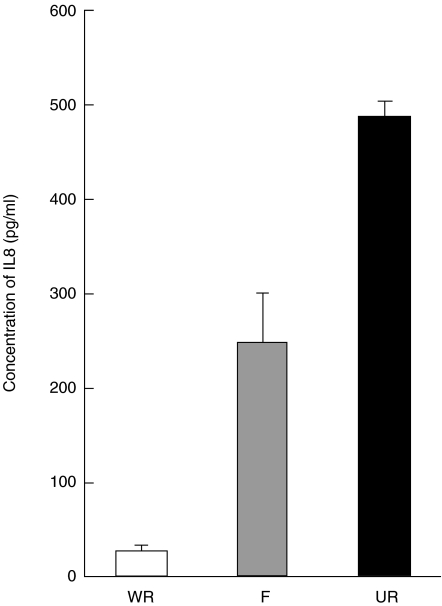
IL-8 was present at significantly higher concentrations (485 pg/ml ± 19·7 SE) in the unwashed RSV preparation than the filtrate sample (248 pg/ml ± 50·2 SE) but the IL-8 in the washed RSV sample was below the limit of accurate detection on the standard curve having a value of ∼26·3 pg/ml (± 4·0 SE) (Fig. 2).
Fig. 2.
A bar graph showing the reduction of IL8 in the RSV for samples (n = 3 + SEM) before washing (UR), in the filtrate sample (F), and in the washed sample (WR). The concentrations were determined by regression using a standard curve of IL8 (from 62·5 pg/ml; where r = 0·998).
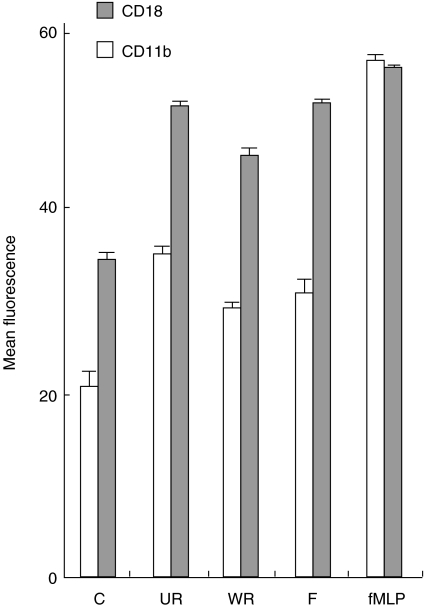
CD11b/CD18 surface expression fMLP-stimulated PMNs expressed high levels of fluorescence for CD11b (55·9 ± 0·3 MFI) and CD18 (54·8 ± 0·4 MFI). PMNs exposed to unwashed RSV expressed a mean MFI value of 35 ± 0·3 units and 50·7 ± 0·4 units, respectively. These were both significantly higher (P < 0·001) compared to both nonstimulated PMNs (20·4 MFI ± 0·1 and 34·9 MFI ± 0·3) and PMNs exposed to washed RSV (28·9 MFI ± 0·2 and 45·9 MFI ± 0·4). Incubation of the PMNs with the filtrate sample at equivalent dilutions gave MFI values of 30·5 ± 0·4 and 50·9 ± 0·4 significantly higher (P < 0·05) than for the control PMNs (Fig. 3).
Fig. 3.
Graph showing the mean (± SE) values of fluorescence as measured by flow cytometry for the expression of CD11b (PE) and CD18 (FITC) on the surface of purified neutrophils. The cells were either left nonstimulated (C), or were stimulated with 1 × 104 pfu/ml washed (WR), or 1 × 104 pfu/ml of unwashed (UR) RSV for 30 min at 37°C. In addition, the change in CD expression was compared to the positive control 1 µm FMLP (fMLP), and the viral specific effects were compared to virus free filtrate (F).
Oxidative burst
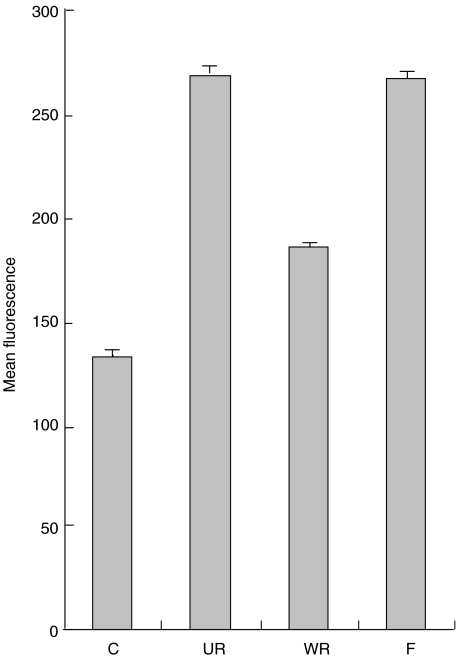
Non-stimulated PMNs produced low levels of free radicals (132·8 ± 1·8 SE MFI) (Fig. 4). When PMNs were exposed to unwashed RSV there was a significant increase (P < 0·001) in free radical production (271·2 ± 1·9 SE MFI). Stimulation with washed RSV resulted in a much smaller increase in free radical production (186·7 ± 1·5 SE MFI), significantly (P < 0·001) lower than the unwashed sample. Free radical production by PMNs when challenged with the virus free filtrate (270·4 ± 1·9 SE MFI) was similar to unwashed virus and this was significantly greater (P < 0·001) than both the control PMNs and PMNs exposed to washed RSV.
Fig. 4.
Graph showing the mean (± SE) values of fluorescence for measurement of free radical production using DC-FDA as measured by flow cytometry over a three minute period. The cells were either left nonstimulated (C) or stimulated with the virus sample filtrate (F); either at 1 × 104 pfu/ml of unwashed (UR) RSV, or 1 × 104 pfu/ml of washed (WR) RSV. The X axis shows the increase in units of FITC fluorescence.
Release of intracellular calcium
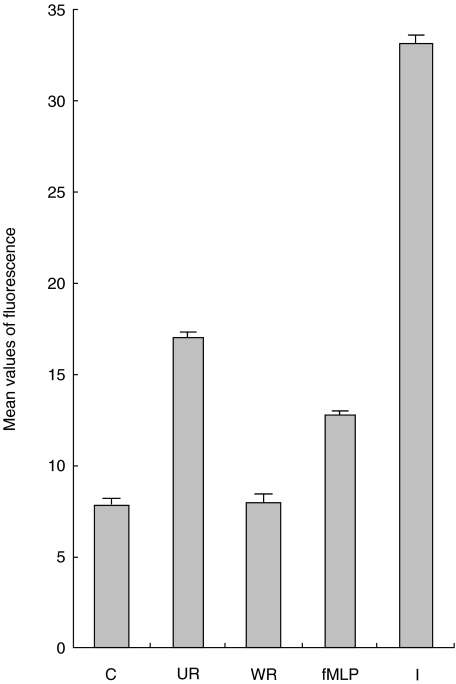
Basal calcium release in nonstimulated PMNs was measured as an Indo-1 fluorescence ratio of 7·6 ± 0·05210/381nm compared to the positive ionomycin control ratio of 32·7 ± 0·03210/381nm. Following the addition of unwashed RSV the mean Indo-1 fluorescence ratio rapidly increased 2·2 fold (16·8 ± 0·06210/381 nm), and then gradually decayed to baseline. The addition of washed RSV did not stimulate an increase in the mean Indo-1 fluorescence ratio (7·8 ± 0·02210/381nm). Addition of filtrate led to a rapid increase in the fluorescence ratio (12·6 ± 0·06210/381 nm) but this response decayed to the basal levels more quickly compared to the effect of an unwashed RSV sample (Fig. 5).
Fig. 5.
Graph showing the peak mean values (± SE) values of Indo-1 fluorescence in purified neutrophils with different stimulants. The samples were compared to the control (C) of no stimulants, positive control of 1 µm fMLP (fMLP) and 100 n m Ionomycin (I). Both washed (WR) and unwashed (UR) RSV preparations were used at 1 × 104 pfu/ml.
When the percentage of PMNs releasing calcium was assessed against the basal control expression, the positive cells increased at a rate similar for both ionomycin and the unwashed RSV preparation. However, the proportion of positive cells decayed quickly from 90 to 70% in response to the unwashed virus preparation compared to the Ionomycin challenge. In contrast no increase to responding cells was observed after treatment with the washed RSV preparation. The virus free filtrate sample stimulated detectable calcium release in 55% of the cells.
Effect of G glycoprotein
Different concentrations of G glycoprotein ranging from 10 to 0·001 µg/ml did not to have any measurable effect on the PMNs. When the PMNs were exposed to 10 µg/ml of purified G-glycoprotein they expressed a mean of 22·4 ± 0·4 SE MFI for CD11b which was not significantly higher than the nonstimulated control PMNs (19 ± 0·2 SE MFI). CD18 expression in response to purified G glycoprotein was also examined but no changes to the expression of this molecule were observed.
Effect of anti-F antibody
More than 100 µg/ml of the anti-F antibody completely blocked viral infection in plaque assays. Pre-incubation of unwashed RSV with 100 µg/ml of the blocking F-glycoprotein antibody did not significantly reduce the expression of CD11b (32·7 MFI ± 0·2 SE units) compared with unwashed RSV alone (35·0 MFI ± 0·2 SE units) or CD18 expression (51·8 MFI ± 0·4 SE units vs. 51·4 MFI ± 0·4).
Discussion
This study indicates that it is possible to purify RSV effectively using a rapid diafiltration technique and that PMN activation in response to RSV infection is largely attributable to the presence of inflammatory cytokines rather than a direct response to virus or viral products. The results emphasis the need to purify virus when attempting explore aspects of the virus host interaction.
Some groups have previously attempted to investigate the possibility that RSV may directly activate PMNs [19,20] but the purity of the viral preparation was not defined. In this present study, incubation of neutrophils with a washed preparation of RSV resulted in weak activation of these granulocytes as compared to the activation seen when unwashed preparations of RSV were added, or the virus free filtrate which contained the inflammatory products from damaged epithelial cells. This was observed across all the markers assessed with only relatively small increases in CD11b, CD18b and in oxidative burst. Washed RSV also had no impact on intracellular calcium release. Furthermore, purified RSV G glycoprotein did not activate the neutrophils. Whilst washed RSV did not activate the cells but the unwashed RSV preparation did, the addition of a neutralizing RSV F-glycoprotein antibody to the unwashed preparation in no way diminished the stimulatory activity of this preparation. This same antibody completely blocked uptake of RSV by the HELA cells used to propagate the virus. These results indicate that neither F of G glycoproteins stimulate neutrophil cytotoxic activity. Further work is required to determine whether host antibodies, lectins, or complement, when bound to RSV activate neutrophil responses. It is also possible that PMNs are more competent to respond to RSV when activated by specific cytokine/chemokine signals.
The rapid method for washing RSV preparations described in this study generated small amounts of infective virus (e.g. ∼106 pfu from starting titres of 108 pfu) but has several advantages compared to existing purification methods. Conventional sucrose gradient methods take significantly longer, and are also associated with a loss of infective virus [22]. Furthermore the final product may require additional manipulation to remove sucrose and the stabilizing agent MgSO4 that can interfere with cellular assays. The measurement of IL-8 concentration demonstrated the effectiveness of this washing procedure. IL-8 is a key chemokine produced by RSV infected airway cell and is involved in the activation and recruitment of granulocytes. Concentrations of IL1α were also measured using a specific ELISA, but we were at the limits of detection both in the unwashed RSV preparations and were undetectable in the washed preparations.
Despite loosing infective virus during diafiltration equal infective titres of washed and unwashed RSV were applied to the HeLa cells. 48 h after inoculation with the virus, many dead HeLa cells were observed across the monolayer but only when the unwashed virus was added. This demonstrated that distinct from cell damage caused to infected cells significant damage was also caused by factors in the unwashed virus preparation. The same cytopathic effects occurred following the addition of unwashed UV irradiated preparations of RSV that were unable to replicate. To measure the onset of inflammatory signalling caused by RSV replication in the epithelial cell it is therefore necessary to use washed preparations of the virus.
The reduced RSV titre following diafiltration was not due to the virus escaping across the 1000 kD pores of the polyethersulphone membrane. Infective virus was not detected in the filtrate samples in any of these experiments. The loss was also not caused by handling of the virus as a control sample of RSV was treated in the same way except for passage through the filter and this showed no loss of infectivity. The most probable reason for loss of titre was adherence of RSV to the upper surface of the filter membrane under the centrifugal pressure. This was minimized but not completely prevented by precoating the filter ultrapure Casein. Despite these caveats, washed RSV was obtained quickly using these filters and of sufficient titre to carry out challenge studies upon PMNs. The recovery of the virus using this method may be improved by using larger filter 60 ml units that can be operated under pressure without centrifugation. Pre-treatment of the membranes with a mild surfactant prior to use has also been shown to reduce nonspecific binding of samples.
These present observations suggest that the activation of neutrophils in response to RSV infection is largely attributable to epithelial derived inflammatory signalling molecules and that neutrophils may not directly recognize intact RSV, or its constituent molecules. However, the results obtained with washed RSV require further investigation since it is possible that neutralizing antibody, lectin, or complement, complexed with RSV may activate neutrophil cytotoxicity.
Acknowledgments
We would like to acknowledge the technical help provide by Mr Simon Hawkins and the financial support provided by the Sheffield Children's Appeal through out this work. We would also like to thanks Dr Sharon Brownlow (Sartorius Ltd) for technical advice regarding the use of the Vivaspin columns.
References
- 1.Everard ML. Respiratory syncytial virus bronchiolitis and pneumonia. In: Taussig L, Landau L, editors. Textbook of Paediatric Respiratory Medicine. St Louis: Mosby; 1998. pp. 580–94. [Google Scholar]
- 2.Gilchrist S, Torok TJ, Gary HE, Jr, Alexander JP, Anderson LJ. National surveillance for respiratory syncytial virus, United States, 1985–90. J Inf Dis. 1994;170:986–90. doi: 10.1093/infdis/170.4.986. [DOI] [PubMed] [Google Scholar]
- 3.Fleming DM, Cross KW. Respiratory syncytial virus or influenza. Lancet. 1993;342:1507–10. doi: 10.1016/s0140-6736(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 4.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. ‘Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection.’. Arch Dis Child. 1994;71:428–32. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith P, Wang SZ, Dowling K, Forsyth K. Leukocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health. 2001;37:146–51. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Harb M, Bell F, Finn A, Rao WH, Nixon L, Shale D, Everard ML. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J. 1999;14:139–43. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans GS, Pettitt E, Elphick HE, Jones A, Everard ML. Identification of Neutrophil Elastase as the Major Uninhibited Protease in the Airways of Children with RSV Positive Bronchiolitis. Am J Respiratory Crit Care Med. 1999;159:A189. [Google Scholar]
- 8.Wang SZ, Xu H, Wraith A, Bowden JJ, Alpers JH, Forsyth KD. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J. 1998;12:612–8. doi: 10.1183/09031936.98.12030612. [DOI] [PubMed] [Google Scholar]
- 9.Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, IL-8 production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991;147:4307–12. [PubMed] [Google Scholar]
- 10.Noah TL, Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993;265:L472–8. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 11.Arnold R, Humbert B, Werchau H, Gallati H, Konig W. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunol. 1994;82:126–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–64. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noah TL, Ivins SS, Murphy P, Kazachkova I, Moats-Staats B, Henderson FW. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin Immunol. 2002;104:86–95. doi: 10.1006/clim.2002.5248. [DOI] [PubMed] [Google Scholar]
- 14.Schultz C, Richter N, Moller JC, Bucsky P. IFN-gamma response and IL-8 plasma levels in neonates with respiratory syncytial virus bronchiolitis. Eur Respir J. 2000;17:321–4. doi: 10.1183/09031936.01.17203210. [DOI] [PubMed] [Google Scholar]
- 15.Jones A, Qui JM, Bataki E, Elphick H, Ritson S, Evans GS, Everard ML. Neutrophil survival is prolonged in the airways of healthy infants and infants with RSV bronchiolitis. Eur Respir J. 2002;20:651–7. doi: 10.1183/09031936.02.00278902. [DOI] [PubMed] [Google Scholar]
- 16.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 17.de Haas M, von Vossebeld PJ, dem Borne AE, Roos D. Fc gamma receptors of phagocytes. J Laboratory Clin Med. 1995;26:330–41. [PubMed] [Google Scholar]
- 18.Faden H, Kaul TN, Ogra PL. Activation of oxidative and arachidonic acid metabolism in neutrophils by respiratory syncytial virus antibody complexes: possible role in disease. J Inf Dis. 1983;148:110–6. doi: 10.1093/infdis/148.1.110. [DOI] [PubMed] [Google Scholar]
- 19.Jaovisidha P, Peeples ME, Brees AA, Carpenter LR, Moy JN. Respiratory syncytial virus stimulates neutrophil degranulation and chemokine release. J Immunol. 1999;163:2816–20. [PubMed] [Google Scholar]
- 20.Konig B, Krusat T, Streckert HJ, Konig W. IL-8 release from human neutrophils by the respiratory syncytial virus is independent of viral replication. J Leukoc Biol. 1996;60:253–60. doi: 10.1002/jlb.60.2.253. [DOI] [PubMed] [Google Scholar]
- 21.Mbiguino A, Menezes J. Purification of human respiratory syncytial virus: superiority of sucrose gradient over percoll, renografin, and metrizamide gradients. J Virol Meth. 1991;31:161–70. doi: 10.1016/0166-0934(91)90154-r. [DOI] [PubMed] [Google Scholar]
- 22.Fernie BF, Gerin JL. The stabilization and purification of respiratory syncytial virus using MgSO4. Virology. 1980;106:141–4. doi: 10.1016/0042-6822(80)90229-9. [DOI] [PubMed] [Google Scholar]
- 23.Ottolini MG, Curtis SR, Mathews A, Ottolini SR, Prince GA. Palivizumab is highly effective in suppressing respiratory syncytial virus in an immunosuppressed animal model. Bone Marrow Transplant: 2002;29:117–20. doi: 10.1038/sj.bmt.1703326. [DOI] [PubMed] [Google Scholar]
- 24.Smith J, Cortes N, Evans G, Read R, Finn A. Induction of beta2 integrin-dependent neutrophil adhesion to human alveolar epithelial cells by type 1 Streptococcus pneumoniae and derived soluble factors. J Inf Dis. 1998;177:977–85. doi: 10.1086/515235. [DOI] [PubMed] [Google Scholar]
- 25.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. Roles of CD18 and intercellular adhesion molecule-1. J Immunol. 1996;156:4774–82. [PubMed] [Google Scholar]
- 26.Wang SZ, Smith PK, Lovejoy M, Bowden JJ, Alpers JH, Forsyth KD. Shedding of 1-selectin and PECAM-1 and upregulation of Mac-1 and ICAM-1 on neutrophils in RSV bronchiolitis. Am J Physiol. 1998;275:L983–L989. doi: 10.1152/ajplung.1998.275.5.L983. [DOI] [PubMed] [Google Scholar]