Abstract
Coxsackie virus RNA has recently been detected in biopsy specimens of minor salivary glands from patients with primary Sjögren's Syndrome (pSS). A peptide derived from Coxsackie virus 2B protein (pepCoxs) presents 87% sequence homology with the 222–229 region of the major linear B-cell epitope of Ro60 kD autoantigen (pep216–232). Synthetic peptides corresponding to pep216–232: 216KALSVETEKLLKYLEAV232 and pepCoxs: 31MVTSTITEKL LKNLVKI47, were prepared. Sera from 42 patients with pSS and 43 patients with systemic lupus erythematosus (SLE) as well as sera from 27 healthy individuals (normal controls) and sera from 30 patients with rheumatoid arthritis (disease controls) were tested against the two homologous peptides. Twenty-five percent of SLE sera and 33·3% of pSS sera reacted against pep216–232, whereas 28% of SLE sera and 37% of pSS sera recognized the pepCoxs. The sera reacting with pep216–232 were apparently the same as those reacting with pepCoxs. Normal sera and disease control sera presented only a limited reactivity against both peptides (ranging from 3·7% to 10%). Both peptides reacted more prominently with anti-Ro/La (+) sera from pSS patients. Thus, pep216–232 was recognized by 17% of the anti-Ro (+) sera and by 42% of the anti-Ro/La (+) sera, whereas pepCoxs was recognized by 28·5% and 38% of the a-Ro(+) and a-Ro/La(+) sera, respectively. Purified anti-pep216–232 antibodies readily reacted with both peptides while inhibition experiments revealed the specificity of this reaction. These results suggest a possible cross-reaction between antibodies to the major linear B-cell epitope of Ro60 kD autoantigen and the homologous pepCoxs in pSS patients. This cross-reaction might potentially play a role in autoantibody formation and the perpetuation of the autoimmune response against Ro/SSA and La/SSB.
Keywords: Autoimmunity, Sjogren's syndrome, epitope, antigen, Coxsackie virus
Introduction
Molecular mimicry between foreign and host protein remains an attractive mechanism to explain the initiation and perpetuation of autoimmune response. Autoimmune disease is the result of an aberrant immune response against self-antigens, leading to tissue damage and loss of function of target organs. The exact mechanisms of the breakdown of self-tolerance are not fully understood; environmental factors, such as infectious agents, have for a long time been considered as the initiating agent of autoimmunity. Therefore, the induction of autoimmunity by infectious agents can be accomplished by molecular mimicry, epitope spreading, activation of T cells by microbial superantigens, bystander activation and activation of lymphocytes by lymphotropic viruses [1,2]. According to the molecular mimicry hypothesis, a cross-activation of autoreactive T- or B-lymphocytes by a foreign antigen sharing a T- or B- cell epitope with a host antigen occurs. Molecular mimicry has been implicated to the pathogenesis of several organ specific autoimmune diseases, including multiple sclerosis, myocarditis, diabetes mellitus type I, acute rheumatic fever, myasthenia gravis, Guillain—Barré syndrome, ankylosing spondylarthropathies and herpes stromal keratitis [3].
Sjögren's Syndrome (SS) is an autoimmune disease characterized by lymphocytic invasion and destruction of exocrine glands. The autoantibody profile of the disease is characterized by the presence of autoantibodies to Ro/SSA and La/SSB ribonucleoproteins. In an attempt to identify the origin of these autoantibodies and elucidate the mechanisms underlying the perpetuation of humoral autoimmune response, others and our laboratories focused on the definition and biologic relevance of B cell epitopes of Ro/SSA and La/SSB autoantigens. Ro/SSA has two linear epitopes spanning the sequences 169–190aa and 216–232aa of the molecule. Protein sequence data search revealed that epitope 216–232aa (216KALSVETEKLLKYLEAV232) possess in 7/8 aminoacids similarity with a region of protein 2B of Coxsackievirus A21 (31MVTSTITEKLLKNLVKI47) and Coxsackievirus A13 (31MVTSVLTEKLLKNLIKI47).
In our laboratory, Coxsackievirus B4 and A13 RNA has been recently detected in all minor salivary gland biopsy samples from patients with primary SS indicating an active involvement of Coxsackievirus in the pathogenesis of the disease [4]. Coxsackieviruses belong to enterovirus genus of picornaviridae family. Their genome codes for a large polyprotein which contains various structural and nonstructural proteins. Protein 2B, a nonstructural small hydrophobic protein of 99 aminoacids, is a product of the P2 precursor molecule of the viral polyprotein. Functionally, it is characterized as viroporin, a protein that interacts with membranes modifying the permeability of cellular membrane [5]. Protein 2B inhibits protein secretion [6], whereas its precursor molecule 2 Bc has been correlated to intracellular membrane remodelling and vesicle formation of the viral replication complex in enterovirus—infected cells [7].
These observations prompted us to investigate whether cross-reactivity between the two homologous peptides occurs. Hereafter is shown that one third of pSS sera with anti-Ro/SSA antibodies possess antibodies reacting with both peptides.
Materials and methods
Peptide synthesis
Aminoacid sequence of the major linear B-cell epitope of human 60 kDRo/SSA autoantigen [8], spanning the sequence 216–232aa (pep216–232), was compared with known sequences in Uniprot Protein Data Bank and was found to share homology with viral antigens. Pep216–232 shares in its 222–229 region a sequence similarity of 87% with the TEKLLKYL peptide of protein 2B of Coxsackievirus.
Synthetic peptides 216KALSVETEKLLKYLEAV232 (pep 216–232) and 31MVTSTITEKLLKNLVKI47 (pepCoxs) which contained the homologous sequences, were synthesized by solid phase peptide synthesis (Biosynthesis Incorporated, Lewisville, TX, USA). In addition, an irrelevant peptide, the 250–257 a.a.region (IASRYDQL) from Leismania glycoprotein gp63 was also purchased by Biosynthesis and used as control peptide (pepCTRL).
Patients and sera
Twenty-seven patients with primary Sjögren's Syndrome (pSS) and 28 patients with Systemic Lupus Erythematosus (SLE) were studied. All patients fulfilled the revised criteria for the classification of pSS [9] and SLE [10]. The autoantibody profile was evaluated in all sera by counterimmunoelectrophoresis (CIE): 28 samples had anti-Ro/SSA autoantibodies and 27 samples had both anti-Ro/SSA and anti-La/SSB autoantibodies. Sera from 27 healthy donors were used as controls. All control sera were age-matched and sex-matched with the 27 pSS sera. In addition, sera from 30 patients with rheumatoid arthritis (RA) [11] and 30 patients with pSS or SLE without anti-Ro/SSA and anti-La/SSB autoantibodies were used as disease controls. All sera had been taken for diagnostic purposes with the full consent of the patients that part of the serum will be used for research purposes.
Purification of specific anti-pep216–232 antibodies
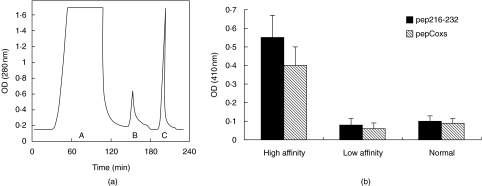
To assess the reactivity of anti-Ro60 kD antibodies against pep216–232 and pepCoxs, specific anti-pep216–232 antibodies were purified from 3 pSS patient sera by affinity chromatography with pep216–232/Sepharose 4B column. For this purification, the synthetic peptide pep216–232 was coupled (coupling buffer pH = 9·9) to CNBr-activated Sepharose 4B gel at a concentration of 10 mg/g of freeze-dried sepharose powder according to the manufacturer's instructions (Pharmacia Biotech, Uppsala, Sweden). Every serum sample from a patient with pSS was diluted in phosphate buffer saline (PBS) pH: 7·4 and were applied to the column. The latter was washed with PBS and bound immunoglobulins were eluted in two steps. Low affinity antibodies were eluted with phosphate buffer pH: 7·4 containing 0·6 M NaCl and subsequently high affinity antibodies were eluted with 0·1 M HCl-Glycine pH: 2·7. Figure 3a shows the sequential purification of low and high affinity anti-pep216–232 antibodies from a representative pSS serum.
Fig. 3.
Anti-peptide reactivity of purified anti-pep216–232 antibodies. (a) Chromatogram representing the purification procedure of specific anti-pep216–232 antibodies from a representative serum by affinity chromatography A: flowthrough, B: low affinity antibodies eluted with phosphate buffer pH:7·4 containing 0·6 M NaCl C: high affinity antibodies eluted with HCl-glycine pH:2·7 (b) Purified anti-pep216–232 antibodies, from 3 patient sera, exhibited high reactivity in anti-pep216–232 (▪), and anti-pepCoxs (□) ELISAs. In contrast, low affinity antibodies (eluted with 0·6 M NaCl) and normal human serum, gave low OD410 values.
Assays for detection of antibodies against pep216–232 and pepCoxs
Sera reactivity against pep216–232, pepCoxs and the control peptide was tested by solid phase assay. Briefly, all three peptides were diluted in phosphate buffer pH:7·8 at a concentration of 5 µg/ml and were used to coat 96-well polystyrene ELISA plates (Corning-Costar, NY, USA) for 6 h, at 4°C. To eliminate nonspecific binding, the plates were blocked with 200 µl/well of bovine serum albumin (BSA) 2% in PBS pH:7·4 for 1 h. Following three washes with PBS, the plates were incubated with human sera (diluted 1 : 120 in blocking buffer) overnight, at 4°C. After 3 cycles of washing with PBS, an alkaline phosphatase-conjugated anti-human IgG (Jackson ImmunoResearch, Pennsylvania, USA) was added in order to detect antibodies bound onto the solid phase. Following an incubation period of 1 h at room temperature (RT), the plates were washed 4 times with PBS and the bound enzymatic activity was measured with p-nitrophenyl-phosphatase disodium substrate (Sigma, St Louis, USA) at 410 nm by an ELISA reader (Dynatech, UK). Sera from 27 healthy individuals were used as controls at a dilution 1 : 120 in 2% bovine serum albumin (BSA). The cut-off points for the assays were calculated from the mean optical density (OD) value of the sera from the healthy individuals plus two standard deviations (SD).
Inhibition assays
The specificity of the immune response against the homologous peptides, was assessed by homologous and heterologous inhibition of (a) a positive pSS serum sample diluted 1 : 120 in 2% BSA and (b) anti-pep216–232 antibodies at a concentration of 25 µg/ml in 2% BSA. Pep216–232 and pepCoxs were used to coat 96-well polystyrene ELISA plates at a concentration of 5 µg/ml, as previously described at the ELISA section. Pep216–232, pepCoxs and pepCtrl were used as inhibitors at increasing concentrations ranging from 0 to 150 µg/ml. The positive serum sample, as well as the anti-pep216–232 antibodies, were preincubated with the inhibitors for 3 h in RT. Subsequently, the mixtures were transferred to the pep216–232 and pepCoxs coated plates and were incubated for 1 h and 30min at RT. Inhibition was expressed as a percentile reduction of antibody binding of the uninhibited positive serum or purified antibodies.
Results
Prevalence of anti-pep216–232 antibodies and anti-pepCoxs antibodies in patients’ sera
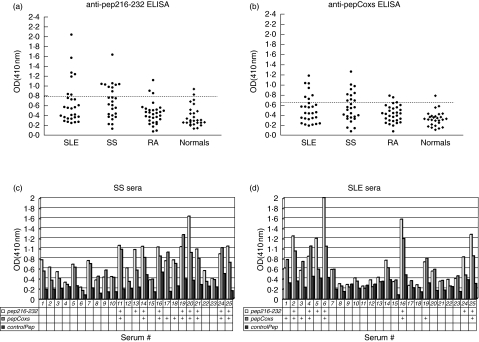
Sera from patients with pSS or SLE or RA as well as from healthy individuals were tested for reactivity against the two homologous peptides pep216–232 and pepCoxs. All results from antipeptide ELISAs are depicted in Fig. 1a,b. It was observed that 25% (7/28) of the SLE sera and 33·3% (9/27) of pSS sera with anti-Ro/SSA or anti-Ro/SSA and anti-La/SSB autoantibodies recognized pep216–232, whereas 28·5% (8/28) of the same SLE sera and 37% (10/27) of the same pSS sera recognized pepCoxs. Such reactivity was detected neither among healthy individuals, nor among disease control patients. Seven point four percent (2/27) and 3·7% (1/27) of healthy controls recognized pep216–232 and pepCoxs, respectively, whereas 10% of disease controls (RA patients) reacted with both peptides. As it is shown in Fig. 1c,d, the reactivity of pSS and SLE sera against pep216–232 and pepCoxs varies in parallel, whereas the reactivity against the control peptide is not significant.
Fig. 1.
Anti-peptide reactivity of autoimmune and normal sera. Twenty-five percent of SLE sera, 33·3% of pSS sera and 10% of RA sera reacted with pep216–232 (a), whereas 28% of SLE sera, 37% of pSS sera and 10% of RA sera reacted with pepCoxs (b) (dotted lines represent the cut off values). The reactivity of pSS (c) and SLE (d) sera against pep216–232 and pepCoxs varied in parallel in contrast to the reactivity against control peptide, which was remained in low levels. Sera with OD values greater than the cut-off value for each peptide are marked with the ‘+’ symbol.
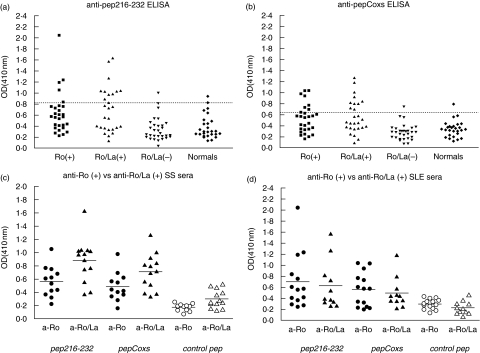
Estimation of the prevalence of anti-pep216–232 and anti-pepCoxs antibodies in patient sera in regard with the autoantibody profile, gave the following results: 17% (5/28) of anti-Ro(+) sera and 42% (11/26) of anti-Ro/La(+) sera gave a positive reaction against pep216–232, whereas the corresponding reactivity against pepCoxs was 28·5% (8/28) and 38% (10/26), respectively. Anti-Ro/La(–) sera demonstrated only a limited reaction with pep216–232 and pepCoxs peptides (Fig. 2a,b). These data demonstrate a stronger recognition of the two homologous peptides by anti-Ro/La(+) sera versus anti-Ro(+) sera. This difference is mostly attributed to the enhanced reactivity of anti-Ro/La(+) sera from patients with pSS (χ2 test, Ro/La(+) versus Ro(+) sera: Ppep216–232 = 0·006, PpepCoxs = 0·02). In contrast, SLE sera did not exhibit significantly different reactivity between anti-Ro/La(+) and anti-Ro(+) groups (χ2 test: Ppep216–232 = 0·55, PpepCoxs = 0·26). Moreover, as is shown in Fig. 2c,d, the median OD410 values of anti-Ro/La(+) sera reactivity is significantly higher from the median OD410 values of anti-Ro(+) sera reactivity only in the case of pSS patients.
Fig. 2.
Anti-peptide reactivity of autoimmune sera according to their autoantibody profile. A stronger recognition of the two homologous peptides by anti-Ro/La positive sera versus anti-Ro positive sera was observed: 17% of anti-Ro positive sera and 42% of anti-Ro/La positive sera gave a positive reaction against pep216–232 (a), whereas the reactivity of anti-Ro positive sera and anti-Ro/La positive sera against pepCoxs was 28·5% and 38%, respectively (b). Amongst pSS patients anti-Ro/La positive sera displayed greater reactivity (median OD410 values) against the two homologous peptides comparing to anti-Ro positive sera (c), whilst SLE patients’ sera did not display statistically different reactivity according to their autoantibody profile (d).
Reactivity of purified anti-pep216–232 antibodies against the two homologous peptides
To further investigate the reactivity of anti-Ro60 kD antibodies against the two homologous peptides, specific anti-pep216–232 antibodies were purified from 3 patient sera by affinity chromatography (Fig. 3a) and then tested in ELISA experiments (Fig. 3b). Purified autoantibodies exhibited high reactivity in anti-pep216–232 and anti-pepCoxs ELISAs. In contrast, low affinity antibodies (eluted with 0·6 M NaCl), as well as serum from a healthy individual, gave low OD410values (Fig. 3b).
Inhibition experiments revealed specificity of this reaction
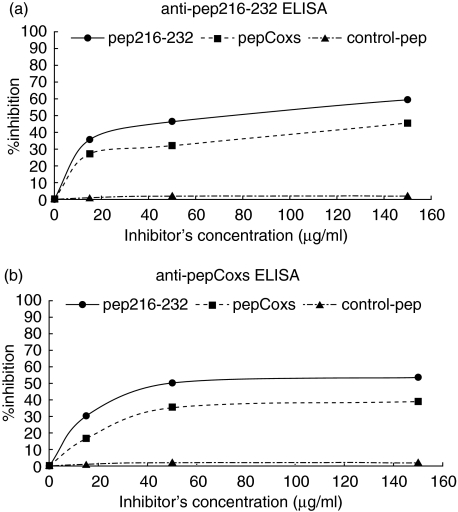
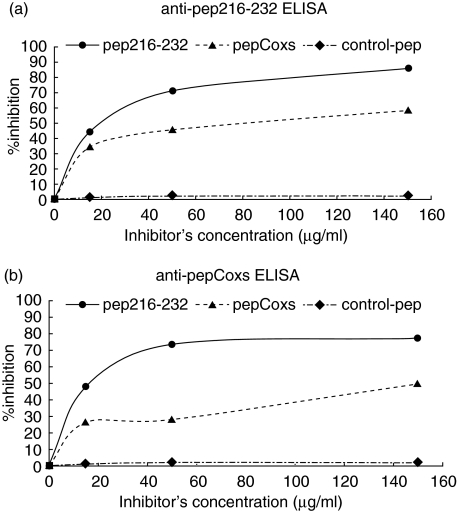
The next step in our study was to investigate the specificity of the recognition of pep216–232 and pepCoxs peptides. For this purpose, homologous and heterologous inhibition experiments were performed. In the anti-pep216–232 ELISA, the reactivity of a serum sample from a patient with pSS was inhibited by 60% and 46% after prior incubation with pep216–232 and pepCoxs, respectively (Fig. 4a). In the anti-pepCoxs ELISA, the inhibition rates were 46% and 40%, when the same serum was preincubated with pep216–232 and pepCoxs, respectively (Fig. 4b). Inhibition rates were further ameliorated, upon usage of purified anti-pep216–232 antibodies instead of the whole patient serum. Using pep216–232 and pepCoxs as inhibitors, the binding of purified anti-pep216–232 antibodies was inhibited by 86% and 60%, respectively, in an anti-pep216–232 ELISA (Fig. 5a). When pepCoxs was used as coating peptide, the inhibition rate was 81% for the pep216–232 and 52% for pepCoxs, respectively (Fig. 5b). Using a control peptide (pepCtrl) as inhibitor, no inhibition was observed.
Fig. 4.
Homologous and heterologous inhibition of a pSS patient serum. Using pep216–232 (•) and pepCoxs (▪) as inhibitors, the reactivity of a serum sample from a patient with pSS was inhibited by 60% and 46%, respectively, in an anti-pep216–232 ELISA (a) and by 46% and 40%, respectively, in an anti-pepCoxs ELISA (b). Using an irrelevant peptide (▴) as inhibitor, no inhibition was observed.
Fig. 5.
Homologous and heterologous inhibition of purified anti-pep216–232 antibodies. (a) Using pep216–232 (•) and pepCoxs (▴) as inhibitors, the binding of purified anti-pep216–232 antibodies was inhibited by 86% and 60%, respectively, in an anti-pep216–232 ELISA. (b) When pepCoxs was used as coating peptide, the inhibition rate was 81% for the pep216–232 (•) and 52% for pepCoxs (▴), respectively. Using an irrelevant peptide (pepCtrl, ♦) as inhibitor, no inhibition was observed.
Discussion
During the past several years, there have been studies linking autoimmunity and viral infection. Molecular mimicry is a mechanism that has been implicated in the generation of cross-reactive antibodies following a viral infection [12–14]. Recently, our laboratory has detected Coxsackievirus RNA in all biopsy specimens of minor salivary glands from patients with pSS, an autoimmune disease characterized by the presence of anti-Ro/SSA and anti-La/SSB autoantibodies [4]. In parallel, a peptide of protein 2B of Coxsackievirus A21 was found having 7/8 (87%) aminoacid homology with a region of the previously identified in our laboratory major linear B-cell epitope of Ro60 kD protein spanning the sequence 216–232aa [8]. These two observations, taken together with the fact that Coxsackievirus infections have been already postulated to play a role in the pathogenesis of human disease such as autoimmune myocarditis [15] and insulin-dependent diabetes mellitus [13], prompted us to investigate a possible cross-reaction between antibodies against the two homologous peptides and hence, possible links between Coxsackievirus and the pathogenesis of SS.
The present study revealed a cross-reaction between antibodies against the homologous pep216–232 and pepCoxs peptides. It was observed that almost the same proportion of pSS sera (33%-37%) reacted with both peptides, SLE sera exhibited a weaker anti-pep216–232 and anti-pepCoxs reactivity (25% and 28%, respectively), while the reactivity of disease controls and normal sera against the homologous peptides was much lower (3·7–10%). The reactivity of pSS and SLE sera against pep216–232 and pepCoxs followed the same pattern: the same patients at a similar extent recognized the homologous peptides. Purified antibodies against pep216–232 of Ro60 kD autoantigen were found to react with both peptides. The specificity of this cross-reaction was demonstrated by homologous and heterologous inhibition experiments. Another observation was that pep216–232 and pepCoxs peptides were preferentially recognized by pSS sera with both anti-Ro/SSA and anti-La/SSB autoantibodies. In contrast, sera from SLE patients with the same autoantibody profile did not demonstrate significant reactivity against the two peptides. Given that anti-La/SSB autoantibodies are more prominent in SS patients (detected in 50% of SS versus 10% of SLE) [16], the stronger recognition of the two peptides by the anti-Ro/La(+) sera from patients with Sjögren's Syndrome, points to a possible stronger correlation between Coxsackievirus and pSS rather than Coxsackievirus and SLE. It should also be noted that the presence of antibodies to pep216–232 linear B-cell epitope of Ro60 kD autoantigen used in this study has been previously correlated with pSS [8]. If there is a cross-reaction between the two homologous peptides, it should be more prominent among SS patients.
The generation of cross-reactive antibodies can be attributed to molecular mimicry, which has been proposed as one of the mechanisms by which viruses could induce or enhance autoimmune responses. From this point of view, an infection with Coxsackievirus could initiate an immune response characterized by production of antibodies against protein 2B, including the determinant shared by Ro60 kD autoantigen. If these antibodies encounter the Ro60 kD autoantigen, the immune response can be perpetuated. In such a case, the antibody affinity for the Ro60 kD autoantigen should be enhanced through a process known as affinity maturation. This hypothesis is consistent with our inhibition experiments in which either sera samples or specific purified anti-pep216–232 antibodies were more efficiently inhibited by pep216–232 independently of the peptide used for the detection of antibodies.
Furthermore, if we consider the function and subcellular distribution of protein 2B and its precursor 2 Bc, we could make some hypotheses about possible interactions between this viral protein and some host cell proteins with possible implications in autoimmunity. One of the main functions that has been attributed to this protein is endoplasmic reticulum (ER) membrane remodeling with formation of the vesicles that are required for the viral replication [7]. It could be possible that during this process, 2 Bc interacts with calreticulin, the molecular chaperone localized in ER. In a previous study in our laboratory, it has been demonstrated that calreticulin binds preferentially with B-cell linear epitopes of Ro60 kD, enhancing their recognition by anti-Ro60 kD autoantibodies, from 30% to 100%[17]. It is suggested that interaction between calreticulin and the viral protein 2 Bc could give a special conformation to its epitopes against which antibodies are generated. The same antibodies possibly cross-recognize the homologous peptide of Ro60 kD autoantigen when the latter is complexed with calreticulin.
In conclusion, we found that a peptide derived from Coxsackievirus 2B protein presents 87% sequence homology with a region of a major linear B-cell epitope of Ro60 kD autoantigen spanning the sequence 216–232 aminoacid. Our experiments demonstrate a cross-reaction between antibodies against the two homologous peptides pointing to the importance of Coxsackievirus infection in pathogenesis of SS. These observations are currently under further investigation in our laboratory.
References
- 1.Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James JA, Harley JB, Scofield RH. Role of viruses in systemic lupus erythematosus and Sjogren syndrome. Curr Opin Rheumatol. 2001;13:370–6. doi: 10.1097/00002281-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–65. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triantafyllopoulou A, Tapinos N, Moutsopoulos HM. Evidence for coxsackievirus infection in primary Sjogren's syndrome. Arthritis Rheum. 2004;50:2897–902. doi: 10.1002/art.20463. [DOI] [PubMed] [Google Scholar]
- 5.Agirre A, Barco A, Carrasco L, Nieva JL. Viroporin-mediated membrane permeabilization. Pore formation by nonstructural poliovirus 2B protein. J Biol Chem. 2002;277:40434–41. doi: 10.1074/jbc.M205393200. [DOI] [PubMed] [Google Scholar]
- 6.Doedens JR, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho MW, Teterina N, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2 BC in human cells. Virology. 1994;202:129–45. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 8.Routsias JG, Tzioufas AG, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM. Epitope mapping of the Ro/SSA60KD autoantigen reveals disease-specific antibody-binding profiles. Eur J Clin Invest. 1996;26:514–21. doi: 10.1046/j.1365-2362.1996.186316.x. [DOI] [PubMed] [Google Scholar]
- 9.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Olson JK, Croxford JL, Calenoff MA, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. J Clin Invest. 2001;108:311–8. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harkonen T, Lankinen H, Davydova B, Hovi T, Roivainen M. Enterovirus infection can induce immune responses that cross-react with beta-cell autoantigen tyrosine phosphatase IA-2/IAR. J Med Virol. 2002;66:340–50. doi: 10.1002/jmv.2151. [DOI] [PubMed] [Google Scholar]
- 14.Panoutsakopoulou V, Sanchirico ME, Huster KM, et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–47. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 15.Huber S. T cells in coxsackievirus-induced myocarditis. Viral Immunol. 2004;17:152–64. doi: 10.1089/0882824041310667. [DOI] [PubMed] [Google Scholar]
- 16.Tzioufas AG, Moutsopoulos HM. Hochberg MC. Rheumatology. Philadelphia: Mosby; 2004. Sjogren's Syndrome. [Google Scholar]
- 17.Staikou EV, Routsias JG, Makri AA, et al. Calreticulin binds preferentially with B cell linear epitopes of Ro60 kD autoantigen, enhancing recognition by anti-Ro60 kD autoantibodies. Clin Exp Immunol. 2003;134:143–50. doi: 10.1046/j.1365-2249.2003.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]