Abstract
Intestinal epithelial cells secrete the chemokine interleukin (IL)-8 in the course of inflammation. Because heat shock proteins (Hsps) and butyrate confer protection to enterocytes, we investigated whether they modulate Salmonella enterica serovar Enteritidis (S. serovar Enteritidis)-induced secretion of IL-8 in enterocyte-like Caco-2 cells. Caco-2 cells incubated with or without butyrate (0–20 m M, 48 h) were infected with S. serovar Enteritidis after (1 h at 42°C, 6 h at 37°C) or without prior heat shock (37°C). Levels of Hsp70 production and IL-8 secretion were analysed using immunostaining of Western blots and enzyme-linked immunosorbent assay (ELISA), respectively. The cells secreted IL-8 in response to S. serovar Enteritidis and produced Hsp70 after heat shock or incubation with butyrate. The IL-8 secretion was inhibited by heat shock and butyrate concentrations as low as 0·2 m M for crypt-like and 1 m M for villous-like cells. In a dose-dependent manner, higher butyrate concentrations enhanced IL-8 secretion to maximal levels followed by a gradual but stable decline. This decline was associated with increasing production of Hsp70 and was more vivid in crypt-like cells. In addition, the higher concentrations abolished the heat shock inhibitory effect. Instead, they promoted the IL-8 production in heat-shocked cells even in the absence of S. serovar Enteritidis. We conclude that heat shock and low concentrations of butyrate inhibit IL-8 production by Caco-2 cells exposed to S. serovar Enteritidis. Higher butyrate concentrations stimulate the chemokine production and override the inhibitory effect of the heat shock. The IL-8 down-regulation could in part be mediated via production of Hsp70.
Keywords: butyrate, heat shock, Hsp70, IL-8, Salmonella serovar Enteritidis
Introduction
The involvement of the intestinal epithelium in host defence is well understood. It serves as an effective physical barrier to protect the internal host milieu against various potentially harmful luminal contents. The enterocytes of this epithelium secrete an array of inflammatory mediators [interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1 and tumour necrosis factor-α (TNF-α)] either constitutively [1,2] or upon stimulation by inflammatory agents (e.g. IL-1β and TNF-α) from other sources and by pathogenic bacteria [3]. These mediators in turn induce a local defensive inflammatory response. Of these mediators, IL-8 is pivotal to and governs the progress of most local intestinal inflammations. IL-8 is a member of the C-X-C family of chemokines that has a diverse spectrum of biological activities including T cell, neutrophil and basophil chemotactic properties [4]. It can be produced by a wide variety of cell types and is believed to play a significant role in the initiation of the acute inflammatory response [5]. In the course of inflammation, IL-8 attracts and activates neutrophils at the site of infection leading to neutrophil infiltration, which may subsequently culminate into epithelial cell damage [6]. Therefore, its down-regulation is vital in the prevention of chronic inflammation.
Within the intestinal compartment, regulation of IL-8 production can be influenced by luminal components such as short chain fatty acids, which are normally present in vivo in the colon lumen as major products of anaerobic fermentation of dietary fibres [7]. Butyrate is the most potent and abundant short chain fatty acid. Its intestinal physiological concentration in man ranges from 5 to 24 mM depending upon the dietary fibre composition [8,9]. Butyrate plays an important role in the homeostasis of the intestinal epithelium. It contributes to modulation of biological processes such as cellular metabolism, growth, differentiation and immunity [10,11]. Butyrate deficiency is linked with the pathogenesis of inflammatory bowel diseases [12]. Restoration of the normal endogenous butyrate levels by intracolonic infusion is used in the treatment of these diseases [12,13], but with limited success [14,15]. This limitation may be due to the possible presence of IL-8 in the intestinal mucosa, especially because butyrate can inhibit [16–18] or stimulate [2,19] IL-8 production by intestinal cells.
Heat shock proteins (Hsps) are a group of evolutionary conserved proteins ranging in size from 8 kDa to 150 kDa that are synthesized rapidly by most cells responding to stress-related events [20,21]. They act as chaperones through protein—protein interaction to protect the already synthesized proteins against further injury. In turn, they protect cells against noxious processes including heat stress, infection and inflammation. They confer protection against inflammation by suppressing the production of inflammatory mediators [22]. The induction of Hsp70 by intestinal cells is known to protect against several stresses including hyperthermia and ischaemia/reperfusion injury [23]. In respiratory epithelial cells, Hsp70 imparts an anti-inflammatory role by inhibiting IL-8 production [24]. This role has not been established in enterocytes.
Considering the protective nature of Hsps, the range of homeostatic processes influenced by butyrate, and the concurrent exposure of intestinal cells to butyrate, it is of interest to know how butyrate and Hsps interact. Because butyrate induces Hsp25 in rat colon cells and in IEC-18 cells (rat intestinal cell line) [25], it is tempting to suggest that butyrate may also induce Hsp70 in human intestinal cells. Subsequently these changes in Hsp70 levels may have an effect on IL-8 synthesis. It is therefore reasonable to hypothesize that butyrate modulates the IL-8 secretion by intestinal cells and that this modulation may be mediated, at least in part, via synthesis of Hsps.
In the experiments presented in this study we focused on the anti-inflammatory properties of the heat-shock response and butyrate. In particular, we investigated the anti-inflammatory potency of Hsp70, induced by either heat shock or butyrate, on Salmonella-induced IL-8 secretion by intestinal cells and established its extent. We used Caco-2 cells, an in vitro model of the human intestinal epithelium, which differentiate in culture and acquire characteristics both structurally and functionally of either crypt cells (5-day-old Caco-2 cells) or villous cells (19-day-old Caco-2 cells) of the small intestine [26–28].
Materials and methods
Cell culture
Human colon adenocarcinoma Caco-2 cells (ATCC HTB 37) were grown in Dulbecco's modified Eagle medium (DMEM) (Flow Laboratories, Amstelstad BV, Amsterdam, the Netherlands) supplemented with 1% (v/v) non-essential amino acids (flow), 10 mM NaHCO3 (flow), 1·7 m M glutamine, 50 µg/ml gentamicin (flow), 25 mM HEPES (flow) and 20% (v/v) fetal calf serum (Sanbio BV, Uden, the Netherlands). Supplemented culture medium devoid of gentamicin and fetal calf serum in the experiments is referred to as plain DMEM. Cell cultures were maintained at 37°C in 95% air−5% CO2 in a humidified atmosphere with three cell culture medium changes per week. Cells were seeded at 40 000 cells/cm2 in 25 cm2 tissue culture flasks (Greiner, Alphen a/d Rijn, the Netherlands) containing 5 ml of cell culture medium.
Heat-shock response
To induce the heat-shock response, Caco-2 cells grown in 25 cm2 tissue culture flasks were exposed to 42°C for 1 h. This was achieved by full immersion of the flasks in a water bath heated by a circulating thermostat DC10 (Haake, Karlsruhe, Germany), which provided a temperature stable within 0·02°C. Temperature equilibration of the cell monolayers took about 30 s. Cells were then allowed to recover at 37°C in a 95% air−5% CO2 humidified incubator for 6 h. The cell culture medium and the Caco-2 cells were then collected.
Incubation of Caco-2 cells with butyrate
Caco-2 cells were cultured to crypt-like (day 5) or villous-like (day 19) enterocytes after which they were incubated in culture medium containing 0, 0·05, 0·1, 0·2, 0·5, 1, 2, 5, 10 and 20 mM butyrate for 48 h. After the first 24 h, cells were exposed to Salmonella serovar Enteritidis 857 (1 h) after or without prior induction of the heat-shock response. Exposure to butyrate was continued with those cells receiving a heat shock, whereas the subsequent exposure to bacteria took place in the absence of butyrate. The cell culture medium and the Caco-2 cells were then collected.
Incubation of Caco-2 cells with trichostatin A and extraction of histones
Caco-2 cells were cultured to crypt-like (day 5) or villous-like (day 19) enterocytes after which they were incubated in culture medium containing 0, 0·1, 0·2, 0·5, 1, 2, 5, 10, 20, 50 and 100 nM trichostatin A (TSA) for 24 h. After this time, culture medium and the cells were collected. Collected cells were divided into two aliquots for Hsp70 and histone acetylation analysis. While Western blotting was performed as described below, histones were extracted according to Cousens and Alberts [29]. Collected histones were separated onto a Triton X-acetic acid—urea gel and the positions of histones were identified with a histone-4 marker (Boehringer Mannheim).
Salmonella serovar Enteritidis 857
S. serovar Enteritidis 857 was grown on Luria—Bertani (LB) agar and one colony was inoculated into 5 ml of LB broth. After growing this inoculum overnight (16 h) with shaking (200 r.p.m.) at 37°C, 1 ml of the resulting bacterial suspension was inoculated into 100 ml of LB broth and incubated with shaking (200 r.p.m.) at 37°C for 2 h to obtain logarithmically growing bacteria. A bacterial suspension was made in 100 ml of plain DMEM after collection of the bacteria by centrifugation (15 min 1500 g at 22°C). From the bacterial suspension a serial dilution was made and cells were exposed to 0–1000 bacteria/cell for 1 h. To stop the exposure, cells were washed twice with 5 ml of plain DMEM supplemented with 50 µg/ml gentamicin.
Cell viability test
Viability of the cells exposed to S. serovar Enteritidis 857 was assessed by trypan blue exclusion and staining with propidium iodide and was 92–95% for cells exposed to 1–200 S. serovar Enteritidis 857 per cell and 70% for cells exposed to 1000 bacteria per cell.
Collection of the cell culture medium and the Caco-2 cells
The cell culture medium was collected in tubes and centrifuged (10 min 600 g at 4°C) to obtain the supernatants. After collecting the cell culture medium, the cell monolayers were rinsed twice with 5 ml of 0·01 M phosphate-buffered saline (PBS) [0·01 M Na2HPO4, 0·01 M NaH2PO4, 0·9% (w/v) NaCl], pH 7·3 at 37°C. The monolayers were then incubated for 30 min at 37°C with 5 ml of transfer medium, pH 7·3 (8 g/l NaCl, 0·2 g/l KH2PO4, 0·2 g/l Na2EDTA.2H2O). The dispersed cells were collected in tubes and washed twice with 10 ml of 0·01 M PBS, pH 7·3 (0°C). The washing procedure involved centrifugation (10 min 300 g at 4°C) and discarding of supernatants. After the last washing step the cells were collected by centrifugation (10 min 600 g at 4°C). The collected cell culture supernatants and the cells were then stored at −70°C until analysis.
Western blot analysis for Hsp70
To the collected Caco-2 cells, 0·5 ml (for crypt-like) or 1·0 ml (for villous-like) of distilled water (4°C) was added and the mixture was sonicated at 0°C for 30 s at an amplitude of 24 µm with an MSE Soniprep 150 (Beun de Ronde BV, Abcoude, the Netherlands). The protein content of the resulting sonicates was determined [30] and found to be equal in all samples. An equal volume of loading buffer (twice the strength) [125 mM Tris (hydroxymethyl) aminomethane-HCl, 4% sodium dodecyl sulphate (SDS), 10%β-mercaptoethanol, 20% glycerol and 0·0015% bromophenol blue, pH 6·8] was added to the protein samples and the mixture was heated at 95°C for 5 min. After loading the slots of the gel with equal amounts of proteins (10 µg) they were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE) (reducing gel) on 10% gels. A protein ladder (Bio-Rad Laboratories, CA, USA) was loaded as a molecular weight marker. Subsequently, the proteins were transferred to Immobilon-P PVDF membrane following the recommendations of the manufacturer (Millipore, Bedford, USA). Protein transfer was confirmed by staining with Ponceau Red stain. The non-specific binding sites on the membrane were blocked by incubating the membrane for 1 h with 10 ml of blocking solution (0·1% Tween/PBS, pH 7·3 containing 5% Boehringer blocking agent) (Amersham Pharmacia Biotech, Buckinghamshire, UK) and the membrane was washed with washing buffer (0·1% Tween/PBS, pH 7·3). The washing procedure consisted of two quick washes followed by three additional ones: one 15-min and two 5-min washes. Subsequently, the membrane was incubated for 1 h with 2·5 µg of mouse anti-Hsp70 monoclonal antibody (SPA-810) (Stressgen Biotechnologies Corporation, Victoria, BC, Canada) in 5 ml of blocking solution (0·1% Tween/PBS, pH 7·3 containing 0·5% Boehringer blocking agent). After having performed the above-mentioned washing procedure again, the blots were incubated for 1 h with goat antimouse IgG alkaline phosphatase secondary antibody (SAB-101) (Stressgen). The washing procedure was repeated again and the blots were incubated for 5 min in alkaline phosphatase detection buffer (0·1 M Tris-HCl, 0·1 M NaCl, 5 mM MgCl2·6H2O, pH 9·5) containing 2% (v/v) NBT-BCIP (nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indoyl-phosphate) (product number 1681451) (Roche Diagnostics GmbH, Mannheim, Germany).
Determination of IL-8 secretion by sandwich enzyme-linked immunosorbent assay (ELISA)
IL-8 concentrations were assayed using the IL-8 Cytosets™ antibody pair kit containing matched, pretitred and fully optimized capture and detection antibodies, recombinant standard and streptavidin—horseradish peroxidase (catalogue number CHC1304) (Biosource Europe SA, Nivelles, Belgium). The assay was conducted according to the manufacturer's specifications.
Statistical analysis
Statistical significance between the mean values of control, heat-shocked, butyrate and S. serovar Enteritidis 857-exposed cells was assessed by one-way analysis of variance (anova) with comparison of means. Differences were considered significant at 95% confidence interval using Student's t-test.
Results
Inhibition of Salmonella-induced IL-8 secretion by heat shock in Caco-2 cells
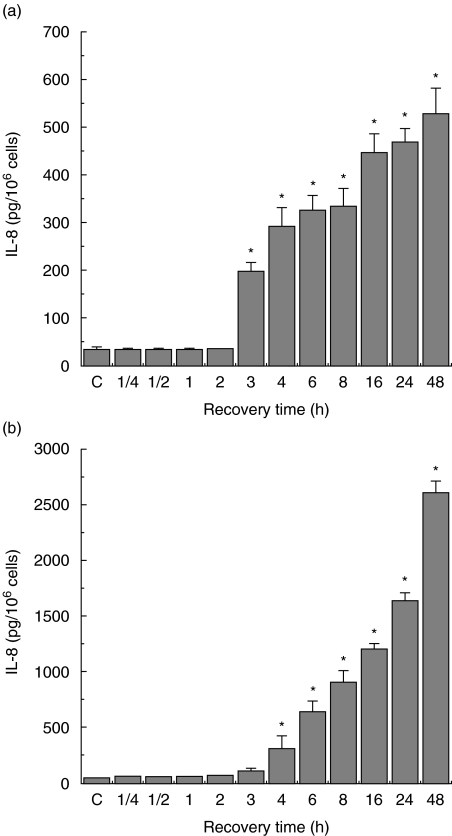
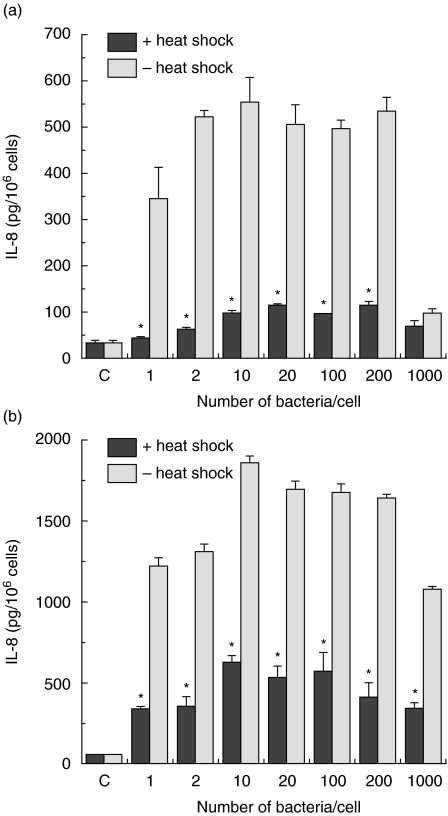
Before investigating the modulatory potency of Hsps on IL-8 production in enterocyte-like Caco-2 cells, a chemoattractant that is pivotal to most intestinal inflammations, the pattern of Salmonella-induced IL-8 secretion was determined. Exposure of the cells to 200 bacteria/cell for 1 h resulted in a linear time-course IL-8 production (Fig. 1a, b). Compared to control cells the secretion is already significantly higher 3 h after infection for both crypt-like (Fig. 1a) and villous-like cells (Fig. 1b). When Caco-2 cells were exposed for 1 h to graded numbers of bacteria (1, 2, 10, 20, 100, 200 and 1000 bacteria/cell) a significant increase in the secretion of IL-8 was achieved in comparison with control cells. Already, on exposure to low numbers of bacteria, the levels of IL-8 secretion rapidly increased and the highest levels were measured after incubation with 10 bacteria/cell for both crypt-like (Fig. 2a) and villous-like cells (Fig. 2b), while further increasing the numbers of bacteria up to 200 bacteria/cell the levels remained approximately constant. Cell cultures exposed to 1000 bacteria/cell showed distinct indications of cell death that was expressed in a sudden decrease of the levels of IL-8 secretion. A cell viability test indicated that only 70% of the cells exposed to 1000 bacteria/cell were viable, while 92–95% of cells exposed to 1–200 bacteria/cell were viable.
Fig. 1.
Interleukin (IL)-8 secretion by Salmonella serovar Enteritidis exposed crypt-like (a) and villus-like (b) Caco-2 cells. Incubation of the cells was performed with 200 bacteria/cell and the IL-8 secretion was measured at various time-points. IL-8 secretion is expressed as pg IL-8/106 cells. Salmonella-induced IL-8 secretion was determined using two cell passages and triplicate cultures per passage. Significant differences (*P < 0·05) between the IL-8 levels of Salmonella exposed cells and control Caco-2 cells are indicated.
Fig. 2.
Inhibition of Salmonella serovar Enteritidis-induced interleukin (IL-8) secretion in crypt-like (a) and villus-like (b) Caco-2 cells by heat shock treatment. Control (1 h 37°C/6 h 37°C) and heat-shocked cells (1 h 42°C/6 h 37°C) were exposed to graded numbers of bacteria for 1 h and allowed to recover from bacterial exposure for 24 h. Salmonella-induced interleukin (IL)-8 secretion is expressed as pg IL-8/106 cells. Inhibition of Salmonella-induced IL-8 secretion was determined using two cell passages and triplicate cultures per passage. Significant differences (*P < 0·05) between the IL-8 levels of heat-shocked and control Caco-2 cells are indicated.
When Caco-2 cells received a heat shock, which is known to induce a significant expression of Hsps, prior to incubation with the same graded numbers of bacteria, this thermal stress attenuated the Salmonella-induced IL-8 production in both crypt-like (Fig. 2a) and villous-like (Fig. 2b) cells. Exposure of Caco-2 cells to a temperature shock alone did not modify the levels of IL-8 (data not shown). The results demonstrated clearly that the Salmonella-induced IL-8 secretion in heat-shocked Caco-2 cells had been markedly inhibited.
Induction by butyrate of Hsp70 expression in Caco-2 cells
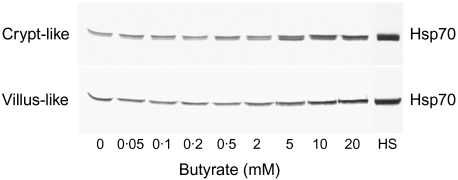
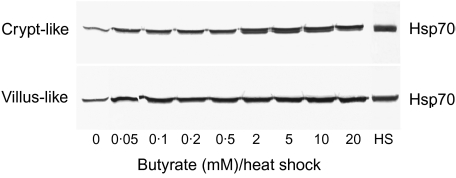
In order to elucidate whether butyrate induces Hsp70 expression, Caco-2 cells were incubated for 48 h in a range of physiological butyrate concentrations (0·05–20 mM). Compared to control cells, Western blot analysis clearly showed that with increasing butyrate concentrations the expression of Hsp70 in both crypt-like and villous-like Caco-2 cells was significantly enhanced (Fig. 3). The response was higher in crypt-like than in villous-like cells. A change in the expression could be observed at concentrations as low as 0·5 mM for crypt-like and 5 mM for villous-like cells. At 20 mM the strongest induction was achieved in both cell types. Although this concentration of butyrate induced a significant expression of Hsp70, as can be judged from the immunostaining intensity of these bands in both crypt-like and villous-like cells, the levels of expression did not reach those displayed by heat-shocked cells (HS).
Fig. 3.
Induction of heat shock protein (Hsp) 70 synthesis in enterocyte-like Caco-2 cells during incubation with butyrate. Cells were incubated for 48 h in a range of physiological butyrate concentrations. HS indicates the level of Hsp70 in cells heat-shocked at 42°C in the absence of butyrate. Subsequently, the cells were processed for Western blotting and immunostaining as described. The blot is a representative of two cell passages and triplicate cultures per passage.
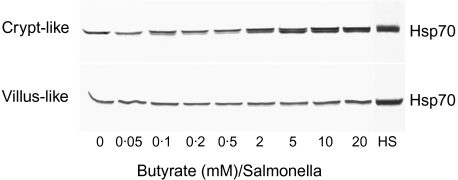
Because the induction of Hsps is generally implicated in cytoprotection during infection and inflammation, the potency of butyrate to induce the expression of Hsp70 in S. serovar Enteritidis 857-infected Caco-2 cells has been investigated. As demonstrated in Fig. 4, a dose-dependent Hsp70 expression was exhibited, especially with the crypt-like cells. At 2 m M the levels were already significantly higher and the highest expression was found at 20 mM (Fig. 4). By quantifying the stained blots using a Bio-Rad GS700 imaging densitometer, we observed a slight difference among the various butyrate concentrations for villous-like cells. Nevertheless, an increase in Hsp70 expression with 5–20 m M could be observed in these cells (Fig. 4). In addition, at the highest butyrate concentration the level of Hsp70 expression in S. serovar Enteritidis 857-infected Caco-2 cells was lower (95% confidence interval of six quantified blots using a Bio-Rad GS700 imaging densitometer) compared to the levels of the thermally induced Hsp70. Because the slots of all gels were loaded with equal amounts of protein (10 µg), the Western blots demonstrate clearly that exposure of the cells to butyrate and bacteria (Fig. 4) revealed a higher expression than by butyrate alone (Fig. 3).
Fig. 4.
Induction of heat shock protein (Hsp) 70 expression in Salmonella serovar Enteritidis exposed enterocyte-like Caco-2 cells after preincubation with butyrate. After preincubation of the cells for 24 h in a range of physiological butyrate concentrations, cells were exposed to Salmonella (200 bacteria/cell) for 1 h and allowed to recover for 24 h in the very same butyrate concentrations. HS indicates the level of Hsp70 in cells heat-shocked at 42°C in the absence of butyrate. After these experimental procedures the cells were processed for Western blotting and immunostaining as described. The blot is a representative of two cell passages and triplicate cultures per passage.
In order to investigate whether thermal stress interfered with the butyrate-induced expression of Hsp70, the enterocyte-like Caco-2 cells were heat-shocked at 42°C. Taking into account that equal amounts of protein are loaded, the staining intensity of the bands (Fig. 5) shows that exposure to butyrate and thermal stress enhances further the expression observed by butyrate alone (Fig. 3). The expression extended to as low as 0·05 m M butyrate, which could not be observed by butyrate-exposed cells (Fig. 3), and exhibited a dose-dependent pattern that was maximal at 20 mM for both cell types. At this concentration the expression level of Hsp70 was only slightly lower than that induced by heat shock.
Fig. 5.
Heat-shock-induced synthesis of heat shock protein (Hsp) 70 in enterocyte-like Caco-2 cells after preincubation with butyrate. Cells were incubated for 48 h in a range of physiological butyrate concentrations. The incubation period was interrupted after 24 h and the cells were heat-shocked (1 h 42°C/6 h 37°C) in the presence of butyrate. HS indicates the level of Hsp70 in cells heat-shocked at 42°C in the absence of butyrate. After these experimental procedures the cells were processed for Western blotting and immunostaining as described. The blot is a representative of two cell passages and triplicate cultures per passage.
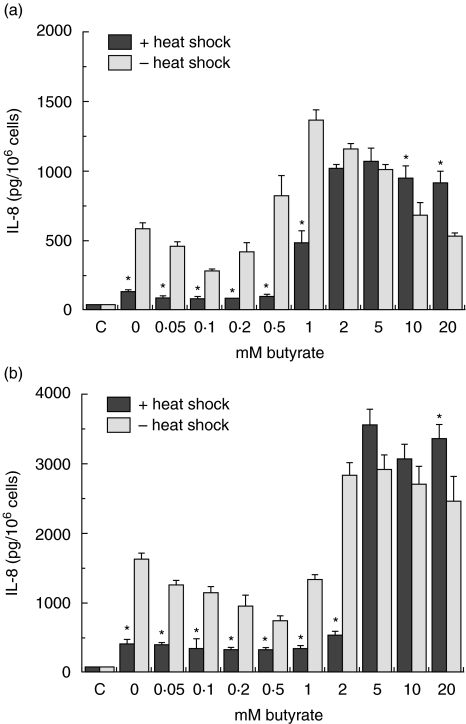
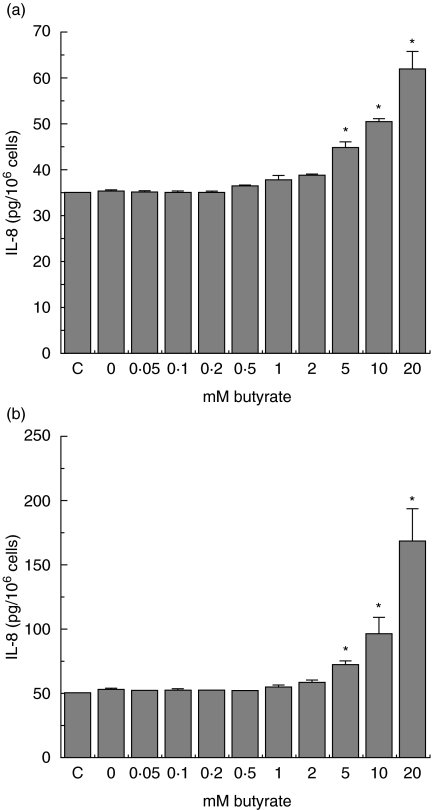
Modulation of IL-8 secretion by butyrate in Salmonella-exposed Caco-2 cells
Butyrate is implicated in interfering with chemokine production in intestinal epithelial cells. After having demonstrated the ability of butyrate to induce Hsp70 (Fig. 3), its effect on IL-8 production in Caco-2 cells was investigated. Exposure to S. serovar Enteritidis 857 (200 bacteria/cell) in the presence of butyrate resulted in a concentration-dependent modulation of IL-8 secretion by both crypt-like (Fig. 6a) and villous-like (Fig. 6b) cells. Both inhibitory and stimulatory effects of butyrate on IL-8 secretion could be clearly observed. Low butyrate concentrations of up to 0·2 m M (crypt-like) and 1 m M (villous-like) inhibited the IL-8 secretion achieving the lowest levels at 0·1 m M and 0·5 m M, respectively. The secretion started rising with further increase in butyrate concentrations and reached its highest level at 1 mM and 2 mM for crypt-like and villous-like cells, respectively. Beyond the 1 mM concentration, the IL-8 levels in crypt-like cells exhibited a stable gradual decline with further increase in butyrate concentrations. At 10 and 20 mM the levels of IL-8 did not differ significantly from the levels in control cells. In villous-like cells, the high butyrate concentrations of 2–20 m M maintained high levels of IL-8 production with little or no difference at all in IL-8 levels.
Fig. 6.
Heat-shock-induced modulation of Salmonella serovar Enteritidis induced interleukin (IL)-8 secretion in crypt-like (a) and villus-like (b) Caco-2 cells during incubation with butyrate. Cells were incubated for 48 h in a range of physiological butyrate concentrations. The incubation period was interrupted after 24 h and the cells were heat-shocked in the presence of butyrate. Control (1 h 37°C/6 h 37°C) and heat-shocked cells (1 h 42°C/6 h 37°C) were then exposed to 200 bacteria/cell for 1 h and allowed to recover from bacterial exposure in the presence of butyrate for the remaining 24 h. Salmonella-induced IL-8 secretion is expressed as pg IL-8/106 cells. Inhibition of Salmonella-induced IL-8 secretion was determined using two cell passages and triplicate cultures per passage. Significant differences (*P < 0·05) between the IL-8 levels of heat-shocked and control Caco-2 cells are indicated.
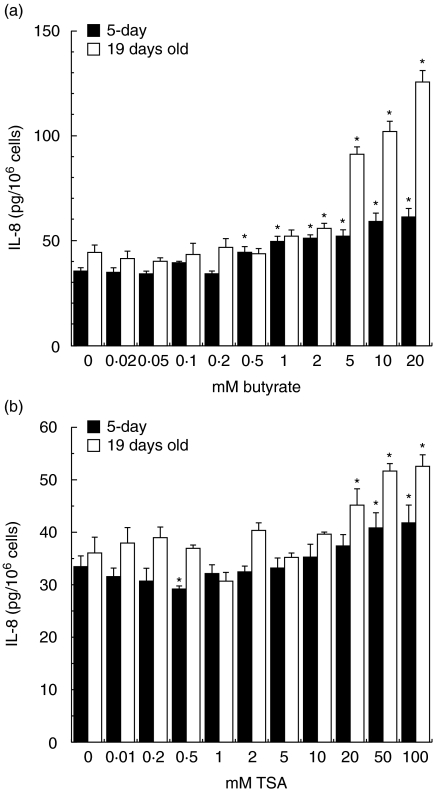
Effect of trichostatin A on IL-8 and Hsp70 production
The clear observation that low concentrations of butyrate did not induce Hsp70 (Fig. 3) and yet inhibited IL-8 production (Fig. 6), alluded to a separate mechanism on IL-8 modulation independent of Hsp70 production. To demonstrate the mechanism, we tested the well-known mechanism of butyrate on IL-8 synthesis. Butyrate acts on IL-8 secretion through its ability to induce histone acetylation by inhibiting histone deacetylase. To determine if histone acetylation inhibited the IL-8 secretion at low butyrate concentrations, we inhibited histone deacetylase by trichostatin-A (TSA). TSA is a specific histone deacetylase inhibitor and is about 1000-fold more potent than butyrate. Figure 7a, b indicates that exposure of Caco-2 cells to TSA at comparative butyrate concentrations required to induce a similar degree of histone acetylation TSA inhibited IL-8 production in a similar manner to butyrate. The inhibitory effect was gradually decreasing as the concentration of TSA increased. These high concentrations of TSA and those of butyrate were associated with a decrease in the acetylation of histones (data not shown).
Fig. 7.
Interleukin (IL)-8 secretion in crypt-like (a) and villus-like (b) Caco-2 cells after incubation with butyrate or trichostatin A. The Caco-2 cells were exposed to physiological butyrate concentrations (0–20 m M) and comparative concentrations of trichostatin A (TSA) (0–100 n M) for 24 h. Culture medium was collected. IL-8 secretion is expressed as pg IL-8/106 cells. The IL-8 secretion was determined using two cell passages and triplicate cultures per passage. Significant differences (*P < 0·05) between the levels of IL-8 for cells exposed to butyrate or TSA and control Caco-2 cells are indicated. Caco-2 cells exposed to butyrate (a) and TSA (b).
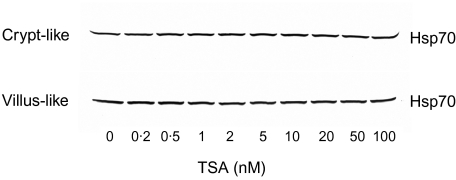
Figure 8 demonstrates the effect of TSA on Hsp70 production. It is evident from the figure that, contrary to butyrate, TSA at comparative butyrate concentrations required to induce Hsp70 production, did not induce the expression of Hsp70. This observation strengthens the hypothesis that the regulation of IL-8 secretion at higher concentrations of butyrate can be explained by its action on induction of Hsp70.
Fig. 8.
Expression of heat shock protein (Hsp) 70 in Caco-2 cells exposed to trichostatin A. Caco-2 cells exposed to trichostatin A (TSA) (0–100 n M) for 24 h were collected and processed for Western blotting and immunostaining as described. The blot is a representative of two cell passages and triplicate cultures per passage.
Effect of thermal stress on IL-8 secretion in Salmonella-exposed Caco-2 cells in the presence of butyrate
To establish whether physiological butyrate concentrations interfere with the anti-inflammatory role of heat stress, Caco-2 cells were incubated for 24 h in 0–20 m M butyrate, heat-shocked at 42°C and exposed to S. serovar Enteritidis 857 (200 bacteria/cell). Subsequently, incubation with the respective butyrate concentrations was continued for 24 h to allow recovery of the cells. Heat-shocking the cells in the presence of butyrate prior to exposure to 200 bacteria/cell inhibited the IL-8 secretion at low butyrate concentrations (0·05–2 m M) and was stimulatory at high concentrations (5–20 m M) in both crypt-like and villous-like cells. This relationship appeared to be more pronounced in crypt-like cells (Fig. 6a, b).
Whereas thermal stress or butyrate alone did not modify the levels of IL-8 (data not shown), thermal stress induced a significant stimulation of IL-8 secretion by cells incubated in high butyrate concentrations (5–20 m M) (Fig. 9a, b).
Fig. 9.
Heat-shock-induced interleukin (IL)-8 secretion in crypt-like (a) and villus-like (b) Caco-2 cells after preincubation with butyrate. After exposure to physiological butyrate concentrations (0–20 m M) for 24 h, the control (1 h 37°C) and heat-shocked (1 h 42°C) cells were allowed to recover for 6 h. During the subsequent heat shock and recovery period, butyrate exposure was continued. IL-8 secretion is expressed as pg IL-8/106 cells. Heat-shock-induced IL-8 secretion was determined using two cell passages and triplicate cultures per passage. Significant differences (*P < 0·05) between the levels of IL-8 of heat-shocked cells and control Caco-2 cells are indicated.
Discussion
Hsps, which evolutionarily have remained remarkably conserved, are a set of proteins involved in coping with chemical and physical stress in all living cells [20,21,31]. The data presented in this study clearly demonstrated a time-dependent expression of IL-8 secretion (Fig. 1) and that the presence of high levels of Hsp70 in cells heat-shocked at 42°C (Figs 3–5) inhibited the IL-8 production by enterocyte-like Caco-2 cells exposed to graded numbers of S. serovar Enteritidis 857 (Fig. 2). Further, it has been shown that butyrate dose-dependently induces the expression of Hsp70 (Fig. 3) and modulates IL-8 secretion ranging from inhibition to stimulation (Fig. 6). Following exposure to S. serovar Enteritidis 857, the heat-shocked cells revealed an augmentation of IL-8 secretion in the presence of butyrate, which was obvious in both crypt-like (2–20 m M) and villous-like (5–20 m M) cells (Fig. 6). It is clear that these high butyrate concentrations override the inhibitory effect of the heat-shock response on IL-8 secretion observed in the absence of butyrate (Fig. 2). The lower butyrate concentrations of 0·05–0·5 mM for crypt-like and 0·05–2 mM for villous-like cells maintained the inhibitory effect of this short chain fatty acid (Fig. 6).
The intestinal epithelial cells are capable of synthesizing Hsps that confer protection against thermal stress, infection and inflammation. Protection against inflammation is mediated partly via inhibition of proinflammatory cytokine production [22]. Inhibition of IL-8 secretion by Caco-2 cells observed by others is due to prevention of IκB degradation [18], an effect ascribed to Hsp70 in respiratory epithelial cells [24]. Undoubtedly, the thermally induced Hsp70 could account for the IL-8 inhibition observed in this study.
We have demonstrated the potential of butyrate to induce Hsp70 expression (Fig. 3) and to modulate IL-8 production by enterocyte-like Caco-2 cells (Fig. 6). The Hsp70 seems not to have taken part in the inhibition of IL-8 production by low butyrate concentrations, because these concentrations did not induce a marked increase in Hsp70 levels (Figs 3 and 4). In addition, the increase in IL-8 production, as revealed by high butyrate concentrations, alludes further that butyrate modulates IL-8 secretion via several mechanisms. It is known that butyrate, through histone hyperacetylation, is capable of switching the pattern of chemokine secretion by Caco-2 cells [2,17] on and off via regulation of the nuclear factor kappa B (NF-κB) and the activator protein (AP)-1 transcription factors. By so doing, butyrate can inhibit [16–18] or enhance [2,19] the IL-8 production by Caco-2 cells. In this study, we showed that low concentrations of both butyrate and TSA do not induce IL-8 production (Fig. 7a, b). We linked this effect with histone hyperacetylation as higher concentrations of both butyrate and TSA were associated with a decrease in the acetylation of histones (data not shown).
It has been shown that the bacterium Escherichia coli induces Hsp70 expression in Caco-2 cells that can be part of the natural mechanism of protection for intestinal epithelial cells in the potentially harmful environment in the intestinal tract [32]. Similarly, the induction of Hsp70 by butyrate observed in our study can be linked with IL-8 modulation and may imply a welfare phenomenon for the intestinal epithelium. It is interesting to note that the decline of IL-8 secretion by increasing butyrate concentrations appears to coincide with increasing Hsp70 expression. After reaching its highest levels, IL-8 secretion started to decline with further increase in butyrate concentrations (Fig. 6a). Although most concentrations retained higher IL-8 levels for villous-like cells (Fig. 6b), the fall in the IL-8 levels of the crypt-like cells exposed to 20 mM butyrate was such that compared to control cells these levels became insignificant (Fig. 6a). This fall was associated with increasing Hsp70 expression for both non-infected (Fig. 3) and S. serovar Enteritidis-infected cells (Fig. 4). Based on the anti-inflammatory role of Hsp70, it is reasonable to assume that this IL-8 down-regulation might be mediated, at least in part, through production of Hsp70. In Fig. 8, TSA could not induce Hsp70 at all concentrations tested, suggesting that the Hsp70 induction was not mediated through histone acetylation and thus was specific for butyrate. The potential of butyrate to induce the production of Hsp25 in rat colon and IEC-18 cell line that is protective against oxidative stress has been demonstrated by others [25]. In consistency, the results of our study demonstrate clearly that butyrate has the potential to induce Hsp70 in enterocyte-like Caco-2 cells.
Induction of the heat-shock response in Caco-2 cells exposed to high butyrate concentrations set aside the heat-shock inhibitory effect on IL-8 despite inducing Hsp70 (Fig. 9a, b). The failure of Hsps to protect intestinal cells against various noxious conditions has been reported. Induction of Hsp60 by thyrotrophin-releasing hormone, for instance, does not protect colonic mucosa against acetic acid-induced lesions in rats [33]. Depending upon the site of the cells along the gut, the condition of the cell, the luminal environment and the stress causative agent, induced Hsps could or could not be protective. In this study we demonstrated that high butyrate concentrations attenuated the anti-inflammatory effect of the thermally induced Hsp70 in Caco-2 cells incubated with S. serovar Enteritidis 857 (Fig. 6).
Our findings, that butyrate dose dependently modulates IL-8 production by enterocyte-like Caco-2 cells, are supported by the in vivo dissemination of intestinal inflammations in the presence of varying butyrate concentrations. It has been reported that high butyrate concentrations are associated with elevated IL-8 levels in the intestinal mucosa of active Crohn's disease patients [9,34]. By contrast, low butyrate concentrations diminish the disease activity [35]. Further, instillation of high concentrations of butyrate into the rectum of healthy mice induces the accumulation of neutrophils and causes inflammatory changes in the colon [6]. Consistent with these facts, our findings also suggest a concentration-dependent butyrate-mediated inhibition or promotion of inflammation via modulation of IL-8 production. The phenomenon implies that low butyrate doses inhibit while higher doses enhance intestinal inflammations. In part, these results may explain the limited therapeutic use of butyrate enemas to patients with Crohn's disease, distal ulcerative colitis and diversion colitis [14,15,35].
The way in which butyrate induces Hsp70 production is enigmatic. However, its ability to induce modulation and binding of transcription factors to their proximal promoter and enhancer elements could be events of the mechanism. In addition, butyrate can mediate its effects through activation of butyrate response elements, which in turn interfere with the rates of gene transcription [36,37]. Butyrate also induces cellular acidification, which is itself a stress condition capable of activating other stress kinases that may culminate into the induction of Hsps [38].
In summary, we have demonstrated the potential of butyrate to induce Hsp70 and the modulation of IL-8 secretion by butyrate and heat-shock response in enterocyte-like Caco-2 cells. The heat-shock response alone and low butyrate concentrations suppress IL-8 production while higher butyrate concentrations enhance the chemokine production. Because IL-8 is pivotal in the course of inflammation by attracting and activating neutrophils, its inhibition by low concentrations of butyrate or heat-shock response contributes to intestinal protection. By contrast, higher butyrate concentrations that enhance IL-8 production suggest a deleterious effect of butyrate. The limitations to the successful therapeutic use of butyrate enemas in colitis may stem from this contrasting butyrate effect.
References
- 1.Stadnyk AW. Cytokine production by epithelial cells. FASEB J. 1994;8:1041–7. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 2.Fusunyan RD, Quinn JJ, Fujimoto M, et al. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med. 1999;5:631–40. [PMC free article] [PubMed] [Google Scholar]
- 3.Eckmann L, Fierer J, Kagnoff MF. Bacterial invasion of epithelial cells induces secretion of the chemoattractant interleukin-8. Gastroenterology. 1993;104:A694. [Google Scholar]
- 4.Oppenheim JJ, Zachariae CO, Mukaida N, et al. Properties of the novel proinflammatory supergene ‘intercrine’ cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 5.Harada A, Sekido N, Akahoshi T, et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukocyte Biol. 1994;56:559–64. [PubMed] [Google Scholar]
- 6.McCafferty DM, Zeitlin IJ. Short chain fatty acid-induced colitis in mice. Int J Tissue React. 1989;11:165–8. [PubMed] [Google Scholar]
- 7.Cummings JH, Branch WJ. Fermentation and production of short chain fatty acids in human large intestine. In: Vahouny GB, Kritchevesky D, editors. Dietary fiber: basic and clinical aspects. New York: Plenum Press; 1990. pp. 131–52. [Google Scholar]
- 8.Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treem WR, Ahsan N, Shoup M, et al. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994;18:159–64. doi: 10.1097/00005176-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bernard JA, Warwick G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 1993;4:495–501. [PubMed] [Google Scholar]
- 11.Frankel WL, Zhang W, Singh A, et al. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106:375–80. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 12.Roediger WEW. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980;2:712–15. doi: 10.1016/s0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- 13.Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–6. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- 14.Harig JM, Soergel KH, Komorowski RA, et al. Treatment of diversion colitis with short chain fatty acid irrigation. N Engl J Med. 1989;320:23–8. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson IR. Diet and gut inflammation. Curr Opin Gastroenterol. 1997;13:518–24. [Google Scholar]
- 16.Huang N, Katz JP, Martin DR, et al. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine. 1997;9:27–36. doi: 10.1006/cyto.1996.0132. [DOI] [PubMed] [Google Scholar]
- 17.Andoh A, Fujiyama Y, Hata K, et al. Counter-regulatory effect of sodium butyrate on tumuor necrosis factor-alpha (TNF-α)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. Clin Exp Immunol. 1999;118:23–9. doi: 10.1046/j.1365-2249.1999.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu GD, Huang N, Wen X, et al. High-level expression of IκB-β in the surface epithelium of the colon: in vitro evidence for an immunomodulatory role. J Leukoc Biol. 1999;66:1049–56. doi: 10.1002/jlb.66.6.1049. [DOI] [PubMed] [Google Scholar]
- 19.Fusunyan RD, Quinn JJ, Ohno Y, et al. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1β and lipopolysaccharide. Pediatr Res. 1998;43:84–90. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–9. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 21.Ovelgönne JH, Koninkx JFJG, Pusztai A, et al. Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins. Gut. 2000;46:679–87. doi: 10.1136/gut.46.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malago JJ, Koninkx JFJG, van Dijk JE. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones. 2002;7:191–9. doi: 10.1379/1466-1268(2002)007<0191:thsrac>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuruma T, Yagihashi A, Matsuno T, et al. The heat-shock protein 70 family reduces ischemia/reperfusion injury in the small intestine. Transplant Proc. 1996;28:2629–30. [PubMed] [Google Scholar]
- 24.Yoo C, Lee S, Lee C, et al. Anti-inflammatory effect of heat shock protein induction is related to stabilization of IκBα through preventing IκB kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–23. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- 25.Ren H, Musch MW, Kojima K, et al. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631–9. doi: 10.1053/gast.2001.27028. [DOI] [PubMed] [Google Scholar]
- 26.Pinto M, Robine-Leon S, Appay MD, et al. Enterocyte-like differentation and polarization of the human colon carcinoma Caco-2 cell line in culture. Biol Cell. 1983;47:323–30. [Google Scholar]
- 27.Koninkx JFJG, Hendriks HGCJM, van Rossum JMA, et al. Interaction of legume lectins with the cellular metabolism of differentiated Caco-2 cells. Gastroenterology. 1992;102:1516–23. doi: 10.1016/0016-5085(92)91709-d. [DOI] [PubMed] [Google Scholar]
- 28.Koninkx JFJG. Enterocyte-like Caco-2 cells as a tool to study lectin interaction. In: Pusztai A, Bardocz S, editors. Lectins: biomedical perspectives. London: Taylor & Francis; 1995. pp. 81–101. [Google Scholar]
- 29.Cousens LS, Alberts BM. Accessibility of newly synthesized chromatin to histone acetylase. J Biol Chem. 1982;257:3945–9. [PubMed] [Google Scholar]
- 30.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;159:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–81. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 32.Deitch EA, Beck SC, Cruz NC, et al. Induction of heat shock gene expression in colonic epithelial cells after incubation with Escherichia coli or endotoxin. Crit Care Med. 1995;23:1371–6. doi: 10.1097/00003246-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Iwabuchi A, Otaka M, Otani S, et al. Specific preinduction of 60-kDa heat shock protein (chaperonin homolog) by TRH does not protect colonic mucosa against acetic acid-induced lesions in rats. Dig Dis Sci. 2000;45:1480–9. doi: 10.1023/a:1005597113024. [DOI] [PubMed] [Google Scholar]
- 34.Dabard J, Hudault S, Saby MA, et al. Production of butyric acid in human premature baby suffering from necrotizing enterocolitis. Proceedings of the 9th International Symposium on Gnotobiology; Versailles. 1987. pp. 90–5. [Google Scholar]
- 35.O'Morain C, Segal AW, Levi AJ. Elemental diet as primary treatment of acute Crohn's disease: a controlled trial. BMJ. 1982;288:1859–62. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lallemand F, Corilleau D, Sabbah M, et al. Direct inhibition of the expression of cyclin D1 gene by sodium butyrate. Biochem Biophys Res Commun. 1996;229:163–9. doi: 10.1006/bbrc.1996.1774. [DOI] [PubMed] [Google Scholar]
- 37.Nakano K, Mizuno T, Sowa Y, et al. Butyrate activates the Waf1/Cip1 gene promoter through Sp1 sits in a p53-negative human colon cancer cell line. J Biol Chem. 1997;272:22199–206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- 38.Amemiya M, Yamaji Y, Cano A, et al. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am J Physiol. 1995;269:C126–33. doi: 10.1152/ajpcell.1995.269.1.C126. [DOI] [PubMed] [Google Scholar]