Abstract
Infiltration by circulating inflammatory cells is a prominent local inflammatory feature of ulcerative colitis (UC). Several trials have suggested that leukocytapheresis by filtration can benefit patients with active UC. We investigated how this therapy might modulate the inflammatory response. Patients with active UC who were beginning repeated filtration leukocytapheresis were studied. Mononuclear cell preparations were obtained from blood before and after the first treatment, and expression of cytokine signalling components and the cell-proliferative response were analysed in vitro. Leukocytapheresis reduced lipopolysaccharide-induced production of proinflammatory cytokines (interleukin-1, -6, -8 and tumour necrosis factor-α, P < 0·05 for all) and activation of intracellular signalling components (nuclear factor-κB, mitogen-activated protein kinases, and signal transducer and activator of transcription-3), as well as surface expression of toll-like receptor-4 (P < 0·05) in mononuclear cells. The therapy also reduced the cell-proliferative response by mononuclear cells stimulated with sonicated bacterial preparations from autologous intestine (P < 0·05). These results indicate that activated mononuclear cells in the peripheral blood of patients with active UC are removed by leukocytapheresis and replaced by cells with a lower activation status. This replacement may partly explain the therapeutic benefit.
Keywords: cytokines, leukocytapheresis, microflora, signal transduction, ulcerative colitis
Introduction
Ulcerative colitis (UC), a disorder of unclear aetiology characterized by chronic relapsing inflammation of the colon, is considered a disease of multifactorial origin reflecting an interplay of genetic, immunological, and environmental factors. A potent inflammatory response, within a genetically susceptible individual is assumed to be triggered by intestinal microflora [1–3]. Intense infiltration of the intestinal mucosa by neutrophils, lymphocytes and macrophages is a hallmark of UC. Not only tissue macrophages but also blood monocytes show high activity, producing large quantities of interleukin (IL)-1, IL-6, and IL-8 and also tumour necrosis factor (TNF)-α. These cytokines, in turn, mediate the inflammatory and immunological responses [4–11]. Studies using radiolabelled monocytes [12] or immunohistochemical techniques [13] found evidence for monocyte recruitment to the affected bowel. During active disease, monocytes newly emigrated from the circulation would be exposed to bacteria in the gut lumen, which could further activate these cells resulting in severe mucosal damage. Removal of circulating monocytes may therefore be an attractive approach to treating UC.
Trials of therapeutic leukofiltration from the peripheral circulation have been carried out by leukocytapheresis (LCAP), which uses a veno-venous extracorporeal apheresis device coupled to a leukofiltration device [14,15]. In several trials, LCAP appeared to attenuate inflammation in UC [16–19] as well as in rheumatoid arthritis [20,21] and rapidly progressive glomerulonephritis [22], without provoking severe complications. Summarizing results of previous clinical reports, Ortolano et al. [16] concluded that 76% of 115 patients with inflammatory bowel disease treated with LCAP showed remission that did not require corticosteroid or cytoablative support for maintenance. More recently, Sawada et al. [17] described a multicentre, randomized, controlled trial in which 80 patients with active UC, were treated with either LCAP or corticosteroids. With weekly treatments for 5 weeks, LCAP showed more clinical benefit than corticosteroid therapy.
Little is known about how LCAP modulates the inflammatory response. We therefore investigated effects of LCAP on cellular expression of cytokines and intracellular signalling components in response to bacterial lipopolysaccharide (LPS). Surface expression of the LPS receptor was assessed simultaneously. Finally, the effect of LCAP on the cell-proliferative response to bacteria from autologous intestine was evaluated. For these in vitro studies, blood samples were collected before and after UC patients’ first LCAP procedure. We found that LCAP efficiently removed activated mononuclear leucocytes from the circulation, and that the repopulating cells showed reduced activation status. These findings may partly explain the previously demonstrated clinical benefits of LCAP [16–19].
Subjects and methods
Patient population
Seven patients with active UC were studied (3 male, 4 female; mean age 34 years; mean disease duration 8·4 years). Patients had either left-sided colitis (n = 3) or pancolitis (n = 4) and were classified into the moderate (n = 6) or severe (n = 1) attack category according to the Truelove and Witts criteria [23]. All patients received standard medical therapy with aminosalicylates and/or corticosteroids (mean total dosage 10·1 g; mean duration 5·0 years). Patients with any of the following features were excluded: age less than 18 or greater than 80 years; haemoglobin less than 8 g/dl; total leucocyte count < 4 × 109/l or coagulation abnormalities, bleeding diathesis, pregnancy or unsuitable peripheral venous access for apheresis. The procedure was well tolerated. No severe complications occurred during treatment. Concomitant clinical assessments [24] revealed a reduction of the mean index from 10·2 to 5·3 after the final LCAP procedure (P < 0·05). Approval for this study was granted by the local ethical committee, and all patients gave informed consent.
LCAP procedure
LCAP was performed using a Cellsorba E column (Asahi Medical, Tokyo, Japan) installed in the extracorporeal circulation system (Plasauto LC, Asahi Medical) [14,15]. For apheresis, venous access was secured via two large peripheral veins, and the blood was anticoagulated with nafamostat mesilate (Torii Pharmaceutical, Tokyo, Japan), a protease inhibitor that inhibits the activity of coagulation factors and platelet aggregation [14,15,17–22]. Heparin was not used since its use has been associated with respiratory distress and palpitations [25]. With a flow rate of 30–50 ml/min for 60 min, a total of approximately 2·5 l of blood was treated during each session. Previous data show that nearly 100% of neutrophils and monocytes that entered the filter, and 40–60% of lymphocytes were removed in one session of LCAP [21]. The LCAP procedure was carried out weekly for 5 weeks. At the end of the final treatment, effectiveness was evaluated in terms of clinical manifestations, laboratory results, and endoscopic findings [24,26].
Cell separation
Peripheral blood samples were collected from patients via the LCAP inlet line just before and after the first LCAP session. In these samples, leucocytes, platelets, and erythrocytes were counted, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density centrifugation. Antibody mixtures were purchased from StemCell Technologies (Vancouver, Canada). Blood samples obtained from healthy volunteers were used as normal controls.
Flow cytometric analysis
Phenotypes of lymphocytes were analysed by laser flow cytometry with a FACScan instrument (Becton Dickinson, Sunnyvale, CA, USA). We examined the proportion of CD3, CD4, CD8, CD20, CD25, and HLA-DR-positive cells, and the proportion of CD3, CD4 and CD8 cells carrying HLA-DR. The following murine monoclonal antibodies were used in either fluorescein or phycoerythrin conjugates: anti-CD3, CD4, and CD8 (Nichirei, Tokyo, Japan); anti-CD20 (DAKO, Glostrup, Denmark); and anti-CD25 and HLA-DR (BD Biosciences, San Jose, CA, USA). In separate experiments the proportion of monocytes carrying the toll-like receptor-4 (TLR4) was examined [27]. Anti-TLR4 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cytokine ELISA
PBMCs at a density of 106 cells/ml in culture medium were stimulated with LPS (Escherichia coli 005: B5, Difco Laboratories, Detroit, MI, USA) at a final concentration of 10 ng/ml or with phytohemagglutinin (PHA, 1 : 100; Difco Laboratories) or left unstimulated. Cells were incubated for 24 h in an humidified atmosphere at 37° C with 5% CO2. The supernatants were then harvested, centrifuged, aliquotted, and stored at −20° C until assayed for specific cytokine levels using enzyme-linked immunosorbent assays (ELISA) [28]. ELISAs for IL-1β, IL-6, TNF-α, and IL-1 receptor antagonist (IL-1RA) were obtained from R & D Systems (Minneapolis, MN, USA); for IL-8, from TFB, Technical Customer Service (Tokyo, Japan); for IL-4, from BioSource International (Camarillo, CA, USA); and for soluble IL-2 receptor (sIL-2R), from Pierce (Rockford, IL, USA). The ratio between IL-1β and IL-1RA was calculated by dividing IL-1RA concentration by IL-1β concentration.
Analysis by cDNA array
PBMCs (106 cells/ml) were stimulated with LPS at 10 ng/ml and incubated for 24 h. Total RNA was extracted from these cells using an RNeasy Kit (Qiagen, Hilden, Germany) [29–31]. Aliquots of RNA (5 µg) were used to analyse gene expression of cytokines by GEArray technology (SuperArray, Bethesda, MD, USA). Relative amounts of mRNA transcript were quantified using NIH Image software (version 1·58) after scanning with an Image Scanner (Epson GT-9000, Tokyo, Japan) [32]. The relative amount of a given gene transcript was estimated by comparing its signal intensity with the signal derived from β-actin.
NF-κB binding
Binding of nuclear factor (NF)-κB p65 subunit to the NF-κB binding consensus sequence 5′-GGGACTTTCC-3′ was measured with an ELISA-based Trans-Am NF-κB kit (Active Motif, Carlsbad, CA), first using cell lysates prepared from normal PBMCs (106 cells/ml) [33,34]. Briefly, the Trans-Am kit employs 96-well microtiter plates coated with an oligonucleotide containing the NF-κB binding consensus sequence. The active forms of the p65 subunit in cell extracts can be detected using antibodies specific for an epitope that is accessible only when the subunit is activated and bound to its target DNA. Preparation of cell extracts closely followed the recommendations of the manufacturer. Specificity was checked by measuring the ability of soluble wild-type or mutation-carrying oligonucleotides to inhibit binding. Results are expressed as absorbance values. Since the specific binding in response to LPS peaked at 24 h after stimulation, the same protocol and the 24-h time point were used to study lysates from control PBMCs, UC pre-LCAP PBMCs and UC post-LCAP PBMCs.
Western blot analysis
PBMCs at a density of 106 cells/ml were homogenized in a lysis buffer containing 50 m m Tris-HCl at pH 8·0 and 0·5% NP-40, 1 m m EDTA, 150 m m NaCl, 10% glycerol, 1 m m sodium vanadate, 50 m m sodium fluoride, 10 m m sodium pyrophosphate, and 1 m m phenylmethylsulphonyl fluoride together with a protease inhibitor cocktail (Sigma Chemical, St. Louis, MO, USA) [35]. Extracts were cleared by centrifugation at 15 000 r.p.m. at 4° C for 15 min and then diluted with lysis buffer to achieve a protein concentration of approximately 2 mg/ml. The cell extracts were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were detected by Western blotting as described [36], using antic-Jun N-terminal kinase (JNK; Santa Cruz Biotechnology), antiphospho-JNK, antip38 kinase, antiphospho-p38 kinase, and antiphospho-signal transducer and activator of transcription-3 (STAT3; Cell Signalling Technology, Beverly, MA, USA).
Proliferation assay
Rectal lavage solution obtained during colonoscopy was immediately diluted with anaerobic buffer. Bacteria in lavage fluid were cultured on nonselective media (M10 agar) in an anaerobic chamber. Colonies on the agar were collected carefully after 3 days culture. After several washes with sterile ice-cold phosphate-buffered saline, these isolated bacteria were sonicated and centrifuged at 10 000 × g for 30 min. Supernatants were used as the source for the cell-proliferation assay. Cell-proliferation assays were performed using PBMCs (2·0 × 105 cells/well) in complete RPMI medium with or without sonicated bacteria [37,38]. By serial dilution, 5 µg of bacterial protein/ml of sonicated bacterial preparations had been shown to induce optimal cell proliferation. As a positive control, cells were stimulated with LPS (1 µg/ml). All cell cultures were performed in triplicate in flat-bottomed 96-well tissue culture plates for 48 h at 37° C in an humidified, 5% CO2 atmosphere. During the final 18 h of culture, 20 ml of a solution containing 25 µCi of [3H]-thymidine (Amersham, Arlington Heights, IL, USA) was added to each well. Cells were collected onto a glass filter using a cell harvester, and [3H]-thymidine uptake was measured with a β-emission counter.
Statistics
Statistical analysis was performed using the t-test, the paired t-test, the Mann—Whitney U-test, or the Wilcoxon's rank-sum test. Results are expressed as mean and SD when data follow a normal distribution, or as median and interquartile range in the case of data not normally distributed. Values of P less than 0·05 were considered to indicate significance.
Results
Blood cell counts and lymphocyte surface marker expression
We considered the effect of LCAP on cell counts and lymphocyte surface marker expression in peripheral blood of patients with UC (Table 1). After LCAP, platelet counts had decreased significantly and lymphocyte and monocyte populations were significantly reduced. Total leucocyte counts tended to show a decrease but the differences were not statistically significant. Phenotypes of PBMCs derived from pre- and post-LCAP samples were characterized by determining surface marker expression using flow cytometry. However, these markers were only slightly affected by LCAP, with a mild decrease of CD8-HLA-DR+ cells.
Table 1. Cell counts and lymphocyte subsets in peripheral blood.
| Unit | Before LCAP | After LCAP | P-value | |
|---|---|---|---|---|
| Erythrocytes | × 104/µl | 392 ± 62 | 357 ± 56 | n.s. |
| Platelets | × 104/µl | 35·6 ± 8·2 | 19·6 ± 3·4 | 0·0004 |
| Total leucocytes | /µl | 9243 ± 2413 | 7200 ± 3060 | n.s. |
| Lymphocytes | /µl | 1657 ± 1024 | 621 ± 354 | 0·0264 |
| % | 18·6 ± 11·5 | 9·3 ± 5·5 | n.s. | |
| Monocytes | /µl | 577 ± 239 | 315 ± 143 | 0·0283 |
| % | 6·4 ± 2·4 | 4·5 ± 1·4 | n.s. | |
| Neutrophils | /µl | 6783 ± 2233 | 6010 ± 2975 | n.s. |
| % | 72·0 ± 14·4 | 82·2 ± 9·6 | n.s. | |
| Eosinophils | /µl | 123 ± 168 | 88 ± 117 | n.s. |
| % | 1·8 ± 2·6 | 1·2 ± 1·6 | n.s. | |
| Basophils | /µl | 28 ± 58 | 6 ± 10 | n.s. |
| % | 0·4 ± 0·7 | 0·1 ± 0·2 | n.s. | |
| CD4+ | % (29–55) | 38 ± 12 | 42 ± 11 | n.s. |
| CD8+ | % (19–41) | 35 ± 6 | 35 ± 10 | n.s. |
| CD4/CD8 | % (0·6–2·4) | 1·1 ± 0·5 | 1·3 ± 0·7 | n.s. |
| CD20+ | % (7–30) | 13 ± 7 | 8 ± 2 | n.s. |
| CD25+ | % (−8) | 9 ± 4 | 10 ± 3 | n.s. |
| DR+ | % (11–36) | 30 ± 10 | 23 ± 8 | n.s. |
| CD3+ DR+ | % (−11) | 4 ± 8 | 11 ± 6 | n.s. |
| CD3+ DR− | % (55–84) | 56 ± 11 | 61 ± 8 | n.s. |
| CD3-DR+ | % (1–16) | 12 ± 7 | 8 ± 4 | n.s. |
| CD3-DR− | % (2–29) | 16 ± 10 | 17 ± 8 | n.s. |
| CD4+ DR+ | % (−4) | 3 ± 2 | 3 ± 1 | n.s. |
| CD4+ DR− | % (28–56) | 35 ± 11 | 37 ± 10 | n.s. |
| CD4-DR+ | % (4–19) | 23 ± 7 | 18 ± 5 | n.s. |
| CD4-DR− | % (25–60) | 38 ± 11 | 40 ± 8 | n.s. |
| CD8+ DR+ | % (−7) | 8 ± 5 | 6 ± 5 | n.s. |
| CD8+ DR− | % (16–39) | 23 ± 5 | 25 ± 7 | n.s. |
| CD8-DR+ | % (4–19) | 17 ± 4 | 13 ± 2 | 0·0280 |
| CD8-DR− | %(46–67) | 50 ± 8 | 54 ± 11 | n.s. |
Values are the mean ± SD of seven patients with ulcerative colitis. Normal values in parentheses were obtained from healthy volunteers. n.s.not significant; LCAP, leukocytapheresis.
Cytokine protein and mRNA expression
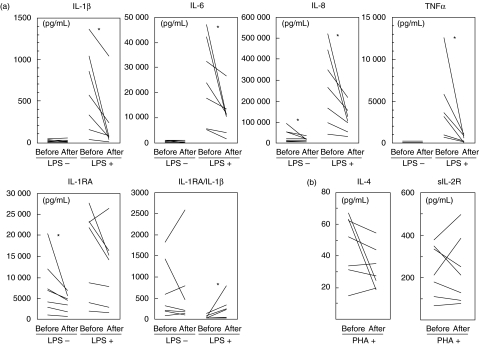
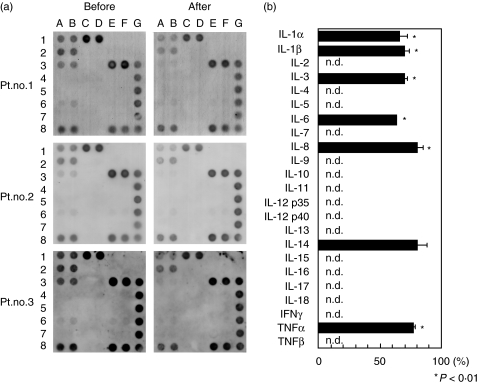
We tested the effect of LCAP on expression of cytokine protein and mRNA transcript in UC patients’ PBMCs. Under unstimulated conditions, release of macrophage-derived inflammatory cytokines IL-1β, IL-6, IL-8 and TNF-α in cultures of pre-LCAP PBMCs was low, and these cytokines were altered little by LCAP. Upon stimulation with LPS, the release of these cytokines in cultures of pre-LCAP PBMCs was markedly increased; this effect was significantly reduced by LCAP. Although the anti-inflammatory cytokine IL-1RA also was mildly decreased by LCAP, the ratio of IL-1RA to IL-1β was increased (Fig. 1a). An LCAP-induced decrease in inflammatory cytokines also was seen in separate experiments using LPS-stimulated monocytes instead of PBMCs (data not shown). In contrast, LCAP did not alter the release of the T cell-derived cytokine IL-4 [39] or the T-cell activation marker sIL-2R [40] in response to PHA stimulation (Fig. 1b). When cytokine gene expression was analysed by cDNA array analysis, expression of IL-1, IL-6, IL-8, and TNF-α mRNA was associated closely with the amounts of cytokine protein expressed (Fig. 2). Similar results were obtained for mRNA encoding of IL-3 [41] and IL-14 [42]. Both of these cytokines act upon the haemopoietic system, and their clinical significance in UC remains to be defined.
Fig. 1.
Changes in cytokine production by PBMCs. Cells (106/ml) were obtained from patients with UC before and after their first LCAP and cultured with LPS (10 ng/ml) or medium alone (a) or with PHA (1/100 dilution) (b) for 24 h. Supernatants were collected and cytokine concentrations were measured using commercial ELISA kits. Data are expressed as the mean of duplicate samples. *P < 0·05. PBMC, peripheral blood mononuclear cell; UC, ulcerative colitis; LCAP, leukocytapheresis; LPS, lipopolysaccharide; PHA, phytohemagglutinin; ELISA, enzyme-linked immunosorbent assay.
Fig. 2.
A cDNA array analysis of cytokine gene expression. PBMCs (106/ml) were obtained from patients with UC before and after first LCAP and cultured with LPS at 10 ng/ml for 24 h. Cell lysates were tested for cytokine mRNA using a GEArray, as described in Subjects and Methods. (a) Representative data for cytokine mRNA expression from three UC patients. IL-1α = 1 A, 1B; IL-1β = 1C, 1D; IL-2 = 1E, 1F; IL-3 = 2 A, 2B; IL-4 = 2C, 2D; IL-5 = 2E, 2F; IL-6 = 3 A, 3B; IL-7 = 3C, 3D; IL-8 = 3E, 3F; IL-9 = 4 A, 4B; IL-10 = 4C, 4D; IL-11 = 4E, 4F; IL-12 p35 = 5 A, 5B; IL-12 p40 = 5C, 5D; IL-13 = 5E, 5F; IL-14 = 6 A, 6B; IL-15 = 6C, 6D; IL-16 = 6E, 6F; IL-17 = 7 A, 7B, IL-18 = 7C, 7D; IFNγ = 7E, 7F; TNF-α = 8 A, 8B; TNFβ = 8C, 8D; β-actin = 3G, 4G; GAPDH = 5G, 6G, 7G, 8E, 8F, 8G; pUC18 = 1G, 2G. (b) Quantitative analysis of cytokine mRNA expression in the same patients. Densitometric analysis was performed, and values are expressed relative to corresponding housekeeping gene transcripts (β-actin). Percent changes in expression of genes in post versus pre-LCAP samples are presented. Similar data were obtained from two additional patients. n.d., not detected. PBMC, peripheral blood mononuclear cell; UC, ulcerative colitis; LCAP, leukocytapheresis; LPS, lipopolysaccharide; IL, interleukin; TNF, tumour necrosis factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Intracellular signalling components
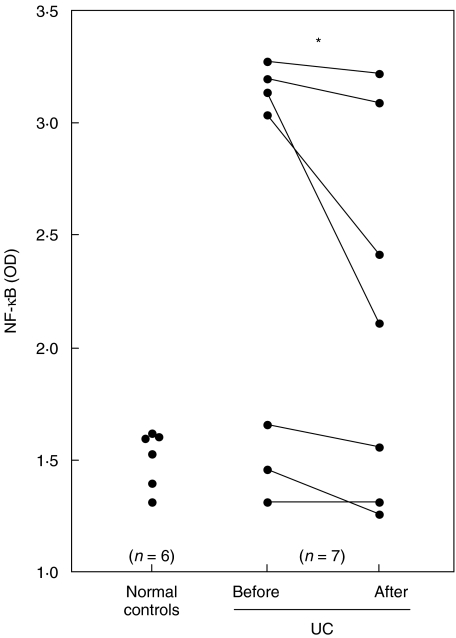
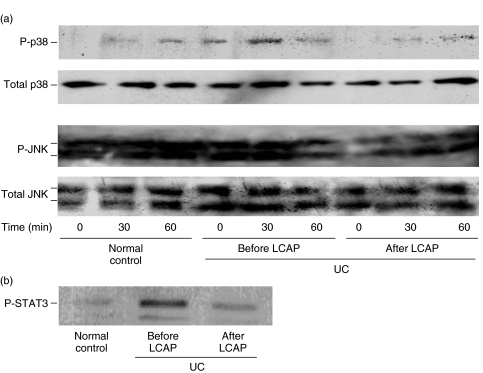
Using LPS-stimulated PBMCs, we investigated whether LCAP-induced reduction of cytokines resulted from alterations in intracellular signalling components that regulate the cytokine genes, including NF-κB [43,44] and mitogen-activated protein kinases (MAPKs) [45,46]. We first focused on the effect of LCAP on binding of the p65 subunit to a consensus NF-κB binding sequence. As shown in Fig. 3, specific binding of active p65 subunit was increased in UC patients’ PBMCs pre-LCAP therapy compared with control subjects’ PBMCs, and LCAP caused a significant decrease in this specific binding. We next examined the effect of LCAP on phosphorylation of MAPKs. Western blot analysis demonstrated a time-dependent phosphorylation of p38 kinases and JNKs in response to LPS, while LCAP reduced this phosphorylation response (Fig. 4a). When we analysed activation of downstream signalling after binding to the cytokine receptor, phosphorylation of STAT3, a key STAT family molecule in intestinal inflammation [36], was inhibited by LCAP (Fig. 4b).
Fig. 3.
Effects of LCAP on binding of the NF-κB subunit to an NF-κB-binding consensus sequence. PBMCs were obtained from normal subjects (n = 6) or UC patients before and after their first LCAP (n = 7) and cultured with LPS at 10 ng/ml for 24 h. Lysates of PBMCs (5 µg) were tested for binding of activated p65 NF-κB subunit to an NF-κB consensus sequence using the Trans-Am NF-κB ELISA kit. Results are expressed as absorbance values. *P < 0·05. OD, optical density; NF, nuclear factor; PBMC, peripheral blood mononuclear cell; ELISA, enzyme-linked immunosorbent assay; LPS, lipopolysaccharide; LCAP, leukocytapheresis.
Fig. 4.
Western blot analysis of p38 kinases and (a) JNKs and (b) STAT3. Lysates of PBMCs (5 µg) obtained from normal subjects and UC patients before and after their first LCAP were harvested after stimulation with LPS at 10 µg/ml for the indicated time periods. Phosphorylation of p38 kinases and (a) JNKs or (b) STAT3 was determined by Western blotting. In STAT3 expression, only data for LPS-stimulated PBMCs were presented because STAT3 phosphorylation was consistently undetectable in unstimulated PBMCs. Data are representative of three separate experiments. JNK, c-Jun N-terminal kinase; STAT, signal transducer and activator of transcription; PBMC, peripheral blood mononuclear cell; UC, ulcerative colitis; LCAP, leukocytapheresis; LPS, lipopolysaccharide.
TLR4 expression
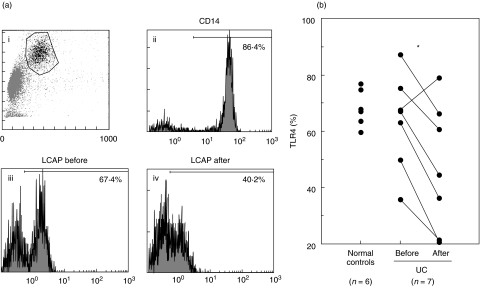
We studied the effect of LCAP on surface expression of TLR4, a crucial component of the signalling receptor complex for LPS [27,47,48] (Fig. 5). In the absence of LPS, flow cytometric analysis showed comparable amounts of TLR4 surface expression on monocytes in UC and normal controls. LCAP caused a reduction in the percentage of TLR4 surface expression from a median value of 63·5% to 46·8% (73·7% of pre-LCAP level). After exposure to LPS, TLR4 surface expression was reduced on normal, pre-LCAP and post-LCAP monocytes, all to a similar extent (data not shown).
Fig. 5.
Flow cytometric analysis of TLR4 surface expression on peripheral monocytes. PBMCs were obtained from normal subjects and UC patients before and after their first LCAP. Cells then were exposed to antibodies against TLR4 and submitted to flow cytometric analysis. (a) Representative histograms of TLR4 surface expression on monocytes in one patient with UC before and after LCAP treatment (iii, iv). Monocytes population was separated by gating using forward scatter and right scatter (i). The expression of CD14 molecules on the gated cell population was > 85% (ii). (b) Quantitative analysis of TLR4 surface expression on monocytes in seven patients with UC and six normal subjects. *P < 0·05. TLR, toll-like receptor; PBMC, peripheral blood mononuclear cell; UC, ulcerative colitis; LCAP, leukocytapheresis; LPS, lipopolysaccharide.
Response to autologous bacteria
In consideration of evidence that LCAP decreased mononuclear cell activity in response to bacterial LPS, we investigated whether LCAP influenced mononuclear cell activity in response to autologous bacteria. In PBMCs exposed to sonicated bacterial preparations from autologous intestine, significant down-regulation of the cell-proliferative response was noted after LCAP (Fig. 6).
Fig. 6.
Cell-proliferative response in PBMCs stimulated with (a) LPS or (b) autologous bacteria. Cells (106/ml) derived from the pre- and post-LCAP samples from UC patients were cultured for 48 h at 37° C in an atmosphere with 5% CO2 in the presence of LPS (1 µg/ml) or autologous bacterial preparations (5 µg bacterial protein/ml). For the final 18 h, 20 ml of a solution containing 25 µCi of [3H]-thymidine was added to each well. Incorporation of [3H]-thymidine was measured with a β-emission counter. Each value represents the mean of triplicate measurements. *P < 0·05. The same symbols in both figures indicate the data from the same patients. PBMC, peripheral blood mononuclear cell; LCAP, leukocytapheresis; UC, ulcerative colitis; LPS, lipopolysaccharide.
Discussion
As LCAP therapy decreases UC activity [16–19], we attempted to determine the underlying mechanisms. The most likely trigger of inflammatory responses in UC is luminal bacteria [49,50]. In the affected bowel, monocytes and macrophages newly emigrated from the circulation are exposed to these luminal bacteria. These bacteria induce further activation of the monocytes and macrophages resulting in severe mucosal damage. Given this background, we investigated whether LCAP modulates the behaviour of mononuclear leucocytes against bacterial components. To emulate the situation in vivo as closely as possible, mononuclear cells rather than monocytes were studied.
A number of studies have suggested that macrophage-derived inflammatory cytokines play a central role in development of UC [1,2,6–11]. Mononuclear cells in UC patients are highly activated while still in the peripheral blood [4,5] and also after entering affected bowel tissues [6,8]. We found that LCAP drastically reduced release of IL-1, IL-6, IL-8, and TNF-α from mononuclear cells stimulated with LPS, one of the most frequent and potent stimuli of the innate immune system. Moreover, the decrease in cytokine protein was accompanied by a decrease in cytokine gene transcripts. Several studies suggest a relative imbalance of IL-1 and IL-1RA in UC, as well as in Crohn's disease [51,52]. LCAP induced a predominant decrease in IL-1 compared to its specific inhibitor, IL-1RA, resulting in beneficial outcomes. Somewhat surprisingly, LCAP had little effect on the expression of lymphocyte surface markers or release of the T cell-related cytokines IL-4 and sIL-2R. This suggests that lymphocyte behaviour is not strongly altered by LCAP. LCAP, then, appears to exert anti-inflammatory activities by down-regulating the capacity of monocytes to produce inflammatory cytokines that enter affected bowel tissue. In the future, the experiment with fractionated populations of leucocytes would be of interest in the view of a more targeted leucocyte-filtration.
The reduction of cytokine gene transcripts seen in the present investigation led us to study expression of the intracellular signalling components that regulate cytokine genes. NF-κB plays a central role in expression of inflammatory cytokine genes after exposure to LPS or other microbial stimuli [43,44]. Of three MAPKs, the p38 kinases and JNKs are involved in cell activation initiated by microbial stimuli including LPS while the extracellular signal-related kinases regulate cell growth and differentiation [45,46]. Some recent reports describe both NF-κB [53–55] and MAPKs [56] as highly activated in UC. Inhibition of both transcription factors could effectively attenuate colonic inflammation [55,56]. We presently demonstrated that an LCAP-induced decrease in cytokines was accompanied by diminished activation of NF-κB as well as p38 kinases and JNKs. Thus, LCAP is likely to inhibit cytokine production by regulating activation of these upstream signalling components.
In addition to upstream signalling, analysis of downstream signalling components, after binding to cytokine receptors, could also be informative with respect to regulation of cytokine function. STATs are activated by a family of cytoplasmic, tyrosine kinases termed Janus kinases that are associated with cell-surface cytokine receptors. After activation, STATs translocate into the nucleus [57,58]. Recently we have demonstrated that among STAT family members, STAT3 plays a key role in the development of intestinal inflammation [36]. We presently demonstrated that LCAP reduced STAT3 activity, indicating a beneficial effect on the target cell as well as the cytokine-producing cell. At present, whether this decrease is caused by a direct inhibitory action on the target cell, or a secondary event following cytokine reduction is not clear.
TLR4 stimulation by LPS activates NF-κB and MAPKs through the signalling molecules MyD88 and IL-1 receptor-associated kinases [59,60]. Since LCAP down-regulated LPS-induced activation of cytokine signalling, we analysed TLR4 surface expression on peripheral monocytes using flow cytometry. Of special interest was the observation that LCAP reduced the amounts of TLR4 on cell surfaces. Such reduction may promote hyporesponsiveness to LPS, resulting in down-regulation of cytokine signalling. Regulatory mechanisms for TLR4 expression are currently a matter of speculation. Monocytes primed with interferon (IFN)-γ, which has been shown to be elevated in the sera from patients with active UC [61], show augmented surface expression of TLR4 [48]. LCAP may decrease the number of such primed monocytes, thus reducing overall monocyte TLR4 surface expression. Why UC and normal subjects showed comparable amounts of TLR4 expression is less clear. One explanation might involve the effects of concomitant medication such as corticosteroids or aminosalicylates. Another possible explanation lies in the coexistence of other receptors binding to LPS, such as the recently described NOD2 [62,63]. These factors may modulate surface expression of TLR4.
The effects of LCAP on mononuclear cell response to bacteria from autologous intestine as a stimulus instead of LPS, were informative. Similarly to responsiveness to bacterial LPS, autologous bacterial preparations also could up-regulate cell proliferation. LCAP, more importantly, down-regulated this response. Intriguing data obtained in both mice and humans indicate that while mucosal mononuclear cells do not ordinarily respond to the antigens of the host's intestinal microflora, this tolerance is abolished in UC [37,38,64]. Taken together, LCAP may enhance tolerance to bacterial antigens by down-regulating mononuclear cell activity, avoiding the immune activation that leads to inflammation in the gut. An important remaining question is how LCAP modulates mononuclear cell activity. Previous studies have shown that treatment with LCAP results in a 50% reduction in circulating leucocytes, including monocyte, lymphocyte and neutrophil subpopulations, over the course of 30–45 min. This is followed by a leukophilia that returns the leucocyte count to pretherapy levels within 90 min [65]. This observation indicates that the elimination of circulating leucocytes is followed rapidly by a reticuloendothelial system cell release response. Mononuclear cells that appear after the LCAP procedure then would be likely to originate predominantly from the marginated pool and/or the bone marrow. Sequestered in their respective compartments, these cells most likely were not primed or activated, thereby lacking up-regulated cytokine production and the capacity to amplify mucosal damage. Since our study was designed to use only first-session samples, we could not demonstrate whether modulation of cytokine signalling in mononuclear cells directly contributed to the positive clinical outcomes of a full course of LCAP. However, from our evidence that LCAP down-regulates mononuclear cell activity, and in view of previous studies showing that activated monocytes contribute to development of UC [1,2], we could at least partly explain the clinical benefit despite the absence of dramatic and sustained decreases in leucocytes.
LCAP can remove neutrophils entering the filter as well as mononuclear cells [21,65]. Previous studies showed that neutrophils also contribute to the development of UC [66,67]. Also, as in previous reports [68,69], our data included an LCAP-induced decrease in platelets, which are activated and overproduced during active UC. Therefore, we cannot rule out the possibility that reductions in these two cell types may be involved in the beneficial effect of LCAP.
In summary, we demonstrated that LCAP exerts an immunomodulatory effect by removing leucocytes from the circulation. This study indicated that highly activated mononuclear cells in patients with UC can be efficiently removed by LCAP. Physiologically, these cells are replaced with cells having a considerably lower activation status with respect to expression of cytokines, intracellular signalling components, and specific receptors. Further study is needed to clarify the molecular mechanisms underlying the clinical effects of LCAP.
Acknowledgments
We thank to Ms. Ritsuko Seki, Mrs Tomoshige Nogami and Hiroshi Shibata, and Drs Osamu Tsuruta, Yasuharu Inayoshi, Hiroshi Yoshida, Masahide Watanabe, Terufumi Sakai, Keigo Emori and Norito Matsukuma for their help during the study. This work was supported in part by grants-in-aid from the Japanese Ministry of Education, Culture, and Science; and the Japanese Ministry of Health and Welfare.
References
- 1.Podolsky DK, Fiocchi C. Cytokines, chemokines, growth factors, eicosanoids, and other bioactive molecules in inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory Bowel Disease. 5. Philadelphia: W. B.Saunders Company; 2000. pp. 191–207. [Google Scholar]
- 2.Katz JA, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15:291–7. doi: 10.1097/00001574-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Madsen KL, Fedorak RN. Naturally occurring and experimental models of inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory Bowel Disease. 5. Philadelphia: W. B.Saunders Company; 2000. pp. 113–43. [Google Scholar]
- 4.Mahida YR, Scott E, Kurlak L, et al. Interleukin 1β, tumour necrosis factor α and interleukin 6 synthesis by circulating mononuclear cells isolated from patients with active ulcerative colitis and Crohn's disease. Eur J Gastroenterol Hepatol. 1992;4:501–7. [Google Scholar]
- 5.Nakamura M, Saito H, Kasanuki J, et al. Cytokine production in patients with inflammatory bowel disease. Gut. 1992;33:933–7. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinecker HC, Steffen M, Witthoeft T. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsuyama K, Sasaki E, Toyonaga A, et al. Colonic mucosal interleikin-6 in inflammatory bowel disease. Digestion. 1991;50:104–11. doi: 10.1159/000200747. [DOI] [PubMed] [Google Scholar]
- 8.Gross V, Andus T, Caesar I, et al. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992;102:514–9. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuyama K, Toyonaga A, Sasaki E, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1994;96:432–6. doi: 10.1111/j.1365-2249.1994.tb06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahida YR, Ceska M, Effenberger F, et al. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci. 1992;82:273–5. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald TT, Hutchings P, Choy MY, et al. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–5. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm MC, Pullman WE, Bennett GM, et al. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995;10:387–95. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 13.Rugtveit J, Brandtzaeg P, Halstensen TS, et al. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–74. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondoh T, Hidaka Y, Katoh H, et al. Evaluation of a filtration lymphocytapheresis (LCP) device for use in the treatment of patients with rheumatoid arthritis. Artif Organs. 1991;15:180–8. doi: 10.1111/j.1525-1594.1991.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 15.Sawada K, Ohnishi K, Fukui S, et al. Leukocytapheresis therapy, performed with leukocyte removal filter, for inflammatory bowel disease. J Gastroenterol. 1995;30:322–9. doi: 10.1007/BF02347507. [DOI] [PubMed] [Google Scholar]
- 16.Ortolano GA, Capetandes A, Wenz B. A review of leukofiltration therapy for decreasing the morbidity associated with cardiopulmonary bypass and acute inflammatory bowel disease. Ther Apher. 2002;6:119–29. doi: 10.1046/j.1526-0968.2002.00338.x. [DOI] [PubMed] [Google Scholar]
- 17.Sawada K, Muto T, Shimoyama T, et al. Multicenter randomized controlled trial for the treatment of ulcerative colitis with a leukocytapheresis column. Curr Pharm Design. 2003;9:307–21. doi: 10.2174/1381612033391928. [DOI] [PubMed] [Google Scholar]
- 18.Yajima T, Takaishi H, Kanai T, et al. Predictive factors of response to leukocytapheresis therapy for ulcerative colitis. Ther Apher. 1998;2:115–9. doi: 10.1111/j.1744-9987.1998.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki M, Tsujikawa T, Fujiyama Y, Bamba T. Leukocytapheresis therapy for severe ulcerative colitis. Ther Apher. 1998;2:101–4. doi: 10.1111/j.1744-9987.1998.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 20.Hidaka T, Suzuki K, Matsuki Y, et al. Filtration leukocytapheresis therapy in rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1999;42:431–7. doi: 10.1002/1529-0131(199904)42:3<431::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Ueki Y, Yamasaki S, Kanamoto Y, et al. Evaluation of filtration leucocytapheresis for use in the treatment of patients with rheumatoid arthritis. Rheumatology. 2000;39:165–71. doi: 10.1093/rheumatology/39.2.165. [DOI] [PubMed] [Google Scholar]
- 22.Furuta T, Hotta O, Yusa N, et al. Lymphocytapheresis to treat rapidly progressive glomerulonephritis: a randomised comparison with steroid-pulse treatment. Lancet. 1998;352:203–4. doi: 10.1016/S0140-6736(05)77809-0. [DOI] [PubMed] [Google Scholar]
- 23.Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–5. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 25.Nagase K, Sawada K, Ohnishi K, et al. Complications of Leukocytapheresis. Ther Apher. 1998;2:120–4. doi: 10.1111/j.1744-9987.1998.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 26.Harig JM, Soergel KH, Komorowski RA, Wood GM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–8. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 27.Sabroe I, Jones EC, Usher LR, et al. Toll-like receptor (TLR) 2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 28.Mao XQ, Kawai M, Yamashita T, et al. Imbalance production between interleukin-1beta (IL-1beta) and IL-1 receptor antagonist (IL-1Ra) in bronchial asthma. Biochem Biophys Res Commun. 2000;276:607–12. doi: 10.1006/bbrc.2000.3516. [DOI] [PubMed] [Google Scholar]
- 29.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 30.Chapoval AI, Ni J, Lau JS, Wilcox RA, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Huang PL, Li JJ, et al. Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis in AIDS-related lymphoma cells infected with Kaposi's sarcoma-associated virus. Biochem Biophys Res Commun. 2001;287:983–94. doi: 10.1006/bbrc.2001.5689. [DOI] [PubMed] [Google Scholar]
- 32.Kanauchi O, Andoh A, Iwanaga T, et al. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J Gastroenterol Hepatol. 1999;14:1173–9. doi: 10.1046/j.1440-1746.1999.02025.x. [DOI] [PubMed] [Google Scholar]
- 33.Mancuso G, Midiri A, Beninati C, et al. Mitogen-activated protein kinases and NF-kappa B are involved in TNF-alpha responses to group B streptococci. J Immunol. 2002;169:1401–9. doi: 10.4049/jimmunol.169.3.1401. [DOI] [PubMed] [Google Scholar]
- 34.Renard P, Ernest I, Houbion A, et al. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucl Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto A, Seki Y, Kubo M, et al. Suppression of STAT5 functions in liver, mammary glands, and T Cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol Cell Biol. 1999;19:6396–407. doi: 10.1128/mcb.19.9.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:483–96. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duchmann R, Schmitt E, Knolle P, et al. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–8. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 38.Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West GA, Matsuura T, Levine AD, et al. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–95. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- 40.Mueller C, Knoflach P, Zielinski CC. T-cell activation in Crohn's disease. Increased levels of soluble interleukin-2 receptor in serum and in supernatants of stimulated peripheral blood mononuclear cells. Gastroenterology. 1990;98:639–46. [PubMed] [Google Scholar]
- 41.Schrader JW. Interleukin-3. In: Thomson AW, editor. The Cytokine Handbook. 2. London: Academic Press; 1994. pp. 81–98. [Google Scholar]
- 42.Thomson AW, Lotze MT. Interleukin-13, 14 and 15. In: Thomson AW, editor. The Cytokine Handbook. 2. London: Academic Press; 1994. pp. 257–63. [Google Scholar]
- 43.Baldwin AS. The NF-kB and I-kB protein: new discoveries and insights. Annu Rev Immunol. 1996;14:649–81. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 44.Baeuerle PA, Baichwal VR. NF-kB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–37. [PubMed] [Google Scholar]
- 45.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Genet. 1996;4:96–101. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 46.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 47.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 48.Bosisio D, Polentarutti N, Sironi M, et al. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–31. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- 49.Sartor RB. Role of the enteric microflora in the pathogenesis of intestinal inflammation and arthritis. Aliment Pharmacol Ther. 1997;11:17–3. doi: 10.1111/j.1365-2036.1997.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald TT, Pettersson S. Bacterial regulation of intestinal immune responses. Inflamm Bowel Dis. 2000;6:116–22. doi: 10.1097/00054725-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Nishiyama T, Mitsuyama K, Toyonaga A, et al. Colonic mucosal interleukin 1 receptor antagonist in inflammatory bowel disease. Digestion. 1994;55:368–73. doi: 10.1159/000201167. [DOI] [PubMed] [Google Scholar]
- 52.Casini-Raggi V, Kam L, Chong YJ, et al. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–40. [PubMed] [Google Scholar]
- 53.Neurath MF, Fuss I, Schurmann G, et al. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci. 1998;859:149–59. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- 54.Waetzig GH, Seegert D, Rosenstiel P, et al. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–51. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 55.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 56.Hommes D, van den Blink B, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology. 2002;122:7–14. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- 57.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 58.Fu XY, Schindler C, Improta T, et al. The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci USA. 1992;89:7840–3. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawai T, Adachi O, Ogawa T, et al. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 60.Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–8. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki T, Hiwatashi N, Yamazaki H, et al. The role of interferon γ in the pathogenesis of Crohn's disease. Gastroenterol Jpn. 1992;27:29–36. [PubMed] [Google Scholar]
- 62.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 63.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 64.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 65.Sawada K, Ohnishi K, Kosaka T, et al. Leukocytapheresis with leukocyte removal filter as new therapy for ulcerative colitis. Ther Apher. 1997;1:207–11. doi: 10.1111/j.1744-9987.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy DA, Rampton DS, Liu YC. Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease. Clin Exp Immunol. 1991;86:489–93. doi: 10.1111/j.1365-2249.1991.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malech HL, Gallin JI. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987;317:687–94. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 68.Eliakim R, Karmeli F, Razin E, Rachmilewitz D. Role of platelet-activating factor in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine and prednisolone. Gastroenterology. 1988;95:1167–72. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- 69.van Wersch JW, Houben P, Rijken J. Platelet count, platelet function, coagulation activity and fibrinolysis in the acute phase of inflammatory bowel disease. J Clin Chem Clin Biochem. 1990;28:513–7. doi: 10.1515/cclm.1990.28.8.513. [DOI] [PubMed] [Google Scholar]