Abstract
The co-stimulatory interactions of the B7 family molecules CD80 and CD86 on antigen-presenting cells, together with their T cell counter receptors CD28 and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), modulate T lymphocyte-mediated immune responses in a reciprocal manner. To investigate whether there is altered expression and the clinical significance of soluble co-stimulatory molecules in asthmatic patients, plasma concentrations of sCTLA-4, sCD28, sCD80 and sCD86 in 51 adult allergic asthmatic adults with or without steroid treatment, and 35 sex- and age-matched control subjects were measured by enzyme-linked immunosorbent assay (ELISA). Cell surface expression of CTLA-4 and CD28 on peripheral blood mononuclear cells (PBMC) were analysed by flow cytometry. Results showed that the plasma sCTLA-4 concentration was significantly higher in all asthmatic patients while sCD28 and sCD86 concentrations were significantly higher in steroid and non-steroid treated asthmatic patients, respectively, compared with control subjects (all P < 0·01). Significantly increased cell surface expression of CD28 but not CTLA-4 on PBMC was found in asthmatic patients compared with controls (P < 0·05). The plasma concentration and cell surface expression of CTLA-4 were found to exhibit positive and significant correlations with those of CD28 (both P < 0·05). Serum total IgE concentration correlated positively and significantly with sCTLA-4 and sCD28 concentrations in allergic asthmatic patients (both P < 0·05). The increased expression of these soluble co-stimulatory molecules may reflect the dysregulation of T cell activation, thereby contributing to the immunopathogenesis of allergic asthma.
Keywords: asthma, ELISA, T cells
Introduction
Allergic asthma is a complex and heterogeneous disease characterized by spontaneous airflow limitation, lung tissue remodelling, increased serum IgE concentration and airway hyperresponsiveness, with the infiltration of lymphocytes and eosinophils into the airway submucosa [1]. Activation and differentiation of T lymphocytes play a central role in mediating the pathogenesis in allergic asthma [2]. Allergen-induced IgE synthesis can trigger eosinophils, basophils and mast cells to release cytokines for the differentiation of T helper (Th) cells into Th2 cells for the secretion of interleukin (IL)-4, IL-5 and IL-13, and the subsequent mediation of allergic inflammation [2].
The initiation of T cell activation requires a primary signal delivered by the antigenic peptide presented by major histocompatibility complex (MHC) molecules, and a non-specific signal generated by the interaction of co-stimulatory molecules [3,4]. The co-stimulatory signal results from the interaction of CD28 on T cells with the B7 family B7-1 (CD80) and B7-2 (CD86) on antigen-presenting cells (APC) [5,6]. Resting APC are negative for CD80 and CD86 expression but monocytes and dendritic cells constitutively express CD86 [7]. Expression of CD80 is mainly activation-induced [7]. Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is a member of the immunoglobulin superfamily and a structural homologue of CD28 [8,9]. CTLA-4 is expressed only on activated Th cells and plays a negative regulatory role in T cell response [8,9]. Both CD28 and CTLA-4 bind the same ligands CD80 and CD86 expressed on APC, but CTLA-4 has a 20- to 50-fold higher affinity than CD28 [10]. Therefore, CD28 provides a critical co-stimulatory signal essential for the initiation and progression of T cell immunity [11], and CTLA-4 can actually down-regulate T cell function [8].
Previous studies have demonstrated that B7, CD28 and CTLA-4 co-stimulatory pathways play important roles in regulating allergen-induced T cell activation in airway inflammation in asthma, probably by T cell recruitment and Th cell differentiation upon allergen provocation [12–15]. In the murine model of asthma, Tsuyuki et al. showed that the treatment with CTLA-4-Ig fusion protein could block the interaction between CD28 and B7 that significantly reduced the recruitment of eosinophils, IgE synthesis and production of IL-4, IL-5, IL-10 and interferon (IFN)-γ from activated T cells [14]. In clinical studies, CTLA-4-Ig or anti-B7-2 antibody could inhibit in vitro allergen-induced proliferation and cytokine production by peripheral blood mononuclear cells (PBMC) from atopic adults [16,17]. Moreover, CTLA-4-Ig could effectively block the allergen-induced production of IL-5 and IL-13 in bronchial biopsy tissue of allergic asthmatic patients [18].
Previous studies have reported elevation of serum CD86 concentration and increased cell surface expression of CD80 on alveolar macrophages in asthmatic patients [19,20]. In molecular studies, there was association of polymorphism in the CTLA-4 gene with asthma severity [21] and the elevation of total IgE in allergic rhinitis [22]. In an attempt to further evaluate the immunopathological roles of T cell co-stimulatory molecules and search for new potential surrogate markers of allergic asthma, we investigated the plasma concentrations of co-stimulatory molecules sCD28, sCTLA-4, sCD80 and sCD86 and cell surface expression of CTLA-4 and CD28 on PBMC in patients with allergic asthma with or without steroid treatment.
Materials and methods
Asthmatic patients, control subjects and blood samples
Fifty-one Chinese adult patients with asthma were recruited from the Asthma Clinic of the Prince of Wales Hospital, Hong Kong. Diagnosis of asthma was based on the guidelines of the American Thoracic Society [23]. Lung function of the subjects was assessed by spirometry (Model S, Vitalograph, Buckingham, UK) according to the American Thoracic Society standards [24]. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and the FEV1/FVC ratio were measured before and 15 min after the inhalation of salbutamol (Glaxo Operations Ltd, Greenford, UK). The results were compared with the local predicted age- and sex-matched values [25]. The severity of asthma in these patients was assessed according to the Global Initiative for Asthma guidelines (GINA) based on daytime symptoms, nocturnal symptoms and lung function [26,27]. All our studied asthmatic patients were on short-acting bronchodilator as needed. Some of them were on maintenance inhaled steroids such as beclomethasone dipropionate (Becloforte; Glaxo Wellcome; Research Triangle Park, NC, USA) or budesonide (Pulmicort, AstraZeneca, London, UK). All patients and control subjects had no oral steroid intake or change of asthma medications 4 weeks prior to recruitment of study. They were also not on theophylline or antileukotriene therapy, and only six (11·8%) of them were on long-acting beta-2 agonist. Thirty-five sex- and age-matched healthy non-allergic Chinese volunteers were recruited as control subjects. The presence of allergic diseases in these subjects was excluded on the basis of negative returns from a detailed questionnaire survey. All subjects were non-smokers and free from upper respiratory tract infection for 2 weeks preceding the study. Nine ml of ethylenediaminetetra-acetic acid (EDTA) venous peripheral blood and 5 ml of clotted blood were collected from each patient and control subject. Aliquots of whole blood from each subject were processed immediately for the analysis of the cell surface expression of CTLA-4 and CD28 on PBMC. Plasma and serum samples were preserved at −70°C for subsequent assays of soluble co-stimulatory molecules, IgE and eosinophilic cationic protein (ECP). The above protocol was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Hospitals Clinical Research Ethics Committee, and informed consent was obtained from all participants.
Assay of serum total IgE, allergen-specific IgE and ECP
The atopic status of patients and control subjects was ascertained by positive serum specific IgE assays to house dust mites, cat, dog, mixed cockroaches and mixed moulds by fluorescence enzyme immunoassay (AutoCAP analyser, Pharmacia Diagnostics AB, Uppsala, Sweden) [28]. Sensitization to local pollens was not tested due to its low prevalence in our community [29]. Subjects were classified as atopic if they had at least one positive test for allergen-specific IgE. Serum total IgE and ECP concentrations were similarly measured (AutoCAP analyser).
Assay of plasma sCTLA-4, sCD28, sCD80 and sCD86
Plasma concentrations of sCTLA-4, sCD28, sCD80 and sCD86 of asthmatic patients and control subjects were measured by enzyme-linked immunosorbent assay (ELISA) using reagent kits for human sCTLA-4, sCD28, sCD80 and sCD86, respectively (Bender Medsystems Diagnostics GmbH, Vienna, Austria).
Flow cytometric analysis of cell surface expression of CTLA-4 and CD28 on PBMC
PBMC were prepared by centrifuging EDTA venous blood using a Ficoll-Paque density gradient (Amersham Pharmacia Biotech Ltd, Uppsala, Sweden). The viability of PBMC was more than 95% as determined by the trypan blue exclusion method. PBMC (1 × 106/ml) were harvested and resuspended in cold phosphate buffered saline (PBS) supplemented with 0·5% bovine serum albumin (BSA). After blocking with 2% human pooled serum for 20 min at 4°C and washed with PBS supplemented with 0·5% BSA, cells were incubated either with mouse anti-human CTLA-4 or CD28 monoclonal antibody or mouse IgG1 isotype for 30 min at 4°C in the dark. After washing, cells were stained with secondary fluorescein isothiocyanate (FITC)-conjugated antimouse antibody for 45 min. The cells were finally resuspended in 1% paraformaldehyde in 1 × PBS as fixative. Cell surface expression of CTLA-4 and CD28 was then analysed by flow cytometry (FACSCalibur, Becton Dickinson, CA, USA). The typical forward- and side-scatter gate for lymphocytes was set to exclude contaminating monocytes from the analysis. A total of 5000 events were collected in the log mode and results were expressed as percentage increase comparing with the isotypic control.
Statistical analysis
Because the expression of sCTLA-4, sCD28, sCD80 and sCD86 and the percentage of cell surface expression of CTLA-4 and CD28 were not in a Gaussian distribution, the Mann–Whitney rank sum test was used to assess the differences between the asthmatic patients and the control subjects. All analyses were performed using statistical software (spss for Windows, version 9·0, SPSS Inc., Chicago, IL, USA). Probability (P) values of less than 0·05 were considered significant. Unless otherwise specified, results are expressed as the median (interquartile range) (IQR).
Results
Asthma severity, atopic status and serum ECP
The age, sex, lung functions, drug treatment and atopic status of the study populations are summarized in Table 1. The mean ± s.d. FEV1 of all asthmatic patients was 2·5 ± 0·9 l/min (85·9 ± 16·9% of predicted normal values), while the FEV1/FVC ratio was 84·1 ± 8·2%. The severity of asthma in these patients according to GINA comprised: intermittent asthma nine patients (17·7%), mild persistent asthma six patients (11·8%), moderate persistent asthma 18 patients (35·3%) and severe persistent asthma 18 patients (35·3%). Atopy, defined as ≥ one positive specific IgE test to inhalant allergens, was found in 76% of the steroid-treated asthmatic patients, 61% of the non-steroid-treated asthmatic patients and 39% of the control subjects. Compared to control subjects, the serum total IgE concentration was significantly elevated in steroid-treated asthmatic patients [median, 114 (IQR, 60–332) versus 48 (10–129) kU/l; P < 0·05] and non-steroid-treated asthmatic patients [161 (93–465) versus 48 (10–129) kU/l; P < 0·001]. Serum ECP concentration was also significantly higher in steroid-treated asthmatic patients than control subjects [3·5 (2·2–5·4) versus 2·0 (2·0–2·1) µg/l; P < 0·001]. A significant positive correlation between serum total IgE concentration and FEV1 was found in allergic asthmatic patients (r = 0·51, P < 0·001).
Table 1. Characteristics of the study populations.*.
| Parameters | Steroid-treated asthmatic patients (n = 28) | Non-steroid-treated asthmatic patients (n = 23) | Control subjects (n = 35) |
|---|---|---|---|
| Sex, male/female | |||
| Male | 15 | 9 | 11 |
| Female | 13 | 14 | 24 |
| Age, years, mean ± s.d. (range) | 43·5 ± 15·7 (21–69) | 37·1 ± 14·0 (20–63) | 40·9 ± 11·9 (22–66) |
| Lung functions | |||
| FEV1, l/min (% predicted) | 2·4 ± 0·8 (82·5 ± 14·9) | 2·6 ± 0·9 (89·9 ± 18·5) | NE |
| FEV1/FVC ratio (%) | 82·0 ± 7·1 | 86·5 ± 8·9 | NE |
| Inhaled steroid treatment | |||
| Daily dose, µg | 1110·7 ± 631·5 | 0 | 0 |
| Atopy (%) | 76 | 61 | 39 |
| Serum total IgE (kU/l)§ | 114 (60–332)† | 161 (93–465)‡ | 48 (10–129) |
| Serum ECP (µg/l)§ | 3·5 (2·2–5·4)‡ | 2·0 (2·0–3·0) | 2·0 (2·0–2·1) |
Values given as median (IQR).
Higher than control subjects [P < 0·001 (Mann–Whitney rank sum test)].
Higher than control subjects [P < 0·05 (Mann–Whitney rank sum test)].
Values given as mean ± s.d., unless otherwise indicated. NE = not examined.
Plasma sCTLA-4, sCD28, sCD80 and sCD86
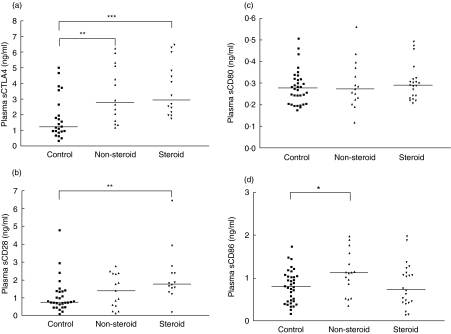
As shown in Fig. 1, sCTLA-4, sCD28, sCD80 and sCD86 were ubiquitously present in plasma. Plasma sCTLA-4 concentrations were significantly higher in non-steroid-treated and steroid-treated asthmatic patients than control subjects [2·8 (1·5–5·2) and 2·9 (2·1–5·4) versus 1·2 (0·9–3·1) ng/ml, P < 0·01 and P < 0·001, respectively] (Fig. 1a). sCD28 concentrations were significantly higher in steroid-treated and sCD86 concentrations were significantly higher in non-steroid-treated asthmatic patients than that of control subjects [sCD28: 1·8 (1·4–2·6) versus 0·7 (0·5–1·4) ng/ml, P < 0·01; sCD86: 1·1 (0·7–1·7) versus 0·8 (0·5–1·0) ng/ml, P < 0·05] (Fig. 1b,d). However, there were no significant differences in sCD80 between non-steroid-treated and steroid-treated asthmatic patients versus controls subjects (all P > 0·05) (Fig. 1c). Plasma concentrations of sCTLA-4 and sCD28 of asthmatic patients were found to have a strongly significant and positive correlation (r = 0·818, P = 0·000) (Table 2). Both co-stimulatory molecules also correlated positively and significantly with serum total IgE concentration (CTLA-4, r = 0·415, P = 0·020; CD28, r = 0·389, P = 0·030) (Table 2). We also observed that there was a significant and positive correlation between the elevation of sCD28 but not other soluble co-stimulatory molecules with the percentage of eosinophils (r = 0·402, P = 0·028).
Fig. 1.
Scatter-plots of plasma concentrations of (a), sCTLA-4 (b) sCD28, (c) sCD80 and (d) sCD86 of control subjects, non-steroid-treated and steroid-treated asthmatic patients. The differences between asthmatic patients and control subjects were determined by non-parametric Mann–Whitney rank sum test. *P < 0·05, **P < 0·01, ***P < 0·001.
Table 2. Correlations among the concentrations of serum total IgE and soluble co-stimulatory molecules in allergic asthmatic patients.
| sCTLA-4 | sCD28 | sCD80 | sCD86 | |
|---|---|---|---|---|
| Serum total IgE | ||||
| Spearman's r | 0·415 | 0·389 | 1·02 | 0·047 |
| P-value | 0·020* | 0·030* | 0·556 | 0·784 |
| sCTLA-4 | ||||
| Spearman's r | 0·818 | 0·100 | −0·211 | |
| P-value | 0·000* | 0·608 | 0·272 | |
| sCD28 | ||||
| Spearman's r | 0·818 | −0·002 | −0·257 | |
| P-value | 0·000* | 0·990 | 0·178 | |
P =0·05.
Cell surface expression of CTLA-4 and CD28 on PBMC
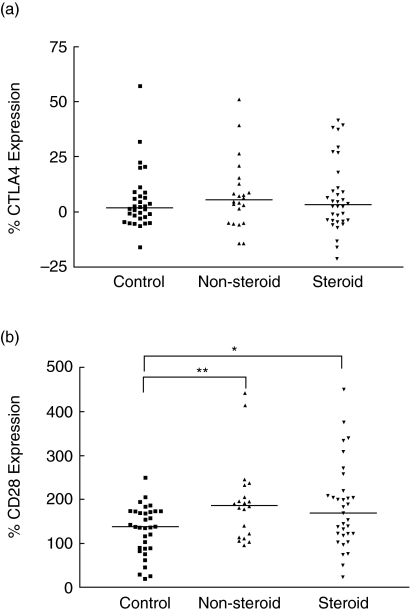
As shown in Fig. 2a, there was no significant difference in cell surface expression of CTLA-4 on PBMC between non-steroid-treated and steroid-treated asthmatic patients and control subjects (P > 0·05). However, there was significant up-regulation in cell surface expression of CD28 on PBMC of asthmatic patients than that of control subjects [non-steroid-treated and steroid-treated patients versus control subjects: 187 (118–219) and 170 (121–214) versus 138 (89–173)%; all P < 0·05] (Fig. 2b). Cell surface expressions of CLTA-4 and CD28 were found to exhibit a positive significant correlation (r = 0·305, P = 0·023). As also observed by us, there was a significant and negative correlation between GINA severity score and cell surface expression of CTLA-4 (r = −0·282, P = 0·041) and CD28 (r = −0·274, P = 0·049) on PBMC in all patients. Using immunophenotyping of CD4 T helper and CD8 suppressor cells plus cytotoxic T cells by flow cytometry, we also observed that the mean percentages of CD4+ CD28+ cells in non-allergic asthmatic patients and allergic asthmatic patients were 94·7 and 94·0%, respectively, and CD8+ CD28+ cells in non-allergic asthmatic patients and allergic asthmatic patients were 47·4 and 70·5%, respectively. Results therefore indicate that CD28 expression is more predominant on CD4 cells but CD28 expression was elevated on CD8 cells of allergic asthmatic patients.
Fig. 2.
The percentage cell surface expression of (a) CTLA-4 and (b) CD28 on peripheral blood mononuclear cells (PBMC) of control subjects, non-steroid-treated asthma subjects and steroid-treated asthmatic patients. The differences between asthmatic patients and control subjects were determined by non-parametric Mann–Whitney rank sum test. *P < 0·05, **P < 0·01.
Discussion
Previous studies have identified the regulatory roles of co-stimulatory molecules CTLA-4 and CD28 for the inflammatory cell recruitment and Th2 cell differentiation after allergen provocation [14,15,17]. Regarding the expression of co-stimulatory molecules, most of the studies investigated the cell surface expression of CD80 and CD86 on eosinophils [30], alveolar macrophages [20,31], dendritic cells [32] and B lymphocytes [33] in patients with allergic asthma or mouse models. Serum sCTLA-4 and sCD28 have also been shown to be elevated in patients with systemic lupus erythematosus [34,35]. However, the production of sCTLA-4, sCD28, sCD80 and sCD86 and the cell surface expression of CTLA-4 and CD28 on PBMC have not been well investigated in patients with allergic asthma. In the present study, we demonstrated that plasma sCTLA-4 concentration was significantly higher in asthmatic patients treated or not treated with steroids, while sCD28 and sCD86 concentrations were significantly higher in steroid-treated and non-steroid-treated asthmatic patients, respectively, than those of control subjects (Fig. 1). Together with the positive correlation of total IgE with plasma sCTLA-4 and sCD28 concentrations, the production of soluble co-stimulatory molecules CTLA-4 and CD28 should be related, at least partly, to the pathogenesis and severity of allergic inflammation.
sCTLA-4 mRNA has been shown to be constitutively expressed on non-stimulated T cells, and its expression is down-regulated after activation [36]. sCTLA-4 plays a more important role than membrane (m)CTLA-4 in the early stage of immune response, because sCTLA-4 is constitutively expressed on non-stimulated T cells and mCTLA-4 is expressed only on activated T cells [34]. Moreover, sCTLA-4 has been shown to have immunoregulatory properties in vitro[36]. In the present study, we observed that there was significant elevation of plasma sCTLA-4 in asthmatic patients treated or not treated with steroids (Fig. 1). sCTLA-4 may block the interaction between B7 on APCs and mCTLA-4 on T cells, thereby interfering with the inhibitory signal sending to T cells for enhancing the immune response [34]. On the other hand, sCTLA-4 may also bind B7 expressed on APC and thereby interfering with B7:CD28-mediated co-stimulation of T cell responses [36]. Therefore, the detailed immunopathological role of plasma sCTLA-4 in allergic asthmatic patients require further investigation. The blockade of CTLA-4 could enhance allergic sensitization and eosinophilic airway inflammation in mice [37]. A fusion protein, CTLA-4-Ig, has been shown to inhibit CD80 (B7-1) and/or CD86 (B7-2) co-stimulation for allergen-induced release of Th2 cytokine, IL-5 and IL-13 in asthmatic patients ex vivo[18]. In view of the above findings, sCTLA-4 could suppress Th1 cell proliferation but deliver a positive signal to Th2 activation [21]. These might explain the higher concentration of sCTLA-4 in asthmatic patients because of the Th2 predominance [38].
sCD28 can be produced either by shedding of the membrane form or alternative mRNA splicing [39], but recent polymerase chain reaction (PCR) analysis has suggested that it is likely to be due to the shedding of the membrane form [35]. In vitro studies indicated that APC such as dendritic cells could be induced by sCD28 to express IL-6 and IFN-γ[40]. sCD28 was also demonstrated to enhance T cell-mediated immunity against tumour and self-peptides, and protection against microbial and tumour challenge [40]. It may also act as inhibitory molecules by competing and interfering the interactions between CTLA-4/CD28 and B7 molecules of APC, perhaps in certain T cell subtypes, e.g. Th1 cells [36]. sCTLA-4 and sCD28 are produced mainly from T lymphocytes whereas sCD80 and sCD86 are from APC including macrophages, B lymphocytes and dendritic cells [31,33,41,42]. Our findings showed that plasma and cell surface expression of CTLA-4 had a very strong positive correlation with that of sCD28 (Table 2). We also observed that there was significant and positive correlation between the elevation of sCD28 and the percentage of eosinophils. These results might highlight close relationships of the expression of CTLA-4 and CD28 in terms of their cellular origins. Our results showing elevated cell surface expression of CD28 on CD8+ CD28+ cells in allergic asthmatic patients is in concordance with previous published results [43], thereby indicating that CD28 pathway plays a role in the development of allergic reactions. Moreover, the significant and negative correlation between GINA severity score and cell surface expression of CTLA-4 and CD28 on PBMC indicated that there was a relationship between the progression of disease and the down-regulation of the cell surface expression of co-stimulatory receptors. Accordingly, it is possible that the elevation of soluble co-stimulatory receptors in allergic asthmatic patients are derived from the cleavage of membrane co-stimulatory receptors along with increasing disease severity.
Our result of the elevation of plasma sCD86 also concurs with that of a recent study [19]. Membrane CD86 is expressed predominantly on monocytes, dendritic cells, lymphocytes, eosinophils and neutrophils [9], but sCD86 has been suggested to be derived from peripheral blood monocytes [19]. CD86 but not CD80 has been proposed for the induction of lung mucosal Th2 immune response and altered airway responsiveness [14]. From our present study, steroid treatment seemed to suppress sCD86 production with alleviation of the disease severity. Therefore, the elevation of sCD86 should relate to the exacerbation of allergic asthma.
Although the exact mechanisms underlying the up-regulation of the production of soluble co-stimulatory molecules in plasma remain largely undefined, they are likely to be influenced by the abnormal production of cytokines and chemokines. Indeed, we have shown significant elevation of plasma inflammatory cytokine IL-18 [18], and Th2-related chemokine thymus and activation-regulated chemokine (TARC) [44] and regulated upon activation normal T cell expressed and secreted (RANTES) (unpublished data) in allergic asthmatic patients. The plasma RANTES also showed significant positive correlation with sCTLA-4 and sCD28 in asthmatic patients (data not shown). The B7-CD28/CTLA-4 co-stimulation has been shown to regulate the T cell chemotaxis and differentiation, probably through the activation of inflammatory cytokines and chemokines [15,45]. Therefore, the dysregulation of T cell functions by the elevation of soluble co-stimulatory molecules such as sCTLA-4, sCD28 and sCD86, and proinflammatory cytokines and chemokines may lead to the recruitment of eosinophils and Th2 cells for the induction of Th2 immune response, particularly in more severe allergic asthmatic patients.
In this study, we have demonstrated elevated concentrations of plasma sCTLA-4, sCD28 and sCD86 but not sCD80 in allergic asthmatic patients, as well as positive correlation between the elevation of plasma sCTLA-4 and sCD28 with total serum IgE concentration and negative correlation between mCTLA-4 and mCD28 with GINA disease severity score. The above results should provide new postulates for potential immunopathological roles of co-stimulatory molecules in the exacerbation of allergic inflammation and the suggestion of new potential surrogate markers of allergic asthma. Further elucidation of the roles and functions of co-stimulatory molecules requires the investigation of samples obtained from or near the site of inflammation, e.g. induced sputum, bronchoalveolar lavage fluid or exhaled breath condensate. In view of recent advances in the exploration of the therapeutic targeting of co-stimulation for the T cell activation in allergic asthma [14–18,36,46], therapeutic agents such as antibody against the soluble form of co-stimulatory molecules CTLA-4, CD28, CD86 and inducible co-stimulator (ICOS) might be potential drugs for treating allergic asthma.
Acknowledgments
This study was supported by a donation from Greater China Technology Group Limited.
References
- 1.Marone G. Asthma: recent advances. Immunol Today. 1998;19:5–9. doi: 10.1016/s0167-5699(97)01187-0. [DOI] [PubMed] [Google Scholar]
- 2.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–63. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 3.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 4.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 co-stimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 5.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azuma M, Ito D, Yagita H, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 7.Sfikakis PP, Via CS. Expression of CD28, CTLA4, CD80, and CD86 molecules in patients with autoimmune rheumatic diseases: implications for immunotherapy. Clin Immunol Immunopathol. 1997;83:195–8. doi: 10.1006/clin.1997.4368. [DOI] [PubMed] [Google Scholar]
- 8.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 co-stimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell co-stimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 12.Green JM. The B7/CD28/CTLA4 T-cell activation pathway. Implications for inflammatory lung disease. Am J Respir Cell Mol Biol. 2000;22:261–4. doi: 10.1165/ajrcmb.22.3.f179. [DOI] [PubMed] [Google Scholar]
- 13.Van Neerven RJ, Van de Pol MM, Van der Zee JS, Stiekema FE, De Boer M, Kapsenberg ML. Requirement of CD28-CD86 co-stimulation for allergen-specific T cell proliferation and cytokine expression. Clin Exp Allergy. 1998;28:808–16. doi: 10.1046/j.1365-2222.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsuyuki S, Tsuyuki J, Einsle K, Kopf M, Coyle AJ. Costimulation through B7-2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J Exp Med. 1997;185:1671–9. doi: 10.1084/jem.185.9.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burr JS, Kimzey SL, Randolph DR, Green JM. CD28 and CTLA4 coordinately regulate airway inflammatory cell recruitment and T-helper cell differentiation after inhaled allergen. Am J Respir Cell Mol Biol. 2001;24:563–8. doi: 10.1165/ajrcmb.24.5.4375. [DOI] [PubMed] [Google Scholar]
- 16.Sayegh MH. Finally, CTLA4Ig graduates to the clinic. J Clin Invest. 1999;103:1223–5. doi: 10.1172/JCI6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larche M, Till SJ, Haselden BM, et al. Costimulation through CD86 is involved in airway antigen-presenting cell and T cell responses to allergen in atopic asthmatics. J Immunol. 1998;161:6375–82. [PubMed] [Google Scholar]
- 18.Jaffar Z, Roberts K, Pandit A, Linsley P, Djukanovic R, Holgate S. B7 co-stimulation is required for IL-5 and IL-13 secretion by bronchial biopsy tissue of atopic asthmatic subjects in response to allergen stimulation. Am J Respir Cell Mol Biol. 1999;20:153–62. doi: 10.1165/ajrcmb.20.1.3255. [DOI] [PubMed] [Google Scholar]
- 19.Shi HZ, Xie ZF, Deng JM, Chen YQ, Xiao CQ. Soluble CD86 protein in serum samples of patients with asthma. Thorax. 2004;59:870–5. doi: 10.1136/thx.2004.021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burastero SE, Magnani Z, Confetti C, et al. Increased expression of the CD80 accessory molecule by alveolar macrophages in asthmatic subjects and its functional involvement in allergen presentation to autologous TH2 lymphocytes. J Allergy Clin Immunol. 1999;103:1136–42. doi: 10.1016/s0091-6749(99)70189-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Lee YH, Shin C, et al. Association of asthma severity and bronchial hyperresponsiveness with a polymorphism in the cytotoxic T-lymphocyte antigen-4 gene. Chest. 2002;122:171–6. doi: 10.1378/chest.122.1.171. [DOI] [PubMed] [Google Scholar]
- 22.Yang KD, Liu CA, Chang JC, et al. Polymorphism of the immune-braking gene CTLA-4 (+49) involved in gender discrepancy of serum total IgE levels and allergic diseases. Clin Exp Allergy. 2004;34:32–7. doi: 10.1111/j.1365-2222.2004.01776.x. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society. Guidelines for the evaluation of impairment/disability in patients with asthma. Am Rev Respir Dis. 1993;147:1056–61. doi: 10.1164/ajrccm/147.4.1056. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 25.Lam KK, Pang SC, Allan WG, et al. Predictive nomograms for forced expiratory volume, forced vital capacity, and peak expiratory flow rate, in Chinese adults and children. Br J Dis Chest. 1983;77:390–6. [PubMed] [Google Scholar]
- 26.National Asthma Education and Prevention Program. Guidelines for the Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. Expert panel report 2. Publication no. 97-4051. [Google Scholar]
- 27.National Heart, Blood, and Lung Institute. Global strategy for asthma management and prevention. Bethesda, MD: National Heart, Lung, and Blood Institute; 1995. WHO/NHLBI workshop report. Publication no. 95-3659. [Google Scholar]
- 28.Lam CWK, Fung HK, Vrijmoed LLP, et al. Aetiology of allergic rhinitis in Hong Kong. Allergol Int. 1998;47:23–8. [Google Scholar]
- 29.Leung R, Ho P, Lam CWK, et al. Sensitization to inhalant allergens as a risk factor for asthma and allergic diseases in Chinese. J Allergy Clin Immunol. 1997;99:594–9. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 30.Mathur M, Herrmann K, Qin Y, et al. CD28 interactions with either CD80 or CD86 are sufficient to induce allergic airway inflammation in mice. Am J Respir Cell Mol Biol. 1999;21:498–509. doi: 10.1165/ajrcmb.21.4.3714. [DOI] [PubMed] [Google Scholar]
- 31.Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy. 2001;31:625–36. doi: 10.1046/j.1365-2222.2001.01068.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheng X, Wang C, Qian G, Zhu B. CD80, but not CD86 were up-regulated on the spleen-derived dendritic cells from OVA-sensitized and challenged BALB/c mice. Immunol Lett. 2003;89:31–8. doi: 10.1016/s0165-2478(03)00107-x. [DOI] [PubMed] [Google Scholar]
- 33.Hofer MF, Jirapongsananuruk O, Trumble AE, Leung DY. Upregulation of B7.2, but not B7.1, on B cells from patients with allergic asthma. J Allergy Clin Immunol. 1998;101:96–102. doi: 10.1016/S0091-6749(98)70199-X. [DOI] [PubMed] [Google Scholar]
- 34.Liu MF, Wang CR, Chen PC, Fung LL. Increased expression of soluble cytotoxic T-lymphocyte-associated antigen-4 molecule in patients with systemic lupus erythematosus. Scand J Immunol. 2003;57:568–72. doi: 10.1046/j.1365-3083.2003.01232.x. [DOI] [PubMed] [Google Scholar]
- 35.Hebbar M, Jeannin P, Magistrelli G, et al. Detection of circulating soluble CD28 in patients with systemic lupus erythematosus, primary Sjogren's syndrome and systemic sclerosis. Clin Exp Immunol. 2004;136:388–92. doi: 10.1111/j.1365-2249.2004.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell Immunol. 2000;201:144–53. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 37.Hellings PW, Vandenberghe P, Kasran A, et al. Blockade of CTLA-4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur J Immunol. 2002;32:585–94. doi: 10.1002/1521-4141(200202)32:2<585::AID-IMMU585>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Wong CK, Ho CY, Ko FW, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magistrelli G, Jeannin P, Elson G, et al. Identification of three alternatively spliced variants of human CD28 mRNA. Biochem Biophys Res Commun. 1999;259:34–7. doi: 10.1006/bbrc.1999.0725. [DOI] [PubMed] [Google Scholar]
- 40.Orabona C, Grohmann U, Belladonna ML, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–42. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 41.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 42.Wang XB, Giscombe R, Yan Z, Heiden T, Xu D, Lefvert AK. Expression of CTLA-4 by human monocytes. Scand J Immunol. 2002;55:53–60. doi: 10.1046/j.0300-9475.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsicopoulos A, Labalette M, Akoum H, et al. CD28 expression is increased in venom allergic patients but is not modified by specific immunotherapy. Clin Exp Allergy. 1996;26:1119–24. [PubMed] [Google Scholar]
- 44.Leung TF, Wong CK, Chan IH, Ip WK, Lam CW, Wong GW. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J Allergy Clin Immunol. 2002;110:404–9. doi: 10.1067/mai.2002.126378. [DOI] [PubMed] [Google Scholar]
- 45.Hidi R, Riches V, Al-Ali M, et al. Role of B7-CD28/CTLA-4 co-stimulation and NF-kappa B in allergen-induced T cell chemotaxis by IL-16 and RANTES. J Immunol. 2000;164:412–8. doi: 10.4049/jimmunol.164.1.412. [DOI] [PubMed] [Google Scholar]
- 46.Wiley RE, Goncharova S, Shea T, Johnson JR, Coyle AJ, Jordana M. Evaluation of inducible co-stimulator/B7-related protein-1 as a therapeutic target in a murine model of allergic airway inflammation. Am J Respir Cell Mol Biol. 2003;28:722–30. doi: 10.1165/rcmb.2002-0220OC. [DOI] [PubMed] [Google Scholar]