Abstract
The aim of this study was to identify immunoreactive domains on human ribosomal P0, P1 and P2 proteins, other than the C-22 peptide, to develop a novel ELISA using a combination of these proteins and to compare this ELISA with one using the C-22 peptide. Human recombinant P0, P1, P2 and mutant P0 lacking the homologous C-22 peptide (N-P0) were produced in bacteria and tested by ELISA and immunoblotting using sera from 48 patients with systemic lupus erythematosus (SLE), 48 with an unrelated inflammatory disorder (Crohn's disease) and 47 healthy controls. ELISA with P0, P1 and P2, premixed at equimolar concentrations, gave higher OD readings than each protein tested individually. Eighteen SLE sera tested positive by ELISA with premixed P0, P1, P2 but only 3 tested positive with the C-22 peptide. Twenty-two SLE sera reacted positively, as determined by immunoblotting, with 5 different P protein combinations: P1P2, P0P1P2, P1, P0P1, P0 and P1. Only sera reactive with all three P proteins reacted with the C-22 peptide, with absent or minimal reactivity with N-P0. Native antigens yielded sensitivity (6/48, 13%) similar to the C-22 peptide assay. An ELISA with premixed P1 and P2 gave higher OD values than the arithmetic means with P1 or P2. Fifteen SLE patients had antibodies to double stranded (ds)-DNA, of which 6 also had antibodies to P0P1P2 by ELISA but 12 reactive with P0P1P2 did not have discernable ds-DNA antibodies. Ribosomal P autoantibodies react mainly with epitopes N-terminal to a homologous C-22 peptide. An ELISA with premixed P0, P1 and P2 has 5-fold greater sensitivity (38%) for SLE than an assay with the conventional C-22 peptide (7%). The combined sensitivity for SLE for antibodies to P0P1P2 and ds-DNA is 56%, higher than C-22 and ds-DNA, 38%. Only one of the SLE patients had neuropsychiatric lupus.
Keywords: anti-ribosomal P, ELISA, SLE
Introduction
Autoantibodies to acidic ribosomal phospho (P) proteins are reported in 10–20% of patients with systemic lupus erythematosus (SLE) [1–5] with higher frequencies in Chinese populations (36–38%) [5–7] and juvenile-onset SLE [8]. There are reports of an association of anti-ribo-P with active SLE [9,10], and disease of the kidney [11,12], liver [13,14] and nervous system [15–18] but associations particularly with neuropsychiatric lupus are contentious [19].
Ribosomal P proteins are proposed to exist as a pentamer of 3 proteins, P0 (38 kD), P1 (19 kD) and P2 (17 kD) with 2 P1/P2 heterodimers binding one molecule of P0 through the P1 molecule [20–22]. The pentamer binds the GTPase-associated domain of 28S rRNA of the 60S subunit and interacts with elongation factors 1 α and 2 [23,45]. It has been suggested that this pentamer is responsible for autoantibody production [24], that autoantibodies recognize both linear and conformational epitope(s) [25,26], and that CD4 T lymphocytes help antibody production [27]. The autoantibodies inhibit protein synthesis [28,44] and to bind and penetrate human plasma membranes causing cellular dysfunction [29,30].
Autoantibodies target all 3 ribosomal P proteins [31] and phosphorylation does not appear necessary for autoantibody recognition [25]. Previously, a homologous 22 amino acid C-terminal (C-22) sequence shared by all 3 ribosomal P proteins was suggested as the dominant immunoreactive domain [32–35], with a major epitope in the 6 C-terminal amino acids [36]. This observation has led to increasing reliance on immunoassays using a synthetic C-22 peptide for autoantibody detection. There is also a report of an assay using 4 copies of the C-13 peptide [37].
To re-examine the question of the autoantibody-reactive domains on all 3 ribosomal P proteins and to cover the full range of putative epitopes we generated full length human recombinant P0, P1 and P2 proteins for application in an ELISA and immunoblotting. To assess the reactive domains we examined reactivity with a mutant human ribosomal P0 protein lacking the homologous C-22 sequence, and with P1/P2 heterodimers. We found that the predominant immunoreactive domains of the 3 ribosomal P proteins are located N-terminal to the homologous C-22 sequence and that an ELISA with premixed P0, P1 and P2 proteins has a sensitivity of 38% for SLE, compared with a sensitivity of 7% for an ELISA using the homologous C-22 peptide.
Materials and methods
Human sera
SLE (n = 48) and Crohn's disease as inflammatory control sera (n = 48) obtained from the University of Calgary, Canada and blinded before testing. The SLE sera were randomly selected from a cohort of patients meeting the ARA criteria for the diagnosis of SLE [38]. Only one of these SLE patients had neuropsychiatric lupus. Anti-ribosomal P positive control serum was determined by Hep-2000 indirect immunofluorescence (ImmunoConcepts®, USA) and anti-ribosomal P ELISA (INOVA®, USA) assays. Normal control serum was obtained from a healthy female. Normal human sera (n = 47) were obtained from an Australian blood bank.
Recombinant constructs and bacterial expression
Ribosomal P0, P1 and P2 cDNA were obtained by RT-PCR using HeLa cell mRNA, Avian Myeloblastosis Virus (AMV) reverse transcriptase (Roche), and appropriate primers for cloning into pET-15b expression vector (Novagen). The cDNA was PCR-amplified using Deep Vent® DNA polymerase (New England Biolab). pET-15b plasmid (Novagen) and P0, P1, P2 and N-P0 were digested with Nde1 and Xho1, dephosphorylated with alkaline shrimp phosphatase (Boehringer Mannheim), ligated using T4 DNA ligase (Promega, USA) and used to transform E. coli DH5α. In-frame DNA sequences were confirmed (ABI Prism™ Big Dye, Baker Institute). Single colonies of pET-P0, pET-N-P0, pET-P1 and pET-P2-transformed E. coli BL21(DE3) were inoculated into bacterial culture broth and induced with IPTG (0·7 mM) at 37°C for 2–3 h.
Ni-NTA affinity chromatography
IPTG induced pET-P0, P1, P2 and N-P0 transformed bacterial cell pellets were frozen overnight, resuspended in buffer (0·1 M NaH2PO4, 10 mM Tris-HCl and 6 M guanidine-HCl, pH 8), incubated at room temperature for 30 min and pelleted. Supernatants were applied to a Ni-NTA column (Qiagen), washed with buffer, pH 6·3 and proteins eluted in pH 4·6 buffer, then dialysed in buffers, 50 mM NaH2PO4, 100 mM NaCl, 0·1% Tween-20, 2 mM DTT, 1 µg/ml leupeptin, 1 µg/ml aprotinin and 100 µg/ml PMSF, pH 7·4 using protein concentrator, Vivaspin20® with a 5 kD cut off (Sartorius). Protein concentrations were determined by the Lowry method (Pearce, USA).
SDS-PAGE and immunoblotting
Protein samples (1 ml) in SDS sample buffer containing 62·5 mM Tris-HCl, 1% SDS, 10% glycerol, 0·001% bromophenol blue (w/v) and 0·7 M β-mercaptoethanol, pH 8, were boiled for 3 min, followed by 12·5% SDS-PAGE [39] at 100 V and 40 mA at 25°C. The gels were stained with 0·25% (w/v) Coomassie blue.
For immunoblotting, proteins were separated on 12·5% SDS-PAGE and transferred to nitrocellulose membranes at 4°C for 16 h at 36 V in buffer (25 mM Tris-HCl, 192 mM glycine and 20% ethanol, pH 7·6), blocked with 5% (w/v) skim milk in the buffer and incubated with anti-ribosomal P positive or negative serum at room temperature for 1 h. Membranes were washed with PBS-T buffer for 20 min, incubated for 1 h with 1 : 10 000 dilution of horseradish peroxidase conjugated goat anti-human IgG, IgM and IgA (DAKO, Denmark) in 1 × TBS-T, reacted with SuperSignal® chemiluminescence substrate (Pierce, USA) and signals detected on Super HR-G30 film (Fuji, Japan) using Kodak RP X-OMAT.
Enzyme-linked immunosorbent assay (ELISA)
His6-P0, -P1 and -P2 preparations (50 µl) were distributed into Costar® microplate wells, incubated overnight, washed seven times with PBS, and blocked for 1 h with buffer containing 2% skim milk and 10% calf serum. Wells were washed once with PBS, incubated for one hour with anti-ribosomal P positive and negative sera (50 µl in blocking buffer), washed seven times with PBS-T and incubated for 1 h with horseradish peroxidase conjugated to goat anti-human IgG (referred to as IgG-HRP) (ImmunoConcepts®, USA) (50 µl). After a further 7 washes, wells were incubated for 5 min with substrate solution (50 µl) containing TMB and H2O2. Reactions were stopped with stop solution (50 µl) containing sulphuric and hydrochloric acids. Optical density at 450 nm (OD450nm) was measured with a Microplate Reader (model 3550) (BIO-RAD). Random SLE sera (n = 48) were also tested by ELISA with either C-22 peptide INOVA® (USA) or native ribosomal-P antigen (Arotec Diagnostics, New Zealand).
Antibody to ds-DNA
Antibody to ds-DNA was determined by standard immunofluorescence with Crithidia luciliae (ImmunoConcepts®, USA) [40].
Statistical analysis
ELISA cut-off value was calculated by mean + 3 standard deviation of the OD values obtained from normal sera using Descriptive Statistics from Microsoft® Excel.
Results
Purified recombinant human ribosomal P proteins are immunoreactive
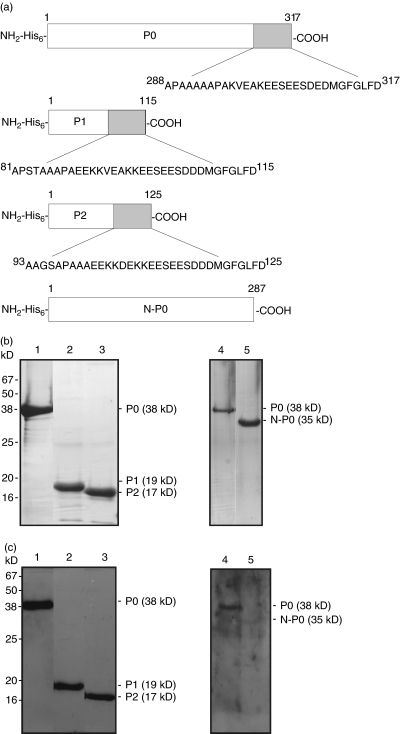
Figure 1a shows features of the three human recombinant P0, P1 and P2 proteins including a N-terminal 6 × His-tag (referred to as His6-) and the common C-22 sequences. The mutant protein N-P0 lacks this C-22 amino acid sequence. Protein purification was monitored by SDS-PAGE and Coomassie blue staining (Fig. 1b). Purified P0 (38 kD), P1 (19 kD), and P2 (17 kD) proteins reacted by immunoblotting with a ribosomal P antibody positive serum from a patient with SLE (Fig. 1c).
Fig. 1.
Human bacterial recombinant ribosomal P proteins. (a) Stick diagrams of recombinant human ribosomal P0, P1, P2 and mutant N-P0 proteins. Recombinant human ribosomal P0, P1, P2 and mutant N-P0 sequences have N-terminal 6 × His-tags. Homologous C-22 sequences shown in P0, P1 and P2 are missing in mutant N-P0. (b) Purity and (c) immunoblot-reactivity of recombinant P proteins. Ni-NTA affinity chromatography-purified P0, P1, P2 and N-P0 proteins were electrophoresed in 12·5% SDS-PAGE and protein bands visualized by (b) Coomassie blue staining and (c) immunoblotting with SLE anti-P positive control serum. Lanes 1 and 4: P0, Lane 2: P1, Lane 3: P2 and Lane 5: N-P0.
Premixed P0, P1 and P2 gives maximal reactivity by ELISA
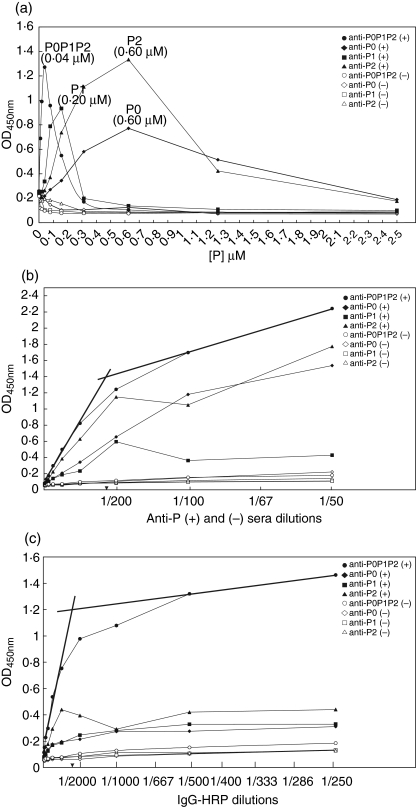
To determine the optimal protein concentration for ELISA, we coated ELISA plates with 0–2·5 µM P0, P1 and P2 proteins or with equimolar concentrations of P0, P1 and P2 mixed together in a molar ratio of 1 : 2 : 2 or 1 : 1 : 1. ELISA was performed with the anti-ribosomal P positive and negative control sera as described in Materials and Methods. Coating concentrations, 0·6 µM P0, 0·2 µM P1, 0·6 µM P2 and premixed P0P1P2 each at 40 nM, gave maximal reactivity with anti-ribosomal P antibody while the normal human serum was nonreactive (< 0·2 at OD450nm, Fig. 2a). No difference was observed between equimolar concentrations of P0, P1 and P2 mixed at molar concentrations of 1 : 1 : 1 compared to 1 : 2 : 2 (data not shown).
Fig. 2.
ELISA optimization. (a) Protein coating concentrations. ELISA plates were coated with each P0, P1 or P2 protein or an equimolar mix of P0, P1 and P2 proteins (premixed P0P1P2) in concentrations ranging from 0 to 2·5 µM. Binding curves were obtained with ribosomal P-positive (closed symbols) or negative serum (open symbols) for P0 (◊, ♦), P1 (□, ▪) and P2 (▵, ▴) or premixed P0P1P2 (○, •). Peak concentration at OD450nm for P proteins, representing optimal coating concentrations, are in parentheses. (b) Serum dilutions. ELISA plates were coated with optimal coating concentrations determined in (a) and tested with ribosomal P positive or negative sera at dilutions ranging from 1 in 50 to 1 in 25 600. IgG-HRP conjugate dilution was 1 in 8000. Binding curves were obtained as in (a). Optimal serum dilution of ribosomal P positive serum (1 : 200) was taken as the point where two linear segments drawn from the P0P1P2 binding curve met. (c) Conjugate dilutions ELISA wells were coated with optimal protein coating concentrations and with optimal serum dilutions determined in (a) and (b) and reacted with IgG-HRP conjugate at dilutions ranging from 1 in 12,800–1 in 250. Binding curves were obtained and optimal conjugate dilution determined as in (b).
Next, we determined the optimal serum dilution of ribosomal P antibody for the optimized P0, P1, P2 and premixed P0P1P2 ELISA by diluting positive and control sera from 1 in 50 to 1 in 25 600 (Fig. 2b). Premixed P0P1P2 ELISA gave higher OD450nm values than those obtained with each ribosomal P protein, used at molar concentrations that were 5–15 times higher than the premixed P0P1P2 preparation. The optimal serum dilution for the premixed protein sample was 1 in 200.
To determine the optimal dilution for IgG-HRP, the conjugate was diluted from 1 in 250 to 1 in 12 800 using optimized ELISA conditions determined previously. 40 nM of premixed P0, P1 and P2 proteins again gave 3–6 fold higher OD450nm values than that observed with each P protein individually. Optimal dilution of the IgG-HRP conjugate was 1 in 2000 (Fig. 2c).
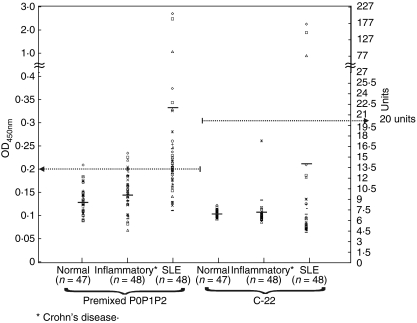
We determined the cut-off value at OD450nm for premixed P0P1P2 ELISA using the optimized experimental conditions by testing serum samples from SLE patients (n = 48), healthy humans (n = 47) and non-SLE inflammatory disease control patients (n = 48) (Fig. 4). The OD450nm value of 0·2 was taken as the cut-off as determined by the mean + 3 standard deviation of the OD values obtained with the normal sera, this was collaborated by the region where the binding curves for OD values with the normal human sera overlapped that for OD values for the SLE sera.
Fig. 4.
Serum reactivity with premixed P0P1P2 and C-22 peptide by ELISA. SLE sera (n = 48), normal human sera (n = 47) and non-SLE inflammatory sera (n = 48) were random tested by ELISA with premixed P0P1P2 proteins using optimized protocol determined in Fig. 2 or with C-22 peptide (INOVA®, USA). OD450nm values are displayed as scatter plots and horizontal bars represent mean values. Horizontal broken line at 0·2 represents the cut-off value, for premixed P0, P1 and P2 ELISA and the line at 20 units is the recommended cut-off for the C-22 ELISA (INOVA®, USA).
Reaction of ribosomal P protein antibody with premixed P and P2 proteins
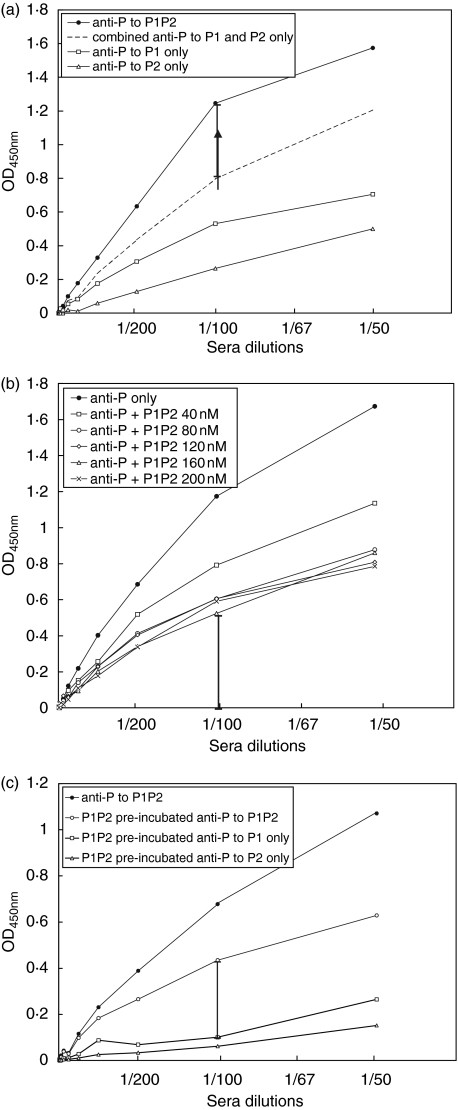
To ascertain whether anti-ribosomal P antibodies react with epitopes in P1/P2 heterodimers, we coated microtiter wells with 40 nM, of individual P1 or P2 proteins or with premixed P1 and P2 proteins for tests with the positive and negative control sera in dilutions ranging from 1 in 50 to 1 in 25 600. Reactivity of positive anti-ribosomal P serum with premixed P1 and P2 proteins was 40% greater than the sum of the arithmetic means of reactivity with individual P1 and P2 proteins at a serum dilution of 1 in 100. (Fig. 3a).
Fig. 3.
(a) Binding curves for premixed P1 and P2. ELISA microwells were coated with 40 nM P1 or P2 or premixed P1P2. Anti-ribosomal P binding signals were background (OD450nm value < 0·2) subtracted. Anti-ribosomal P versus individual P1, P2 and premixed P1P2 binding curves are marked in open rectangle, open triangle and closed circle, respectively. Broken line represents the combined anti-P1 and -P2 binding intensity at 450 nm. Stick bar at 1 in 100 dilution represents 40% of additional signal for premixed P1P2. (b) Binding curves for 40 nM premixed P1 and P2 coated on microwells following pre-incubation with premixed P1 and P2 at concentrations ranging from 40 nM to 200 nM. Stick bar at 1 in 100 dilution represents 40% residual reactivity after pre-incubation with 200 nM P1P2. (c) Binding curves for premixed 40 nM P1P2, P1 or P2 following pre-incubation with 80 nM P1P2. Stick bar represents residual 40% anti-ribosomal P reactivity that does not react with either P1 or P2 alone. Background signals were measured using normal human serum for individual P1, P2 and premixed P1P2 samples. Positive and negative control sera concentrations were diluted from 1 in 50 to 1 in 25 600.
Next, anti-ribosomal P positive and negative sera were pre-incubated with 40–200 nM premixed P1 and P2 proteins, and binding curves of each serum sample to microtitre wells coated with premixed P1P2 (40 nM each) were obtained in the same dilution range as in Fig. 3a. Reactivity of anti-ribosomal P antibody was reduced by pre-incubation with increasing concentrations of premixed P1 and P2 proteins. However a 40% residual reactivity was evident after pre-incubation with 200 nM premixed P1 and P2 (or P1P2) proteins (Fig. 3b). Following pre-incubation with 80 nM premixed P1P2 proteins, while a residual 40% reactivity to premixed P1 and P2 proteins was also evident, the serum was largely non reactive with either P1 or P2 protein alone in 1 in 100 or 1 in 200 serum dilution (Fig. 3c).
ELISA with P0, P1, P2 is more sensitive than with C-22 peptide
Of the 48 SLE sera, 18 sera tested positive for anti-ribosomal P antibody in the ELISA assay using the recombinant antigens (Fig. 4). Of the positive sera, only 3 sera tested positive for the C-22 peptide with OD450nm values ranging from 0·9 to 2·4. Fifteen anti-ribosomal P immunoreactive signals observed with premixed P0P1P2 ELISA were distributed between OD450nm values 0·2 and 0·4, but none of these sera gave OD450nm values > 0·2 by C-22 peptide ELISA. On the basis of these observations, the sensitivity of the ELISA for detection of SLE with the premixed P0P1P2 is 38% while the sensitivity with the C-22 peptide ELISA is 7% (Table 1).
Table 1. ELISA sensitivities and specificities for SLE using premixed P0P1P2 or C-22.*.
| P0P1P2 | C-22* | Sensitivity | Specificity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sera | n | (+) | (−) | (+) | (−) | P0P1P2 | C-22* | P0P1P2 | C-22* |
| Random SLE‡ | 48 | 18 | 30 | 3 | 45 | 38% | 7% | – | – |
| Normal human | 47 | 0 | 47 | 0 | 47 | – | – | 100% | 100% |
| Inflammatory† | 48 | 3 | 45 | 0 | 48 | – | – | 94% | 100% |
INOVA® (USA).
Non-SLE (Crohn’s disease).
Sensitivity for SLE with native ribo-P ELISA (Arotec Diagnostics, New Zealand) is 6/48, 13%.
Antibodies to ribosomal antigens exhibit diverse reactivity with P proteins
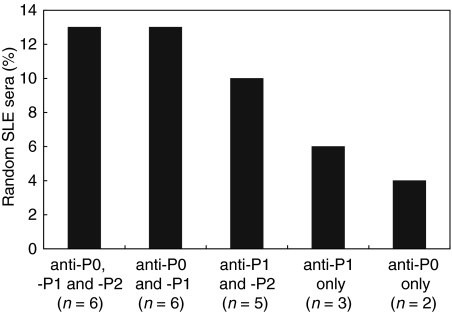
Immunoblotting of the 48 SLE sera showed that overall, 22 tested positive for the P proteins, giving a sensitivity of 46% (Table 2). Of these, 13% gave anti-P0P1P2 reactivity (n = 6), 13% anti-P0P1 (n = 6), 10% anti-P1P2 (n = 5), 6% anti-P1 only (n = 3) and 4% anti-P0 only (n = 2) (Fig. 5). The 6 sera that were reactive with P0, P1 and P2 included the 3 sera that were reactive with the C-22 peptide by ELISA.
Table 2. Comparison of sensitivity for SLE using premixed P0P1P2 ELISA or immunoblotting.*.
1 µg of each P0, P1 and P2 protein.
17 sera tested (+) by ELISA and immunoblotting.
Fig. 5.
Immunobloting with P proteins. The figure shows the distribution of immunoblot-reactivity for 22 SLE random sera with individual or combinations of P proteins. Of 22 sera immunoblotted to all three P proteins, 6 were found immunoreactive to either all three P proteins (anti-P0, P1 and P2) or to P0 and P1 (anto-P0 and –P1). Five were found immunoreactive to both P1 and P2 (anti-P1 and -P2). Anti-P0 and -P1 were found in 2 and 3 sera, respectively.
Only one out of the 18 SLE sera that tested positive in the premixed P0P1P2 ELISA was nonreactive by immunoblotting with the ribosomal P proteins. Four SLE sera that tested negative by the ELISA were reactive with the P proteins by immunoblotting. The 3 sera from patients with Crohn's disease that tested positive by the ELISA were nonreactive with P proteins by immunoblotting.
Antibodies to ribosomal antigens predominantly react with domains N-terminal to the homologous C-22 peptide
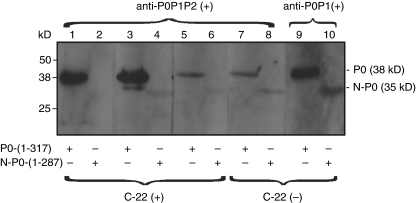
Six sera that were reactive with all 3 P0, P1 and P2 proteins, of which 3 were also reactive with the C-22 peptide by ELISA, were further examined by immunoblotting for reactivity with P0 compared with mutant N-P0 lacking the homologous C-22 peptide (Fig. 6). The 3 sera that were nonreactive with the C-22 peptide by ELISA were immunoblot-reactive with mutant N-P0 lacking the C-22 peptide. Of the 3 sera that were reactive with the C-22 peptide (lanes 1–6), one was nonreactive with the mutant (lane 2) while the other two retained reactivity with the N-P0 mutant albeit with diminished intensity (lanes 4 and 6). Serum reactive with all three P proteins, but not to C-22 peptide was reactive to both P0 and N-P0 (lanes 7–8). Immunoblots were also carried out with representative sera that were immunoblot-reactive only to P0P1 or P1P2, but not to C-22 peptide. Serum reactive with P0P1 reacted with both full length P0 and mutant N-P0 (lanes 9 and 10) while serum reactive with P1P2 was non reactive with P0 or N-P0 (data not shown).
Fig. 6.
Immunoblotting with wild type and mutant P0 (N-P0) protein. Full length P0 (lanes 1, 3, 5, 7 and 9) or mutant P0 (lanes 2, 4, 6, 8 and 10) were immunoblotted with sera containing anti-P0P1P2 (lanes 1–8) or anti-P0P1 (lanes 9 and 10). Anti-P0P1P2–reactive sera reacted only with P0 (lanes 1, 3 and 5); anti-P0P1-reactive serum reacted with both P0 and N-P0 (lanes 9 and 10).
Combination of antibodies to ribosomal P antibodies and ds-DNA gives increased sensitivity for SLE
Fifteen of 48 SLE sera were positive for antibody to ds-DNA as determined by immunofluorescence reactivity with Crithidia luciliae, giving a sensitivity of 31% for the diagnosis of SLE (Table 3). Of these 15 sera, 6 were also reactive with ribosomal P0P1P2 ELISA whereas 9 were nonreactive. Of the 18 sera that were positive for ribosomal P0P1P2, 12 were negative for antibodies to ds-DNA. Hence the detection of antibodies to either P0P1P2 and ds-DNA, or both, gave a sensitivity of 56% for the diagnosis of SLE.
Table 3. Results of premixed P0P1P2 ELISA and ds-DNA IF* with SLE sera.
IF, immunofluorescence using Crithidia luciliae.
Combined sensitivity for SLE with the two assays is 27/48 (56%).
Discussion
We have produced bacterial recombinant human ribosomal P0, P1 and P2 proteins of high quality and sufficient quantity to examine the immunoreactive domains of these proteins that react with autoantibodies that segregate with SLE. Previously, a homologous C-22 peptide sequence shared with all 3 proteins was deemed the dominant immunoreactive domain [32–35] and has become the conventional antigen in diagnostic kits from a variety of manufacturers. However, our studies indicate the immunodominant domains are predominantly N-terminal to this homologous sequence. This suggestion is supported by the following observations accruing from our present study.
First, we found that an ELISA using all 3 ribosomal proteins (P0P1P2) that were premixed at equimolar concentrations in solution gave a 5-fold greater sensitivity of detection of SLE (38%) compared to an ELISA with the common C-22 peptide (sensitivity of 7%). Moreover, we found anti-C-22 reactivity only with high titre antibodies that reacted with all 3 P proteins. Clearly the 5-fold increased sensitivity with all 3 proteins is likely a reflection of antibody reactivity with domains in each of the 3 proteins in addition to those at the common C-22 region. The suggestion is further supported by the observation that the ELISA with the 3 P proteins at equimolar coating concentrations collectively gave > 3-fold higher OD values compared to ELISAs with individual P proteins used at much higher coating concentrations. Our observations are consistent with a previous study with rat ribosomal P proteins that have also predicted the presence of epitope(s) other than those on the common C-22 sequence [41]. It is possible that molecular interactions between the 3 proteins arising from pentamer formation may have contributed to the enhanced sensitivity of the premixed P0P1P2 ELISA. However, mixing the 3 proteins at molar concentrations of P0, P1 and P2 of 1 : 2 : 2 did not give any increased sensitivity for the assay. Reduction of OD450nm values with increasing protein coating concentrations (0·6–2·5 µM) is likely to be the consequence of protein aggregation in solution that resulted in reduced binding of the P proteins to microtitre wells. This suggestion is supported by our observation of immunoreactive 76 and 30 kD bands that correspond to P0 and P2 dimeric protein aggregates at higher ribosomal protein P concentrations (data not shown). This suggestion is also consistent with previous reports of protein aggregation with high concentrations of recombinant ribosomal P proteins [20].
Second, our observation that premixed ribosomal P1 and P2 proteins gave greater reactivity by ELISA than the sum of the arithmetic mean with each protein suggest that premixing may have resulted in the formation of molecular complexes such as P1 and P2 heterodimers, that in turn may have generated additional epitopes. This suggestion is further supported by our observation that attempts to neutralize serum reactivity by pre-incubation with increasing concentrations of premixed P1 and P2 proteins failed to completely neutralize reactivity with premixed P1 and P2 proteins but instead largely neutralized reactivity with individual P1 and P2 proteins. The results suggest that pre-incubation with premixed P1 and P2 proteins removed reactivity with epitopes on the individual proteins but not those on the P1/P2 heterodimer. Our data suggests that at least 40% reactivity with P1 and P2 proteins can be attributed to reactivity with P1P2 heterodimers. Indeed, mixing of P1 and P2 proteins results in formation of P1/P2 heterodimers as demonstrated by gel mobility shift and chemical cross-linking assays [22]. Generation of P1/P2 heterodimers may have revealed site(s) masked within each individual protein or conformational sites apparent only on the dimer(s). This suggestion is further supported by the observation that immunization of mice with P1/P2 heterodimers but not with P1 and P2 depleted-ribosomes produced antibody to all three ribosomal proteins and that pre-incubation of antibody with premixed P1/P2 abrogated antibody-mediated inhibition of protein synthesis by 70%[43]. In this context it should be noted that of 22 sera that gave immunoblot reactivity with the ribosomal P proteins, 11 sera (50%) were reactive with combinations that included P1 and P2 proteins.
Third, the heterogeneity of the antibody reactivity with the ribosomal P proteins does not support the tenet that the dominant immunoreactive domain is restricted to the homologous C-22 sequence. We found that 16 (73%) of 22 sera were reactive with a single ribosomal P protein or pairs of these proteins, an unlikely outcome if the dominant domain is a sequence shared by all 3 proteins. This suggestion is consistent with the observation that only monoclonal antibodies reactive to all 3 proteins, and not those reactive with P1 or P2 alone, recognized the homologous C-22 sequence [42].
Fourth, of 48 SLE sera, only 6 reacted by immunoblotting with each of the 3 ribosomal P proteins. Of these, only 3 reacted by ELISA with the common C-22 peptide and were nonreactive with mutant N-P0 lacking the C-22 peptide sequence (lanes 2, 4 and 6). The other 3 appear to react with epitopes N-terminal to the C-22 peptide because they were nonreactive with the C-22 peptide by ELISA but reacted with mutant P0 lacking this sequence. Moreover, the observation that 3 of the 6 sera reactive with the 3 proteins were nonreactive with the C-22 peptide but reactive with N-P0 indicates that sera reactive with the 3 proteins are not necessarily restricted to reactivity with the C-22 peptide.
In conclusion, we have found that the predominant immunoreactive domains on ribosomal P proteins reside N-terminal to a homologous C-22 peptide sequence shared with all 3 proteins. Further we have established a novel and sensitive ELISA using all 3 proteins together that has a 5-fold enhanced sensitivity for SLE than an ELISA with the C-22 peptide or native ribo-P proteins. The observation of a combined sensitivity of 56% for diagnosis of SLE by detecting autoantibodies to ribosomal P proteins and/or to ds-DNA by the Crithidia assay suggest that both reactivities should be tested in the laboratory investigation of patients with this disease. Finally, the observation that only one patient of the SLE cohort had neuropsychiatric lupus suggest that the presence of antibodies to ribosomal P proteins is not associated with this complication. However, given the small size of the cohort of SLE patients in the present study, the enhanced diagnostic value of this assay for SLE and its lack of association with neuropsychiatric lupus require confirmation with a study with a larger group of patients.
Acknowledgments
This work was supported by funds provided by ImmunoConcepts Inc. (USA). We thank Ian Mackay, Bob Boyes, Jim Smith and Eric Hoy for critical reading of the manuscript.
References
- 1.Ghirardello A, Caponi L, Franceschini F, et al. Diagnostic tests for antiribosomal, p protein antibodies: a comparative evaluation of immunoblotting and ELISA assays. J Autoimmun. 2002;19:71–7. doi: 10.1006/jaut.2002.0595. [DOI] [PubMed] [Google Scholar]
- 2.Gerli R, Caponi L, Tincani A, et al. Clinical and serological associations of ribosomal P autoantibodies in systemic lupus erythematosus: prospective evaluation in a large cohort of Italian patients. Rheumatology. 2002;41:1357–66. doi: 10.1093/rheumatology/41.12.1357. [DOI] [PubMed] [Google Scholar]
- 3.Mahler M, Kessenbrock K, Raats J, Fritzler MJ. Technical and clinical evaluation of anti-ribosomal P protein immunoassays. J Clin Laboratory Anal. 2004;18:215–23. doi: 10.1002/jcla.20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isenberg DA, Garton M, Reichlin MW, Reichlin M. Long-term follow-up of autoantibody profiles in black female lupus patients and clinical comparison with Caucasian and Asian patients. Br J Rheumatol. 1997;36:229–33. doi: 10.1093/rheumatology/36.2.229. [DOI] [PubMed] [Google Scholar]
- 5.Desbos A, Gonzalo P, Monier JC, Tebib J, Reboud JP, Perrier H, Bienvenu J, Fabien N. Autoantibodies directed against ribosomal proteins in systemic lupus erythematosus and rheumatoid arthritis: a comparative study. Autoimmunity. 2002;35:427–34. doi: 10.1080/0891693021000045714. [DOI] [PubMed] [Google Scholar]
- 6.Teh LS, Lee MK, Wang F, et al. Antiribosomal P protein antibodies in different populations of patients with systemic lupus erythematosus. Br J Rheumatol. 1993;32:663–5. doi: 10.1093/rheumatology/32.8.663. [DOI] [PubMed] [Google Scholar]
- 7.Arnett FC, Reveille JD, Moutsopoulos HM, Georgescu L, Elkon KB. Ribosomal P autoantibodies in systemic lupus erythematosus. Frequencies in different ethnic groups and clinical and immunogenetic associations. Arthritis Rheum. 1996;39:1833–9. doi: 10.1002/art.1780391109. [DOI] [PubMed] [Google Scholar]
- 8.Reichlin M, Broyles TF, Hubscher O, et al. Prevalence of autoantibodies to ribosomal P proteins in juvenile-onset systemic lupus erythematosus compared with the adult disease. Arthritis Rheum. 1999;42:69–75. doi: 10.1002/1529-0131(199901)42:1<69::AID-ANR9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Uchiumi T, Ozawa T, Kikuchi M, Nakano M, Kominami R, Arakawa M. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J Rheumatol. 1991;18:1681–4. [PubMed] [Google Scholar]
- 10.Martin AL, Reichlin M. Fluctuations of antibody to ribosomal P proteins correlate with appearance and remission of nephritis in SLE. Lupus. 1996;5:22–9. doi: 10.1177/096120339600500106. [DOI] [PubMed] [Google Scholar]
- 11.Reichlin M, Wolfson-Reichlin M. Evidence for the participation of anti-ribosomal P antibodies in lupus nephritis. Arthritis Rheum. 1999;42:2728–9. doi: 10.1002/1529-0131(199912)42:12<2728::AID-ANR34>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Reichlin M, Wolfson-Reichlin M. Correlations of anti-ds-DNA and anti-ribosomal P autoantibodies with lupus nephritis. Clin Immunol. 2003;108:69–72. doi: 10.1016/s1521-6616(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 13.Koren E, Schnitz W, Reichlin M. Concomitant development of chronic active hepatitis and antibodies to ribosomal P proteins in a patient with systemic lupus erythematosus. Arthritis Rheum. 1993;36:1325–8. doi: 10.1002/art.1780360917. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Reichlin M. Lupus hepatitis: an under-recognized disease feature associated with autoantibodies to ribosomal P. Am J Med. 1995;99:465–72. doi: 10.1016/s0002-9343(99)80221-6. [DOI] [PubMed] [Google Scholar]
- 15.Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Eng J Med. 1987;317:265–71. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 16.Press J, Palayew K, Laxer RM, Elkon K, Eddy A, Rakoff D, Silverman ED. Antiribosomal P antibodies in pediatric patients with systemic lupus erythematosus and psychosis. Arthritis Rheum. 1996;39:671–6. doi: 10.1002/art.1780390420. [DOI] [PubMed] [Google Scholar]
- 17.Georgescu L, Mevorach D, Arnett FC, Reveille JD, Elkon KB. Anti-P antibodies and neuropsychiatric lupus erythematosus. Ann NY Acad Sci. 1997;823:263–9. doi: 10.1111/j.1749-6632.1997.tb48399.x. (1997) [DOI] [PubMed] [Google Scholar]
- 18.Reichlin M. Ribosomal P antibodies and CNS lupus. Lupus. 2003;12:916–8. doi: 10.1191/0961203303lu502oa. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood DL, Gitlits VM, Alderuccio F, Sentry JW, Toh BH. Autoantibodies in neuropsychiatric lupus. Autoimmunity. 2002;35:79–86. doi: 10.1080/08916930290016547. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalo P, Lavergne J, Reboud J. Pivotal role of the P1 N-terminal domain in the assembly of the mammalian ribosomal stalk and in the proteosynthetic activity. J Biol Chem. 2001;276:19762–9. doi: 10.1074/jbc.M101398200. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalo P, Reboud JP. The puzzling lateral flexible stalk of the ribosome. Biol Cell. 2003;95:179–93. doi: 10.1016/s0248-4900(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu T, Nakagaki M, Nishi Y, Kobayashi Y, Hachimori A, Uchiumi T. Interaction among silkworm ribosomal proteins P1, P2 and P0 required for functional protein binding to the GTPase-associated domain of 28S rRNA. Nucl Acids Res. 2002;30:2620–7. doi: 10.1093/nar/gkf379. (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchiumi T, Honma S, Nomura T, Dabbs ER, Hachimori A. Translation elongation by a hybrid ribosome in which proteins at the GTPase center of the Escherichia coli ribosome are replaced with rat counterparts. J Biol Chem. 2002;277:3857–62. doi: 10.1074/jbc.M107730200. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Uchiumi T, Arakawa M, Kominami R. Serological association of lupus autoantibodies to a limited functional domain of 28S ribosomal RNA and to the ribosomal proteins bound to the domain. Clin Exp Immunol. 1994;98:35–9. doi: 10.1111/j.1365-2249.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasler P, Brot N, Weissbach H, Danho W, Blount Y, Zhou JL, Elkon KB. The effect of phosphorylation and site-specific mutations in the immunodominant epitope of the human ribosomal P proteins. Clin Immunol Immunopathol. 1994;72:273–9. doi: 10.1006/clin.1994.1141. [DOI] [PubMed] [Google Scholar]
- 26.Caponi L, Giordano A, Bartoloni EB, Gerli R. Detection of anti-ribosome antibodies: a long story of lights and shadows. Clin Exp Rheumatol. 2003;21:771–8. [PubMed] [Google Scholar]
- 27.Crow MKI, Giudice-Asch G, Zehetbauer JB, Lawson JL, Brot N, Weissbach H, Elkon KB. Autoantigen-specific T cell proliferation induced by the ribosomal P2 protein in patients with systemic lupus erythematosus. J Clin Invest. 1994;94:345–52. doi: 10.1172/JCI117328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stacey DW, Skelly S, Watson T, Elkon K, Weissbach H, Brot N. The inhibition of protein synthesis by IgG containing anti-ribosome P autoantibodies from systemic lupus erythematosus patients. Arch Biochem Biophy. 1988;267:398–403. doi: 10.1016/0003-9861(88)90045-8. [DOI] [PubMed] [Google Scholar]
- 29.Koren E, Reichlin MW, Koscec M, Fugate RD, Reichlin M. Autoantibodies to the ribosomal P proteins react with a plasma membrane-related target on human cells. J Clin Invest. 1992;89:1236–41. doi: 10.1172/JCI115707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koscec M, Koren E, Wolfson-Reichlin M, Fugate RD, Trieu E, Targoff IN, Reichlin M. Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. J Immunol. 1997;159:2033–41. [PubMed] [Google Scholar]
- 31.Elkon KB, Parnassa AP, Foster CL. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985;162:459–71. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkon K, Bonfa E, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1986;83:7419–23. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonfa E, Elkon KB. Clinical and serological associations of the anti-ribosomal P protein antibody. Arthritis Rheum. 1986;29:981–5. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- 34.Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. Association between lupus pyschosis and anti-ribosomal antibodies. New Eng J Med. 1987;317:265–71. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 35.Tzioufas AG, Tzortzakis NG, Panou-Pomonis E, Boki KA, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM. The clinical relevance of antibodies to ribosomal-P common epitope in two targeted systemic lupus erythematosus populations: a large cohort of consecutive patients and patients with active central nervous system disease. Ann Rheum Dis. 2000;59:99–104. doi: 10.1136/ard.59.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahler M, Kessenbrock K, Raats J, Williams R, Fritzler MJ, Bluthner M. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. J Mol Med. 2003;81:194–204. doi: 10.1007/s00109-003-0423-1. [DOI] [PubMed] [Google Scholar]
- 37.Caponi L, Pegoraro S, Di Bartolo V, Rovero P, Revoltella R, Bombardieri S. Autoantibodies directed against ribosomal P proteins: use of a multiple antigen peptide as the coating agent in ELISA. J Immunol Meth. 1995;179:193–202. doi: 10.1016/0022-1759(94)00285-5. [DOI] [PubMed] [Google Scholar]
- 38.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Aarden LA, de Groot ER, Feltkamp TE. Crithidia luciliae, a simple substrate for the determination of anti-ds-DNA with the immunofluorescence techniquE. Ann N Y Acad Sci. 1975;254:505–15. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- 41.Fabien N, Moreira A, Lavergne JP, et al. Autoantibodies directed against the ribosomal P proteins are not only directed against a common epitope of the P0, P1 and P2 proteins. J Autoimmun. 1999;13:103–10. doi: 10.1006/jaut.1999.0291. [DOI] [PubMed] [Google Scholar]
- 42.Uchiumi T, Traut RR, Kominami R. Monoclonal antibodies against acidic phosphoproteins P0, P1 and P2 of eukaryotic ribosomes as functional probes. J Biol Chem. 1990;265:89–95. [PubMed] [Google Scholar]
- 43.Hines JJ, Weissbach H, Brot N, Elkon K. Anti-P autoantibody production requires P1/P2 as immunogens but is not driven by exogenous self-antigen in MRL mice. J Immunol. 1991;146:3386–95. [PubMed] [Google Scholar]
- 44.Zampieri S, Mahler M, Bluthner M, et al. Recombinant anti-P protein autoantibodies isolated from a human autoimmune library. reactivity, specificity and epitope recognition. Cell Mol Life Sci. 2003;60:588–98. doi: 10.1007/s000180300050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abo Y, Hagiya A, Naganuma T, et al. Baculovirus-mediated expression and isolation of human ribosomal phosphoprotein P0 carrying a GST-tag in a functional state. Biochem Biophys Res Commun. 2004;322:814–9. doi: 10.1016/j.bbrc.2004.07.196. [DOI] [PubMed] [Google Scholar]