Abstract
Toll-like receptors (TLRs) are involved in the recognition of bacterial products and thus participate in the induction of the inflammatory cascade. However, much less is known about the evolution of leucocyte TLR expression during human inflammatory stress. We hypothesized that a decrease in leucocyte TLRs could account for the so-called tolerance or hyporesponsiveness state to subsequent stimulation with bacteria-derived products. Because of the profound monocytopenia that ensues after in vivo lipopolysaccharide (LPS) challenge, we also compared monocyte TLR expression using two different techniques of flow cytometric gating. In a first set of experiments, 17 healthy volunteers underwent LPS challenge. Blood was drawn at different time-points and analysed by flow cytometry using light scatter gating and one-colour analysis to assess the expression of the tumour necrosis factor receptor (TNFR) and TLR2 and TLR4 on both monocytes and granulocytes. In a second set of experiments, the assessment of those receptors was made using a more specific gating method that utilized light scatter and CD14 immunofluorescence in a two-colour analysis. This was performed using whole blood drawn from five healthy volunteers and incubated ex vivo for different time periods with or without LPS and in 12 volunteers who underwent LPS challenge in vivo. The pattern of expression for monocyte TNFR was similar for both types of gating. Using only the light scatter gating, an initial drop of TLR 2 and 4 was observed on monocytes. By contrast, when using light scatter × immunofluorescence gating, an up-regulation of these two receptors following both in vivo and in vitro LPS exposure was observed. LPS up-regulates the expression of TLRs on monocytes and granulocytes. Depending upon the methodology utilized, contrasting results were obtained with respect to TLR2 and TLR4 expression. The flow cytometric gating technique used is of importance in determining cellular TLR2 and TLR4 expression, especially in blood samples exhibiting significant monocytopenia.
Keywords: flow cytometry, lipopolysaccharides, receptors, Toll-like receptors 2 and 4, tumour necrosis factor
Introduction
The incidence of severe sepsis is estimated to be 751 000 cases a year in the United States, with an acute mortality rate of 28·6%, and an average cost per case of $22 100 [1]. Despite a recent decrease, Gram-negative bacterial infections still account for about 38% of infectious episodes in the United States [2].
Lipopolysaccharide (LPS, endotoxin), a major component of the Gram-negative bacteria outer cell wall, is a key factor in eliciting the systemic inflammation associated with Gram-negative infection. Low-dose endotoxin administration to human volunteers is frequently utilized as a model to evaluate some aspects of acute systemic inflammation. It induces clinical changes such as fever, headache and general malaise, change in white blood cell count [3] and a release of proinflammatory and anti-inflammatory mediators [4,5].
At the cellular level, recognition of LPS by the immune system has been demonstrated to be a multi-faceted process. Lipoprotein binding protein (LBP) is an acute phase reactant protein, which acts as a principal LPS carrier [6]. CD14, which can be found either soluble in the plasma (sCD14) or on the cell surface, binds the LPS-LBP complex [7]. However, as CD14 lacks a trans-membrane domain, other cellular receptors are involved in intracellular signal transduction.
More recently, a new family of receptors first described in Drosophila and known as Toll-like receptors (TLR) was demonstrated as being able to induce the activation of NF-κB and the expression of NF-κB controlled genes for inflammatory cytokines [8] through different intracellular pathways involving the intracellular domain of the TLR (TIR domain for TLR-IL1 receptor), an adaptator protein (MyD88) and various kinases.
Eleven different TLRs have been identified in humans, each activated by varying protein motifs, known collectively as pathogen-associated molecular patterns (PAMPs). For instance, TLR 4 recognizes LPS, while TLR 2 recognizes peptidoglycans from Gram-positive bacteria (for review see Akira and Takeda [9]).
Many factors including macrophage migration inhibitory factor (MMIF) [10], hypoxia [11], interleukin (IL)-2, IL-4 [12], tumour necrosis factor (TNF), IL-6 [13] and interferon gamma [14] appear to modulate the expression of the TLRs. In vivo regulation of various TLRs is under active investigation during conditions of clinical relevance. One relevant model of human inflammation is acute endotoxaemia, for which a single study [15] has suggested an initial down-regulation of TLR2 and TLR4 on circulating monocytes. These results tend to support our working hypothesis, i.e. the decrease in TLRs in this situation might explain the so-called tolerance phenomenon, defined as a state of acute hyporesponsiveness to a second challenge to bacterial products such as LPS. However, one issue that confounds the study of receptors expression on human leucocytes is the dramatic change in white blood cells counts observed after LPS administration [3]. This complicates the gating on monocytes using only light-scattering characteristics of these cells. Hence, we directly compared light scatter gating flow—single-colour analysis and a more definitive gating using two-colour analysis in which monocytes and granulocytes were identified by a combination of side-scatter and CD14 immunofluorescence. Assessment of TLR2 and TLR4 on granulocytes and monocytes was performed.
In addition to TLRs, TNF receptors (TNFRs) also play a key role in the inflammatory response. We have reported previously that the down-regulation of TNFR expression on leucocytes was a predictor of risk in human sepsis and that these receptors are also reduced after in vivo LPS treatment of human volunteers [16,17]. Those data were generated using a light scatter gating flow—single-colour analysis. Therefore, in this study we also assessed TNFRs in the human LPS model and in a whole blood ex vivo stimulation, in order to compare the two flow-cytometry techniques.
Materials and methods
Human LPS model
Adult male and female subjects were recruited by public advertisement for entry into a protocol approved by the Institutional Review Board of UMDNJ—Robert Wood Johnson Medical School. Inclusion criteria were (1) good general health as demonstrated by medical history, physical examination and laboratory tests within 6 weeks of the study, (2) ages between 18 and 40 years and (3) written informed consent prior to the performance of any study related procedure. Exclusion criteria were (1) history of cancer, rheumatoid arthritis or immunological, renal, hepatic, endocrine, neurological, heart disease or hypertension, (2) recent history of alcohol or drug abuse, (3) exposure to any experimental agent or procedure within 30 days of study, (4) pregnancy or breast-feeding and (5) previous intravenous endotoxin administration.
On study day 1, the subjects were admitted to the Clinical Research Center at UMDNJ—Robert Wood Johnson Medical School and underwent a physical examination. An intravenous catheter was placed in one upper extremity, and a continuous intravenous infusion of 5% dextrose in 0·45% sodium chloride (100 ml/h) was begun and continued until the subject tolerated a regular meal following completion of the acute phase of the study on the following day. On the morning of study day 2, a radial artery was cannulated percutaneously with a 20-gauge catheter and connected to a pressure transducer. Haemodynamic and respiratory parameters, rectal temperature and subjective symptoms of distress (chills, muscle aches, headache, nausea, perceived fever, sensitivity to light and arterial line discomfort) were monitored every 30 min for the next 6 h. Following baseline monitoring, subjects were administered NIH Clinical Center Reference Endotoxin (CC-RE-Lot 2) at a dose of 2 ng/kg over a 5-min period through the intravenous catheter. Blood samples were collected before endotoxin infusion (0 h) and at post-infusion times as indicated. On study day 3, following collection of a 24-h blood sample, the subject was discharged.
Ex vivowhole blood LPS model
After obtaining written informed consent, blood was drawn from five separate healthy subjects and was diluted 1 : 2 with sterile RPMI-1640 solution (BioWhittaker, Walkersville, MA, USA) supplemented with l-glutamine (Sigma Chemicals, Irvine, UK).
It was then incubated at 37°C, 5% CO2 in non-pyrogenic sterile polystyrene tubes (Becton Dickinson, Franklin Lakes, NJ, USA) for up to 24 h with or without LPS (final concentration: 10 ng/ml) as described previously [18]. At the time-points indicated, tubes were removed from the incubator and the leucocytes subjected to flow cytometric analysis as described below.
One-colour flow cytometry
One-colour flow cytometry was performed on blood samples from 17 LPS-challenged volunteers. Briefly, erythrocytes in 100 µl aliquots of blood were lysed with 2 ml of lysing buffer [1000 ml H2O, 8·26 g of ammonium chloride, 1 g of potassium bicarbonate and 0·003 g of sodium ethylenediamine tetra-acetic acid (EDTA)] and washed twice with buffer [1000 ml phosphate-buffered saline (PBS), 10 g bovine albumin (BSA) and 1 g sodium azide] for 5 min at 4°C. The supernatant was decanted and the remaining cells were incubated for 60 min on ice with biotinylated human recombinant TNF-α (1 µg), biotinylated TLR-2 antibody, biotinylated TLR-4 antibody or biotinylated isotype control (all three: eBioscience, San Diego, CA, USA; 2 µg per sample). Cells were then washed twice and incubated on ice for 30 min with streptavidin R-PE (Caltag Laboratories, Burlingame, CA, USA; 60 ng per sample). After a final wash, cells were suspended in PBS solution and analysed with an Epics Profile II flow cytometer (Beckman-Coulter, Miami, FL, USA). Monocytes and granulocytes were gated by their simultaneous forward- × side light-scatter intensities, i.e. monocytes by forward-scatter high and side-scatter intermediate and granulocytes by forward-scatter intermediate and side-scatter high. Results are expressed by mean channel fluorescence.
Two-colour flow cytometry
Two-colour flow cytometric analyses were performed on blood samples from 12 LPS-challenged volunteers and the five normal control blood samples used for ex vivo whole blood stimulation. Two-colour staining was performed exactly as described above for the one-colour analyses except that prior to lysis of the erythrocytes, the 100 µl aliquot of blood was first stained with PerCp-conjugated CD14 antibody (BD Biosciences, San Jose, CA, USA; 5 µl per sample) for 30 min on ice. Analyses were performed with a FACSCalibur flow cytometer (BD Biosciences). Monocytes and granulocytes were gated by their side-scatter and FL3 fluorescence intensities, i.e. monocytes by side-scatter intermediate and FL3 high and granulocytes by side-scatter high and FL3 dim.
Statistical analyses
Because two different flow cytometers were used for these studies, the mean channel fluorescence (MCF) data from one flow cytometer were normalized to those of the other by multiplication by a constant (C), where C = baseline MCF from cytometer 1/baseline MCF from cytometer 2. Data are presented as the mean ± s.e.m. Data were analysed by repeated measures anova using one or two variables. Differences between individual means were assessed by Fisher's least squares difference (LSD) post-hoc test (Statistica, Tulsa, OK, USA). P < 0·05 was considered to represent a statistically significant difference.
Results
Clinical changes observed in the in vivo LPS model
All the subjects who underwent intravenous LPS injection exhibited increased temperature (from 36·7 ± 0·4 to 38·5 ± 0·5; P < 10−6) and increased heart rate (from 64 ± 9·5 to 98·8 ± 10; P < 10−6), and reported symptoms of headache, muscle ache, chills and exacerbated sensitivity to light. As described previously [3–5], they also manifested a profound monocytopenia with a mean baseline value of 415 ± 121 that decreased maximally to 33 ± 54 (P < 10−6). There were no differences in the magnitude of the monocytopenia when samples analysed with the light scatter-only gating and those analysed with the side-scatter × CD14 immunofluorescence were compared (data not shown).
Analyses of the TNF receptor
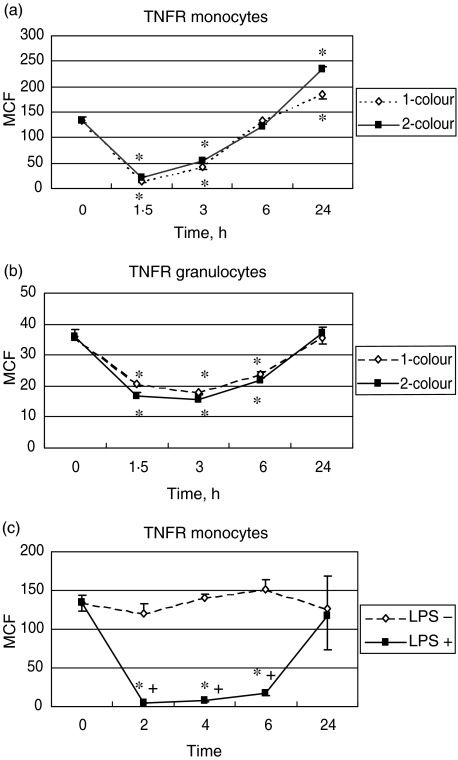
The method for assessment of the TNFR using forward × side light-scatter gating and one-colour analyses in LPS-challenged subjects has been described previously [16]. In summary, baseline TNFR was approximately fourfold higher on monocytes than on granulocytes. A significant decrease (P < 0·05) in this receptor was seen in both types of cells, the nadir being reached at 1·5 h. The decrease was proportionally more pronounced for monocytes. After this time, an increase was observed, this increase being sharp from 2–6 h and then being more progressive until 24 h, when the value of TNFR was statistically higher than baseline. Using the side-scatter × CD14 immunofluorescence gating to assess this receptor in volunteers who underwent in vivo LPS stimulation (12 volunteers) or ex vivo LPS stimulation (five volunteers), we showed the same pattern of curves, i.e. an initial dramatic decrease followed by a progressive increase with a significant ‘overshoot’ compared to baseline at 24 h in the in vivo model. Figure 1 shows the comparison of the two techniques for the in vivo model with the assessment of the TNFR on monocytes (Fig. 1a) and granulocytes (Fig. 1b). Figure 1c depicts the monocyte data from the ex-vivo stimulation study.
Fig. 1.
(a) Comparison of the mean channel fluorescence (MCF) for the tumour necrosis factor receptor (TNFR) analysed by forward- × side-scatter gating on monocytes in 17 human volunteers (dashed line) after lipopolysaccharide (LPS) challenge, compared to TNFR analysed by side-scatter × CD14 immunofluorescence gating on monocytes in 12 human volunteers after LPS challenge (full line); (b) comparison of the MCF for the TNFR studied by forward- × side-scatter gating on granulocytes in 17 human volunteers (dashed line) after LPS challenge and studied by side-scatter × CD14 immunofluorescence gating on granulocytes in 12 human volunteers after LPS challenge (full line); (c) MCF for TNFR studied by side-scatter × CD14 immunofluorescence gating on monocytes from whole blood drawn from five healthy volunteers and exposed ex vivo to LPS (full line) or not (dotted line). *P < 0·05 versus baseline; +P < 0·05 between groups.
Analyses of the Toll-like receptor 2
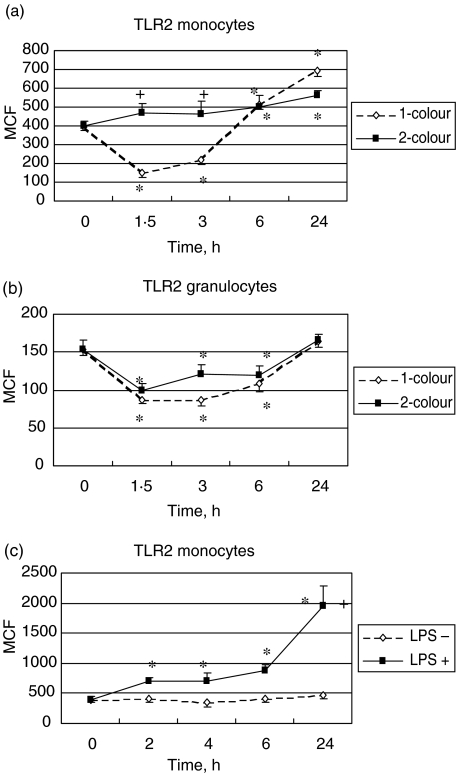
We first determined the TLR2 expression by using the one-colour analyses in 17 volunteers who underwent in vivo LPS challenge. It was threefold higher on monocytes than on granulocytes at baseline (Fig. 2a, b). Within 2 h after LPS administration, a significant decrease in TLR2 expression was observed on monocytes and granulocytes (P < 0·05), the decrease being more pronounced for monocytes. After this initial drop, a dramatic increase of TLR2 expression was observed for monocytes while a less abrupt increase was observed for granulocytes.
Fig. 2.
(a) Comparison of the mean channel fluorescence (MCF) for the Toll-like receptor 2 (TLR2) studied by forward- × side-scatter gating on monocytes in 17 human volunteers (dashed line) after lipopolysaccharide (LPS) challenge and studied by side-scatter × CD14 immunofluorescence gating on monocytes in 12 human volunteers after LPS challenge (full line); (b) comparison of the MCF for the Toll-like receptor 2 (TLR2) studied by forward- × side-scatter gating on granulocytes in 17 human volunteers (dashed line) after LPS challenge and studied by side-scatter × CD14 immunofluorescence gating on granulocytes in 12 human volunteers after LPS challenge (full line); (c) MCF for TLR2 studied by side-scatter × CD14 immunofluorescence gating on monocytes from whole blood drawn from five healthy volunteers and exposed ex vivo to LPS (full line) or not (dotted line). *P < 0·05 versus baseline; +P < 0·05 between groups.
By contrast, when we used side-scatter × CD14 immunofluorescence gating in both in vivo and ex vivo stimulation, we did not reproduce in monocytes the initial decrease described above with the forward- × side-scatter gating (Fig. 2a). In fact, a progressive increase reaching statistical significance at 6 h and 24 h compared to baseline in the in vivo model, and being statistically significant from the 2 h in the ex vivo model, was observed. The kinetics of the TLR2 on granulocytes as assessed by the two-colour analyses was characterized by a significant down-regulation during the first 6 h (P < 0·05) followed by a return to the baseline value at 24 h in volunteers who underwent in vivo stimulation (Fig. 2b). No statistically significant change was observed in the ex vivo model (data not shown).
Analyses of the Toll-like receptor 4
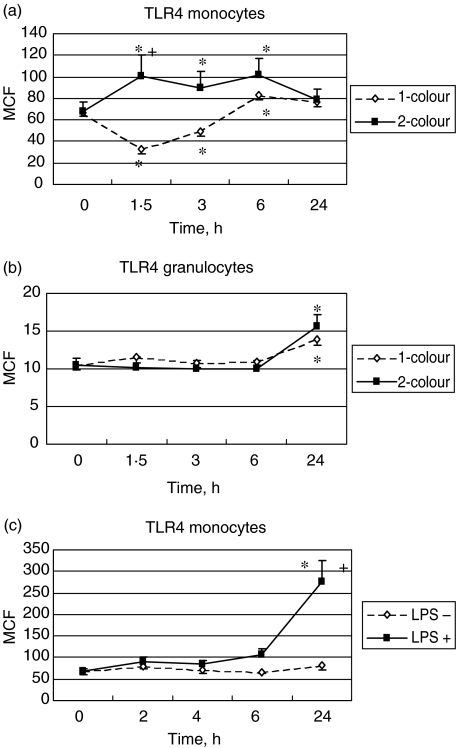
At baseline, monocyte expression of TLR4 was approximately sixfold higher than that of granulocytes. As for the TLR2 expression, using forward- × side-scatter gating, monocyte TLR4 levels decreased significantly from 1 to 2 h after LPS administration and then increased significantly at 6 h (P < 0·05 for all time-points until the 6 h) (Fig. 3a). There were no changes in granulocyte TLR4 levels except for a significant increase at 24 h (P < 0·05) (Fig. 3b). Down-regulation of TLR4 was not observed on monocytes using side-scatter × CD14 immunofluorescence gating in both in vivo and ex vivo experiments. Indeed, we observed a progressive up-regulation reaching statistical significance at 12 and 24 h in the ex vivo stimulation (Fig. 3c) and a statistically significant up-regulation in the in vivo model during the first 6 h (P < 0·05) (Fig. 3a). There was no significant effect of LPS on the expression of TLR4 on granulocytes except for an increase at 24 h in the in vivo model. No difference was observed compared to the LPS negative group on granulocytes stimulated with LPS in the ex vivo model (data not shown).
Fig. 3.
(a) Comparison of the mean channel fluorescence (MCF) for the Toll-like receptor 4 (TLR4) studied by forward- × side-scatter gating on monocytes in 17 human volunteers (dashed line) after lipopolysaccharide (LPS) challenge and studied by side-scatter × CD14 immunofluorescence gating on monocytes in 12 human volunteers after LPS challenge (full line); (b) comparison of the MCF for the TLR4 studied by forward- × side-scatter gating on granulocytes in 17 human volunteers (dashed line) after LPS challenge and studied by side-scatter × CD14 immunofluorescence gating on granulocytes in 12 human volunteers after LPS challenge (full line); (c) MCF for TLR4 studied by side-scatter × CD14 immunofluorescence gating on monocytes from whole blood drawn from five healthy volunteers and exposed ex vivo to LPS (full line) or not (dotted line). *P < 0·05 versus baseline; +P < 0·05 between groups.
Analyses of the isotype control
The isotype control (biotinylated mouse IgG2a) for the TLR antibodies did not show any significant variation over time after LPS stimulation in either in vivo or ex vivo models (data not shown).
Discussion
These results have demonstrated that the flow cytometric gating technique used to assess the surface expression of the TLR2 and TLR4 is of importance. TNFR expression on both monocytes and granulocytes showed a similar expression kinetic pattern whichever of the two gating techniques was used: forward- × side-scatter gating or the more specific side-scatter × CD14 immunofluorescence gating. Therefore it appears that the decrease of the TNFR after LPS challenge in human is related to inflammatory changes induced by LPS itself, rather than a decrease in the circulating monocyte population. This may be important because the assessment of the surface monocyte TNFR expression has been shown to be a prognostic indicator for patients with sepsis who are at increased risk of death [17].
By contrast, the method of analysis appears to be of importance for the assessment of TLR2 and TLR4 expression during similar clinical conditions. While reduced monocyte expression was observed with the forward- × side-scatter gating technique, a significant up-regulation was observed for monocyte expression of both receptors after ex vivo and in vivo LPS stimulation utilizing the side-scatter × CD14 immunofluorescence technique.
Thus, we have shown that the flow cytometry gating technique is of major importance for the assessment of TLR expression on monocytes cell surface and less important for TNFR expression. The putative mechanism behind this is somewhat complex. We believe that when extreme monocytopenia develops at ∼2–3 h after LPS [3–5] and light-scatter gating-only is used, the percentage of errant neutrophils in the scatter gate is increased (incidentally, this is exacerbated by the extreme neutrophila at these same time-points). If we assume that the TNF receptor is truly > 90% down-regulated at these time-points as was evidenced in the CD14+ × side-scatter gating in the present manuscript, errant neutrophils in the scatter gate, which have much lower levels of TNF receptors than do monocytes, would thus add a little to the ‘apparent’ down-regulation. Indeed, in Fig. 1, the light-scatter-only analysis of TNF receptors on monocytes shows marginally lower levels than does the CD14+ × side-scatter analysis. On the other hand, if we consider the TLRs where the CD14+ × side-scatter gating shows them to be increased modestly, albeit significantly, by about 20–30%, errant neutrophils in the scatter gate, which have only ∼35% of TLR2 and ∼15% of TLR4 compared to monocytes, could now very well change this modest increase in TLRs to an apparent decrease, as was shown in the light-scatter gating-only analyses. Further support for errant neutrophils in the scatter gate when analysing monocytes is given by the fact that there were no significant differences between the two gating methods when neutrophils were analysed for TLRs. This is because the profound neutophilia minimizes the effects of any other errant cells in the scatter gate.
The expression of different TLRs, in particular TLR2 and TLR4, has been studied in various models and, at different levels (gene, mRNA and protein expression), especially as it was hypothesized that a decrease of TLR4 would allow further understanding of the so-called LPS-tolerance phenomenon, described initially by Beeson [19] and defined as a state with reduced capacity of whole blood or peripheral blood mononuclear cells (PBMC), to produce proinflammatory cytokines after a short-term re-exposure to a microbiological product such as LPS. The tolerance phenomenon is manifested in the in vivo human endotoxin model used in the present study where hyporesponsiveness to rechallenge with LPS is present for at least up to 6 h and recovery occurs by 24 h [18].
Results of those studies were somewhat conflicting. LPS treatment was shown to decrease the TLR4 mRNA in the RAW 264.7 cell line [20–22] and in mouse peritoneal macrophages [21,23]. Similarly, the expression of theTLR4-MD2 complex on the surface of mouse peritoneal macrophages [21,24,25], as well as on RAW264.7 cells exposed to LPS [22], was shown to decrease after stimulation. The expression of TLR4 on human monocytes was also decreased after a 24-h stimulation with LPS [14], or even after a shorter period with it then returning to baseline value after 12 h [26]. By contrast, no change in TLR2 or TLR4 expression was observed in endotoxin-tolerant mouse peritoneal macrophages [23], in Chinese hamster ovary CD14 cells (clone 3E10) over-expressing TLR2 or TLR4 [27] and in human HEK293 T cells transfected with TLR4 [28]. The TLR2 mRNA was even enhanced in one of these studies [23], as was the TLR4 mRNA in human cardiac myocytes [29] and in different white blood cell populations [14,30] stimulated with LPS. It is notable that some of the experiments assessing the TLR4 expression on cell surface were performed using an antibody directed against the complex TLR4-MD2 rather than TLR4 alone [21,24,28]. Nevertheless, because those experiments were performed with cultured cells, a decrease in the number of cells after LPS stimulation was not expected, as occurs for monocytes in the in vivo human LPS model. Therefore, other factors such as cell specificity, species and time of LPS stimulation could account for those conflicting results.
The human LPS model is a well-characterized and reproducible model that exhibits a dramatic decrease in circulatory monocytes during the first 2 h after the injection of the endotoxin [3,5].
Using a one-colour analysis with light-scatter-only gating, Marsik et al. [15] demonstrated that LPS modulates the TLR expression on both monocytes and granulocytes. The data suggested an initial down-modulation of TLR2 and TLR4 on monocytes 2 h after the LPS in vivo challenge, and a further up-regulation reaching statistically significance for TLR2 by 8 h. Similarly, using the light-scatter-only gating technique, the present study reproduced the same type of expression pattern of TLR after LPS stimulation, especially the initial decrease in TLR2 and TLR4 cell surface expression. Importantly, in the present study, gating based on side-scatter × CD14 immunofluorescence demonstrated that expression of TLR2 and TLR4 on monocytes was up-regulated in the first hours, rather than down-modulated. The decreased expression of TLR observed after LPS challenge with the light-scatter-only gating may be secondary to the monocytopenia, rather than being an effect induced by the LPS itself, as they are concomitant. The results obtained with the two-colour analyses are consistent with those from Armstrong et al., who showed increased monocyte TLR2 and TLR4 mRNA in septic patients with Gram-positive (TLR2 and TLR4) and Gram-negative infection (only TLR2) compared to critical care patients without infection or healthy volunteers [31]. They also showed an increase in TLR2 expression on purified monocyte cell surface for septic critical care patients compared to non-infected patients. The TLR4 expression was not different between groups but this might be explained by the timing of blood sampling. Finally, these data from the human LPS model are also consistent with a report by Calvano et al. [32], who showed an increase in TLR4 expression on monocyte cell surface in patients with systemic inflammatory response syndrome (SIRS) or with SIRS and infection as compared to healthy controls.
Because CD14 is part of the LPS receptor complex, along with TLR4 and MD2, it could be proposed that the use of CD14 antibody as a marker of monocytes might interfere with assessment of expression of TLR4 on these cells. However, similar interference with TLR2 cell surface expression would not be expected and results for TLR2, as with those for TLR4, showed an up-regulation. In addition, the anti-TLR antibodies used are specific for TLR2 or TLR4 receptors and not for a complex of different receptors such as TLR4—MD2 complex used in some of the studies described above [21,24,28].
In summary, it has been shown that LPS stimulation induces an up-regulation of the TLR2 and TLR4 expression on monocytes cell surface. The use of the two-colour analyses employing side-scatter × CD14 immunofluorescence allows more specific staining of monocytes and therefore seems to be a preferable method, especially in cases where significant monocytopenia is present. The up-regulation of the TLR expression on monocytes demonstrated here in the human LPS model does not support the concept that the tolerance phenomenon in monocytes is associated with a decrease of those receptors.
Acknowledgments
This work was supported by United States Public Health Service grant no. GM34695.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Richardson RP, Rhyne CD, Fong Y, et al. Peripheral blood leukocytes kinetics following in vivo lipopolysaccharide (LPS) administration to normal human subjects. Ann Surg. 1989;210:239–45. doi: 10.1097/00000658-198908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Poll T, Lowry SF. Biological responses to endotoxin in humans. In: Tellado JM, Forse RA, Solomkin JS, editors. Modulation of the inflammatory response in severe sepsis. Vol. 200. Basel: Karger; 1995. pp. 18–32. [Google Scholar]
- 5.Lin E, Lowry SF. The human response to endotoxin. Sepsis. 1998;2:255–62. [Google Scholar]
- 6.Schumann RR, Leong SR, Flaggs GW, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–31. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 7.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptative immunity. Nature. 1997;338:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–4. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 11.Ishida I, Kubo H, Suzuki S, et al. Hypoxia diminishes Toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–75. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- 12.Mita Y, Dobashi K, Endou K, et al. Toll-like receptor 4 surface expression on human monocytes and B cells is modulated by IL-2 and IL-4. Immunol Lett. 2002;81:71–5. doi: 10.1016/s0165-2478(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 13.Tamandl D, Bahrami M, Wessner B, et al. Modulation of Toll-like receptor 4 expression on human monocytes by tumor necrosis factor and interleukin 6: tumor necrosis factor evokes lipopolysaccharide hyporesponsiveness, whereas interleukin 6 enhances lipopolysaccharide activity. Shock. 2003;20:224–9. doi: 10.1097/00024382-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bosisio D, Polentarutti N, Sironi M, et al. Stimulation of Toll-like receptor 4 expression in human mononuclear phagocytes by interferon-γ: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–31. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- 15.Marsik C, Mayr F, Cardona F, Derhaschnig U, Wagner OF, Jilma B. Endotoxaemia modulates Toll-like receptors on leucocytes in humans. Br J Haematol. 2003;121:653–6. doi: 10.1046/j.1365-2141.2003.04350.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Poll T, Calvano SE, Kumar A, et al. Endotoxin induces down regulation of tumor necrosis factor receptors on circulating monocytes and granulocytes in humans. Blood. 1995;86:2754–9. [PubMed] [Google Scholar]
- 17.Calvano SE, van der Poll T, Coyle SM, Barie PS, Moldawer LL, Lowry SF. Monocyte tumor necrosis factor receptor levels as a predictor of risk in human sepsis. Arch Surg. 1996;131:434–7. doi: 10.1001/archsurg.1996.01430160092020. [DOI] [PubMed] [Google Scholar]
- 18.van der Poll T, Coyle SM, Moldawer LL, Lowry SF. Changes in endotoxin-induced cytokine production by whole blood after in vivo exposure of normal humans to endotoxin. J Infect Dis. 1996;174:1356–60. doi: 10.1093/infdis/174.6.1356. [DOI] [PubMed] [Google Scholar]
- 19.Beeson PB. Tolerance to bacterial pyrogen. J Exp Med. 1947;86:29–38. doi: 10.1084/jem.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 21.Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down regulation of surface Toll-like receptor 4 expression. J Immunol. 2000;164:3476–9. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 22.Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. J Immunol. 2003;170:1052–61. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- 23.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–74. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 24.Akashi S, Shimazu R, Ogata H, et al. Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–5. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Nomura F, Kawai T, et al. Synergy and cross-tolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–01. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 26.Moreno C, Merino J, Ramirez N, Echeverria A, Pastor F, Sanchez-Ibarrola A. Anti-inflammatory cytokines induce lipopolysaccharide tolerance in human monocytes without modifying Toll-like receptor 4 membrane expression. Scand J Immunol. 2004;59:553–8. doi: 10.1111/j.0300-9475.2004.01445.x. [DOI] [PubMed] [Google Scholar]
- 27.Medvedev AE, Henneke P, Schromm A, et al. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J Immunol. 2001;167:2257–67. doi: 10.4049/jimmunol.167.4.2257. [DOI] [PubMed] [Google Scholar]
- 28.Medvedev AE, Vogel SN. Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J Endotoxin Res. 2003;9:60–4. doi: 10.1179/096805103125001360. [DOI] [PubMed] [Google Scholar]
- 29.Frantz S, Kobzik L, Kim YD, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leucocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong L, Medford AR, Hunter KJ, Uppington KM, Millar AB. Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol. 2004;136:312–19. doi: 10.1111/j.1365-2249.2004.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvano JE, Agnese DM, Um JY, et al. Modulation of the lipopolysaccharide receptor complex (CD14, TLR4, MD-2) and Toll-like receptor 2 in systemic inflammatory response syndrome-positive patients with and without infection: relationship to tolerance. Shock. 2003;20:415–19. doi: 10.1097/01.shk.0000092269.01859.44. [DOI] [PubMed] [Google Scholar]