Abstract
Recent studies have suggested that not only αβ+ T cells, but also the less common γδ+ T cells may play a role as effectors and immunoregolatory cells in the development and perpetuation of allergic inflammation. The objective of this study was to focus on the role of γδ+ T cells in atopic dermatitis (AD), a chronic relapsing inflammatory disease of the skin, often associated with allergic bronchial asthma. The present study employed flow cytometric analysis to compare numbers and phenotypic characteristics of γδ+ T cells in the peripheral blood of children with atopic dermatitis and age-matched healthy controls. The percentage of circulating Vγ 9Vδ2+ T lymphocytes was significantly increased in AD patients with respect to the age-matched controls, with a positive correlation with clinical score severity. The prevalent phenotype in both AD patients and controls was CD45RO+, with no differences observed in the percentage of Vδ2+ CD45RO+ between these groups. Conversely, memory CD45RO+ CD62L+ Vδ2+ lymphocytes were significantly lower in AD patients. Furthermore, naive circulating Vδ2+ T lymphocytes were significantly lower in AD children than in aged-matched controls. No correlation was observed between circulating Vγ 9Vδ2+ expansion and IgE serum levels. It was concluded that an association exists between the levels of circulating γδ+ T lymphocytes and atopic dermatitis, with a positive correlation with clinical score but no link with IgE serum levels. The pathophysiological role of γδ T lymphocytes in atopic dermatitis awaits further investigation.
Keywords: allergy, atopic dermatitis, IgE, inflammation, γδ+ T cells
Introduction
Atopic dermatitis (AD) is a common, chronic, relapsing, inflammatory cutaneous disease, genetically determined, characterized by typically distributed eczematous skin lesions, dry skin, intense pruritus and a wide variety of pathophysiological aspects [1–3].
A strict relationship between genetic, skin barrier, immunological factors and trigger events such as environmental, pharmacological, psychological and infectious, may be elicited and considered to be involved in the development and severity of AD [4].
Since World War II, AD prevalence has been steadily increasing, as reported in population studies, and more than 10% of children are affected at some point during childhood [5]. Moreover, later in childhood nearly 60% may develop allergic rhinitis and/or asthma [6].
AD has been classified recently by the European Academy of Allergy and Clinical Immunology (EAACI) Nomenclature Task Force into non-allergic atopic eczema/dermatitis syndrome (AEDS) and allergic AEDS, the later being divided further into two subgroups: IgE-associated allergic AEDS and non-IgE-associated allergic AEDS [7].
In the past 10 years the immune system has represented the object of scientific attention in AD, alterations in IgE production, Th1/Th2 lymphocyte activity balance and dendritic cell functions have been widely documented [8–10]. Conversely, few data are available in literature on the possible role of the cells bearing the γδ receptor.
γδ+ T cells perform different functions according to their tissue distribution, antigen-receptor structure and local microenvironment [11,12]. Two main populations of γδ+ are present in human peripheral blood. The first population is represented by the γδ+ cells expressing the Vδ2 chain; they are associated mainly with the Vγ9 chain, and constitute more than 70% of circulating γδ+ 0. The other population is represented by γδ+ cells expressing the Vδ1 chain, associated with a γ chain that differs from Vγ9; they account for 30% of the peripheral γδ+, and constitute the majority of mucosal γδ+ lymphocytes [13,14].
The population that expresses the Vγ9Vδ2 receptor recognizes different classes of microbial non-peptidic antigens showing a wide reactivity towards bacteria [15,16], protozoa [17] and viruses [18]. These lymphocytes respond to the antigenic stimulus with a polyclonal pattern [19], proliferating, producing a large amount of Th1 cytokines [20], particularly interferon (IFN)-γ[21,22], and differentiating into cytotoxic effectors [18,23].
Recently, some authors have suggested that γδ+ T lymphocytes may play a role in the course and outcome of the allergic inflammatory process, occurring in bronchial asthma and rhinitis, by their ability to interact with several immune cells influencing their activity [24–29].
The aim of this study was to evaluate the significance and possible role of circulating γδ+ T cells in allergic AEDS.
Materials and methods
Patients
Fifteen children, nine males and six females, aged between 7 and 121 months (mean: 58; median: 65), affected by chronic allergic AEDS, were enrolled in this study. Nine of 15 (60%) were suffering from mild AD (SCORAD index ranging between 10 and 25) and 6 (40%) from moderate/severe AD (SCORAD index over 25). Five had suffered from AD from at least 6 months of age. Six of 15 (40%) presented a positive family history for atopic disease and five of 15 (33%) presented with secondary allergic symptoms such as seasonal rhinocongiuntivitis (three of 15) and bronchial asthma (three of 15).
At the time of sampling, all the children were in an acute phase of the cutaneous disease, with a bacterial super-infection in the case of moderate/severe AD. None of them had taken either antibiotics or corticosteroids for at least 10 days. Fifteen age-matched children (10 boys and five girls) without a history of AD or respiratory allergy were selected as the control group.
SCORAD
The severity of AD was evaluated by using the SCORAD index [30]. This index consists of several evaluation criteria (objective and subjective) of variable importance, giving a global quantitative score representing the intensity of disease at a given time. The objective criteria include the extent and intensity of the disease. The subjective signs assess the pruritus and sleep loss related to AD.
The extent of the disease was assessed by using the scale ‘rule of nine’ (long used as an application for burns) adapted according to age and expressed in percentage of surface altered. The intensity of the involved area for erythema, oedema/papulation, oozing/crusts, excoriation and lichenification of the uninvolved area for dryness was evaluated using a four-point scale: 0 = absence, 1 = mild, 2 = moderate, 3 = severe.
The subjective signs, pruritus and sleep loss related to AD in the last 3 days/nights preceding a scheduled visit was evaluated by the care person on a 10 cm visual analogue scale (VAS) with: 0 = absence, 10 = severe.
Modified SCORAD index = (extent/5) + (intensity × 3·5) (where the extent is rounded to the nearest multiple of 5 if larger than 5%).
Samples
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Ficoll-Hypaque gradient centrifugation and were resuspended at 4 × 106 cells/ml in cold RPMI-1640.
Monoclonal antibodies and flow cytometry
Monoclonal antibodies (mAb) coupled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridin chlorophyll protein (PerCP) and allophycocyanin (APC) were used in combination for simultaneous staining. The anti-Vδ2 mAb, which recognizes the Vδ2 region of the γδTCR, was coupled with FITC (IgG1, clone IMMU1464, Immunotech, Marseille, France) or with PE (IgG1, clone B6, Becton Dickinson, Mountain View, CA, USA). The anti-CD25 mAb (IgG1, clone M-A251, Becton Dickinson), the anti-CD27 mAb (IgG1, clone L128, Becton Dickinson) and the anti-CD94 mAb (IgG1, clone HP-3D9, Becton Dickinson) were coupled with FITC. The anti-CD62L mAb (IgG1, clone Dreg56, Becton Dickinson) and the anti-NKG2-A mAb (IgG1; Immunotech, Marseille, France) were coupled with PE. The anti-CD3 mAb (IgG1, clone SK7, Becton Dickinson) was coupled with PerCP. The anti-CD45RA mAb (IgG2b, clone HI100, Becton Dickinson) was coupled with Cy-Chrome and the anti-CD45-RO (IgG2a, clone UCHL-1, Becton Dickinson) and anti-CD69 (IgG1, clone L78, Becton Dickinson) were coupled with APC.
Analysis of surface antigen expression was performed as described previously [31]. Briefly, 3 × 105 PBMC were washed in phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0·1% sodium azide and were incubated for 15 min at 4°C with the indicated FITC-, PE-, PerCP- or APC-conjugated mAbs. Samples were processed immediately using a FACSCalibur flow cytometer (Becton Dickinson). A total of 50 000 events was acquired for each sample and analysed with the CellQuest software (Becton Dickinson).
Serum IgE levels
Serum samples were blind-coded prior to the analysis of the total IgE contents and the titres were expressed by KUA/l. A panel of inhalant and food allergens were tested with Pharmacia CAP-RAST, according to the manufacturer's instructions.
Statistical analysis
All results are expressed as mean ± standard deviation (s.d.). Differences were been analysed by the unpaired two-tailed Student's t-test. A P-value < 0·05 was considered significant.
Results
Vγ9Vδ2+ T lymphocytes in peripheral blood
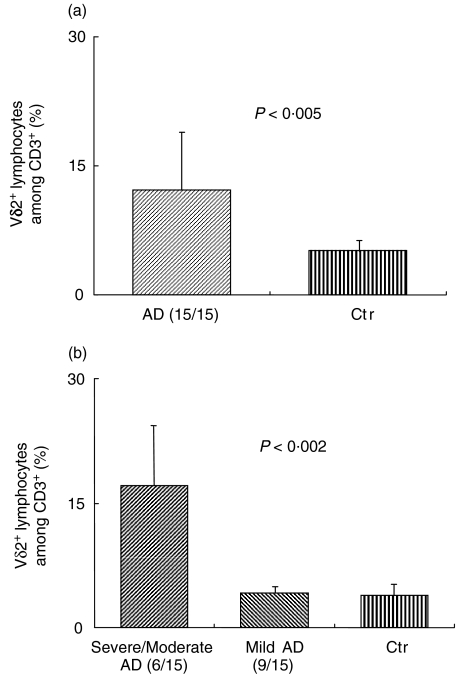
The percentage of circulating Vγ9Vδ2+ T lymphocytes was increased (P < 0·005) in AD children compared to age-matched controls (Fig. 1a). The percentage of Vγ9Vδ2+ T lymphocytes among CD3+ cells was found to be 11·3% ± 8·6% in AD patients versus 4·1% ± 1·7% in healthy subjects. When AD children were divided into two groups, based on the clinical score of disease, the difference between severe and mild AD became striking (Fig. 1b). The Vγ9Vδ2+ T lymphocyte percentage among CD3+ cells was 17·12 ± 7·4% in the severe AD group versus 4·2% ± 1·0% in the mild AD group (P = 0·002), while there was no difference between the mild AD group and the healthy controls (4·1% ± 1·7%).
Fig. 1.
Peripheral blood mononuclear cells (PBMC) freshly isolated from atopic dermatitis (AD) patients or healthy controls were analysed by flow cytometry for the size of the Vδ2+ population. (a) AD patients were grouped altogether or divided into two groups based on clinical score (b). Data shown in (b) revealed significant values only in severe/moderate AD versus mild AD and controls.
Analysis of phenotypical characteristics of Vδ2+ T lymphocytes revealed no statistically significant differences between AD patients and age-matched controls for the expression of most of the membrane markers evaluated (Table 1). Vδ2+ T cells from both AD patients and controls showed very low expression of activation markers such as CD69 and CD25, the latter being virtually absent. Vγ9Vδ2+ T lymphocytes from patients and controls showed no difference in the expression of the inhibitory CD94/NKG2A receptor. Moreover, virtually all CD94 appeared to be associated with NKG2A, the inhibitory isoform of the NKG2-A-G family.
Table 1. Phenotypic characterization of the Vδ2+ T lymphocyte subset among freshly isolated peripheral blood mononuclear cells (PBMC) in atopic dermatitis (AD) patients and age-matched healthy controls.
| AD | CTR | ||
|---|---|---|---|
| CD45RA+ | 13·1 ± 3·4 | 16·9 ± 6·1 | |
| CD45RO+ | 83·8 ± 7·4 | 83·9 ± 8·6 | |
| CD62L+ | 58·0 ± 24·5 | P < 0·002 | 84·8 ± 5·9 |
| CD45RA+/CD62L+ | 8·7 ± 4·1 | P < 0·05 | 13·5 ± 4·3 |
| CD45RO+/CD62L+ | 45·2 ± 19·2 | P < 0·003 | 73·1 ± 8·1 |
| CD45RA−/CD27− | 9·2 ± 2·8 | 8·5 ± 3·5 | |
| CD69+ | 0·9 ± 0·5 | 1·6 ± 0·9 | |
| CD25+ | 0·05 ± 0·05 | 0·08 ± 0·1 | |
| CD94+ | 54·3 ± 17·6 | 58·7 ± 16·8 | |
| NKG2A+ | 54·6 ± 17·6 | 59·3 ± 15·3 |
Naive/memory γδ T lymphocyte markers were also analysed by flow cytometry. The prevalent phenotype in both AD patients and controls was CD45RO+, with similar frequencies of the Vδ2+ CD45RO+ subset and Vδ2+ CD45RA+ 0 percentages in both groups. We found a significant difference in the CD62L and CD45RO+/CD62L+ populations, with significantly fewer such cells in AD patients than in controls, as shown in Figs 2 and 3.
Fig. 2.
Peripheral blood mononuclear cells (PBMC) freshly isolated from atopic dermatitis (AD) patients or healthy controls (CTR) were analysed for the expression of CD62L/CD45RO markers on the Vδ2+ T cell population. The histograms represent a comparison of mean data obtained from 19 AD patients and 15 CTR. Result are represented as mean ± s.d.
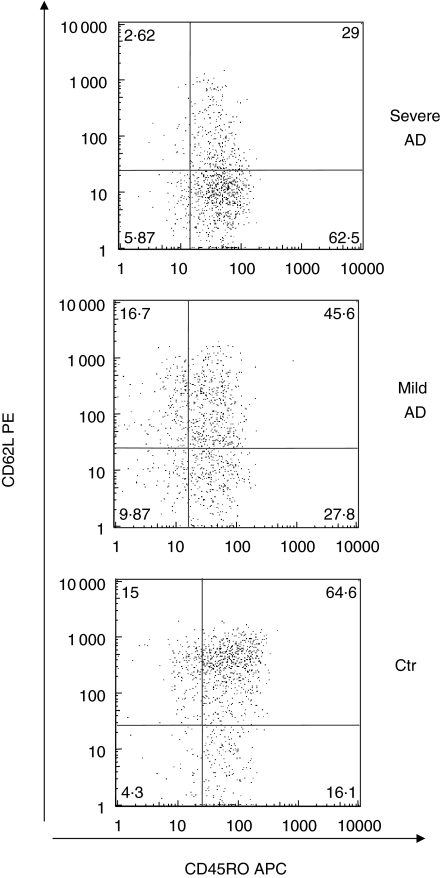
Fig. 3.
FACS analysis of peripheral blood mononuclear cells (PBMC) from severe or mild atopic dermatitis (AD) patients and healthy controls (CTR). Cells were gated on the Vδ2+ T cell population and were analysed for the expression of CD62L/CD45RO markers. Numbers in the boxes indicate the percentage of positive cells within the Vδ2+ T cell population. Data are from representative experiments.
Naive Vδ2+ T lymphocytes, defined as CD45RA+/CD62L+, were slightly less frequent (P < 0·05) in AD patients than in the healthy controls (Fig. 2).
Serum IgE
Eight of 15 children (53%) affected by AD showed higher total IgE serum values (IgE-associated allergic AEDS) with a mean of 250 KUA/l (range 125–610 KUA/l).
Five of eight (62%) showed positive specific IgE values (> 0·35 KUA/l) to house dust mite and/or grass pollen and/or olive and/or Alternaria and/or cat, and six (75%) to cow's milk and/or egg.
No correlation was observed between circulating Vγ9Vδ2+ expansion and IgE serum levels.
Discussion
The aim of our study was to evaluate the relevance and possible role of γδ T lymphocytes in an allergic inflammatory disease, such as AD. This aspect of AD has not really been investigated, except for a recent study carried out on a mouse model suggesting that αβ T cells but not γδ T cells, B cells or CD40L/CD40 interactions are critical for skin inflammation and the Th2 response in AD [32].
Clinical and experimental data, both from humans and mice, suggest that γδ T lymphocytes may play a role as effectors and immunoregulatory cells in allergic disease, particularly in bronchial asthma and rhinitis, in spite of some conflicting opinions [24–29,32–35], and also by pathways separate from the classical Th2 immune activation. Spinozzi et al. [24] have detected an increase in T lymphocytes bearing the γδ1 receptor in the bronchoalveolar lavage fluid of asthmatic adults. Furthermore, Chen et al. [36] have documented a decreased amount of γδ1 T lymphocytes in the peripheral blood of patients with allergic asthma, thus suggesting a recruitment of these cells by bronchial mucosa. Both authors underlined the absence of a relationship between γδ T cells and IgE levels, suggesting that γδ T cells might be implicated in allergic inflammation.
Svensson et al. indicated that γδΤ cells promote allergic airway inflammation by enhancing the systemic IgE response and local antibody reactivity without a specific role in the shift of the immune response towards Th2 [28]. Similarly, Pawankar et al. have observed in the nasal mucosa of subjects with allergic rhinitis an increased proportion of T lymphocytes bearing the γδ1 receptor, allergen- and organ-specific, and able to produce mainly interleukin (IL-4), IL-5 and IL-13 [25,37]. In the same patients no alterations were detected in the subset of circulating γδ bearing the Vγ9Vδ2 receptor [25]. Aoyagi et al., on the other hand, found that infants affected by respiratory syncytial virus (RSV)-bronchiolitis have lower frequencies of IFN-γ-producing γδ T lymphocytes in peripheral blood when compared to age-matched controls. Moreover, they noticed a normalization of this frequency during the convalescent phase, suggesting an important role for the defective IFN-γ production by γδ T cells in the development of asthma in selected subjects after RSV-bronchiolitis [27]. Other authors, less recently, found no differences in the number of γδ T cells between asthmatic and control subjects, both in peripheral blood and in bronchial mucosa [34].
Our study was carried out on a group of children suffering from allergic AEDS, both IgE-associated and non-IgE-associated; however, the group included patients in a chronic and relapsing acute phase, with bacterial superinfection clinically evident in children with moderate/severe dermatitis, and probable in the others. Indeed, it is known that in more than 90% of patients with AD Staphylococcus aureus colonizes the skin, apart from those with clinical features of impetigo.
We evaluated the peripheral blood Vγ9Vδ2 T lymphocyte subset rather than that of Vδ1, which has already been investigated widely in other studies in this field. In fact, the Vγ9Vδ2 population represents not only the prevalent subset among circulating γδ T cells, but is also known to recognize the enterotoxin A released by S. aureus (SEA) and to proliferate following an in vitro stimulation by this superantigen (Sag) [38].
As expected, we detected a significant increase in the percentage of circulating Vγ9Vδ2+ lymphocytes in children with AD compared with their age-matched healthy controls, with a positive correlation noted between their expansion and the severity of the disease (moderate/severe versus mild). The observed expansion may be due to the prolonged cutaneous stimulation by SEA, as can occur in children with moderate/severe AD and by S. aureus colonization.
The spreading of lymphocytes Vγ9Vδ2+, documented in peripheral blood, seems to suggest an increased number of these cells even in the skin. Indeed, in all the children affected by AD, a significant reduction in the numbers of Vγ9Vδ2+ CD45RO+/CD62L+ lymphocytes, the memory subset able to perform homing to secondary lymphoid tissues, with respect to healthy controls and related positively to the severity of the cutaneous disease, was observed. The Sag effect is limited mainly to those lymphocytes that are able to home to the skin, as reported by experimental evidence [39]. This and our findings may lead to the hypothesis that in AD patients a larger proportion of Vγ9Vδ2+ lymphocytes performs homing to the cutaneous district. The next step will be to evaluate the presence and phenotype of this subset in situ, at the level of skin lesions, by cutaneous biopsy.
Recently, Eberl et al. identified two different population of memory γδ T cells: non-polarized central memory T cells (Tcm CD45RA− CD45RO+ CD62L+ CD81+ CD94+ CD95−) representing lymph-node-homing cells that lack inflammatory and cytotoxic functions and polarized effector/memory T cells (Tem: CD45RA− CD45RO+ CD62L− CD81− CD94+ CD95− CXCR5− CCR5+ CCR7−) representing tissue-homing cells with a variety of effector functions [12]. At present, we cannot exclude the existence of these two distinct memory γδ subsets in AD as well; other studies are in progress in our laboratory to clarify this topic.
In children with AD, no correlation was detected between the percentage of Vδ2+ and the plasma level of IgE, as already reported for the Vδ1 population by other authors investigating different allergic disease.
In conclusion, our findings show that, as suggested for other allergic conditions, an alteration of the γδ+ population may also occur in AD, although the physiopathological role of this phenomenon awaits further investigation.
References
- 1.Wuthrich B. Clinical aspects, epidemiology and prognosis of atopic dermatitis. Ann Allergy Asthma Immunol. 1999;83:464–70. doi: 10.1016/S1081-1206(10)62852-9. [DOI] [PubMed] [Google Scholar]
- 2.Wollenberg A, Bieber T. Atopic dermatitis: from the genes to skin lesions. Allergy. 2000;55:205–13. doi: 10.1034/j.1398-9995.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnetson RS, Rogers M. Childhood atopic eczema. BMJ. 2002;328:1376–9. doi: 10.1136/bmj.324.7350.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung DYM. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–72. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 5.European Allergy White Paper Update. Brussels, Belgium: UCB Institute of Allergy; 2000. Allergic diseases as public health problem in Europe. [Google Scholar]
- 6.Schmid P, Simon D, Simon HU, Adkis CA, Wuthrich B. Epidemiology, clinical features and immunology of the intrinsic (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis) Allergy. 2001;56:841–9. doi: 10.1034/j.1398-9995.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 7.Johansson SGO, Hourihane JOB, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:841–9. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 8.Leung DYM. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–19. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S. The role of lymphocytes in allergic diseases. J Allergy Clin Immunol. 1999;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 10.Banfield CC, Callard RE, Harper JI. The role of cutaneous dendritic cells in the immunopathogenesis of atopic dermatitis. Br J Dermatol. 2001;144:940–6. doi: 10.1046/j.1365-2133.2001.04179.x. [DOI] [PubMed] [Google Scholar]
- 11.Carding SR, Egan PJ. γδ T cells. functional plasticity and heterogeneity. Nature. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 12.Eberl M, Engel R, Beck E, Jooma H. Differentiation of human γδ T cells towards distinct memory phenotypes. Cell Immunol. 2002;218:1–6. doi: 10.1016/s0008-8749(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 13.Casorati G, De Libero G, Lanzavecchia A, Migone N. Molecular analysis of human gamma/delta clones from thymus and peripheral blood. J Exp Med. 1989;170:1521–7. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triebel F, Herchend T. Subpopulation of human peripheral T gamma/delta lymphocytes. Immunol Today. 1989;10:186–8. doi: 10.1016/0167-5699(89)90321-6. [DOI] [PubMed] [Google Scholar]
- 15.Modlin RL, Primez C, Horman FM, et al. Lymphocytes bearing antigen specific gamma/delta T cell receptor accumulate in human infectious disease lesions. Nature. 1989;339:554–68. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 16.Molne L, Corthay A, Holmdahl R, Tarkowski A. Role of gamma/delta receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. Clin Exp Immunol. 2003;132:209–15. doi: 10.1046/j.1365-2249.2003.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho M, Tongtawe P, Kriangkum J, et al. Polyclonal expansion of peripheral gamma/delta T cell receptor in human Plasmodium falciparum malaria. Infect Immun. 1994;62:855–62. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace M, Malkowski M, Carding SR. Gamma/delta T lymphocyte in viral infections. J Leukoc Biol. 1995;58:277–83. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 19.De Libero G. Sentinel function of broadly reactive human gamma-delta T cells. Immunol Today. 1997;18:22–6. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 20.Poccia F, Battistini L, Cipriani B, et al. Phosphoantigen-reactive Vγ9Vδ2 T lymphocytes suppress in vitro human immunodeficiency virus type I replication by cell-released antiviral factors including CC chemokines. J Infect Dis. 1999;180:858–61. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 21.Battistini L, Borsellino G, Sawicki G, et al. Phenotypic and cytokine analysis of human peripheral blood γδ T cells expressing NK cell receptors. J Immunol. 1997;169:3723–30. [PubMed] [Google Scholar]
- 22.Wisnewski AV, Herrick CA, Liu Q, Chen L, Bottomly K, Redlich CA. Human γ/δ T-cell proliferation and IFN-γ production induced by hexamethylene diisocyanate. J Allergy Clin Immunol. 2003;112:538–46. doi: 10.1016/s0091-6749(03)01865-7. [DOI] [PubMed] [Google Scholar]
- 23.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ 9Vδ 2 T lymphocytes. J Immunol. 1997;159:6009–17. [PubMed] [Google Scholar]
- 24.Spinozzi F, Agea E, Bistoni O, Forenza N, Bertotto A. γδ T cells, allergen recognition and airway inflammation. Immunol Today. 1998;19:22–6. doi: 10.1016/s0167-5699(97)01182-1. [DOI] [PubMed] [Google Scholar]
- 25.Pawankar R. γδ T cells in allergic airway diseases. Clin Exp Allergy. 2000;30:318–23. doi: 10.1046/j.1365-2222.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 26.Holt PG, Sly PD. γδ T cells provide a breath of fresh air for asthma research. Nature Med. 1999;5:1127–8. doi: 10.1038/13447. [DOI] [PubMed] [Google Scholar]
- 27.Isogai S, Rubin A, Maghnu K, et al. The effects of CD8+γδ T cells on late allergic airway responses and airway inflammation in rats. J Allergy Clin Immunol. 2003;112:547–55. doi: 10.1016/s0091-6749(03)01720-2. [DOI] [PubMed] [Google Scholar]
- 28.Svensson L, Lilliehook B, Larsson R, Bucht A. Gamma/delta T cells contribute to the systemic immunoglobulin E response and local B cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108:98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoyagi M, Shimojo N, Sekine K, Nishimuta T, Kohno Y. Respiratory syncytial virus infection suppresses IFN-γ production of γ/δ T cells. Clin Exp Immunol. 2003;131:312–17. doi: 10.1046/j.1365-2249.2003.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Dermatology. 1993;186:23–3. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 31.Gioia C, Agrati C, Casetti R, et al. Lack of CD27-CD45RA- Vgamma9 Vdelta2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol. 2002;168:1484–9. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- 32.Woodward AL, Spergel JM, Alenius H, et al. An obligate role for T cell receptor αβ+ T cells but not T cell receptor γδ+ T cells, B cells or CD40/CD40L interaction in a mouse model of atopic dermatitis. J Allergy Clin Immunol. 2001;107:359–66. doi: 10.1067/mai.2001.112695. [DOI] [PubMed] [Google Scholar]
- 33.Zuani-Amorin C, Ruffie C, Hailé S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–7. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 34.Lahn M, Kanehio A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nat Med. 1999;5:1150–6. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 35.Fajac I, Roisman GL, Lacronique J, Polla BS, Dusser DJ. Bronchial γδ T lymphocytes and expression of heat shock proteins in mild asthma. Eur Respir J. 1997;10:633–63. [PubMed] [Google Scholar]
- 36.Chen KS, Miller KH, Hengehold D. Diminution of T cells with γδ receptor in the peripheral blood of allergic asthmatic individuals. Clin Exp Allergy. 1996;26:295–302. [PubMed] [Google Scholar]
- 37.Pawankar R, Okuda M, Suzuki K, Okumura K, Ra C. Phenotypic and molecular characteristics of nasal mucosal γδ T cells in allergic and infectious rhinitis. Am J Respir Crit Care Med. 1996;153:1655–65. doi: 10.1164/ajrccm.153.5.8630617. [DOI] [PubMed] [Google Scholar]
- 38.Morita CT, Li H, Lamphear JG, Rich RR, Fraser JD, Mariuzza R, Lee HK. Superantigen recognition by γδ T cells: SEA recognition site for human Vδ2 T cell receptors. Immunity. 2001;14:331–44. doi: 10.1016/s1074-7613(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 39.Strickland I, Hauk PJ, Trumble AE, Picker LJ, Leung DYM. Evidence for superantigen involvement in skin homing of T cells in atopic dermatitis. J Invest Dermatol. 1999;112:249–53. doi: 10.1046/j.1523-1747.1999.00502.x. [DOI] [PubMed] [Google Scholar]