Abstract
Natural killer (NK) cell cytotoxic activity and cell frequency, expressed as a percentage of total lymphocytes, have been determined in peripheral blood mononuclear cells from first-degree relatives of patients with systemic lupus erythematosus (SLE), the patients themselves, a group of rheumatoid arthritis (RA) patients and controls. Low levels of killing activity relative to controls were found in some members of all groups with the extent of depression falling into two ranges. Moderate reductions were seen in female (3/31, 10%) and male (4/14, 29%) relatives of SLE patients, female (12/60, 20%) and male (3/4, 75%) SLE patients and female RA patients (6/17, 35%). A more profound depression of killing activity was confined to other female SLE patients (15/60, 25%). There were strong correlations in all groups between killing activity and percentage of NK cells, but analysis of the ratio of these parameters and studies with purified preparations of NK cells suggest that the reduced activity in SLE frequently involves a defect in the killing capacity of the individual cells in addition to the reduced levels of NK cells. Azathioprine (AZA), which was used in treatment of 12 SLE patients, was invariably associated with low values of killing activity. It appears to substantially reduce the percentage of NK and B cells in an action unconnected with the NK cell abnormalities associated with SLE. The finding of low killing activity in relatives and a correlation between their activity and that of their patients support the view that NK cell deficiency is a genetic determinant of SLE. NK cells in SLE may produce insufficient levels of cytokines required for the regulation of IgG production.
Keywords: SLE patients and relatives, NK cells, natural killing activity, blood lymphocytes, azathioprine
Introduction
Patients with systemic lupus erythematosus (SLE) have low numbers of natural killer (NK) cells circulating in the blood [1,2]. NK cells expressed as a percentage of total blood lymphocytes (% NK cells) are depressed as is natural killing activity of blood mononuclear cells (PBMC) [3,4].
Does this low NK cell level play a part in causation of the disease or is it a consequence of the disease process or drug treatment? We have attempted to answer this question by determining percentage NK cells and natural killing activity in PBMC from healthy first-degree relatives of SLE patients. If these NK parameters were found to be depressed in relatives this would provide support for the view that low NK activity plays a primary role, along with other factors, in the development of the disease. In addition to SLE patients, their relatives and controls we have also tested a group of rheumatoid arthritis patients, another autoimmune condition in which low NK cell activity has been reported [5].
When PBMC are tested for killing activity it is difficult to distinguish effects due to reduced numbers of NK cells from those due to altered cell activity. Previous studies directed at determining NK cell activity in patients with SLE have mostly involved complex single-cell assay procedures in which cell death is determined by microscopy [6,7]. In this study we have taken two approaches to the problem. First, we have analysed the ratio of killing activity to percentage NK cells in the different groups. If variation in killing activity was due solely to variation in NK cell frequency one might expect such ratios to be independent of donor type. Secondly, we have compared directly the killing activity of purified preparations of NK cells from SLE patients and controls.
Finally, we have looked at the relationship between NK cell parameters and clinical disease activity and therapy of the SLE patients. A dramatic effect of the drug azathioprine on the levels of NK and B cells was observed.
Methods
Patients, relatives and controls
SLE patients were attending clinics at St Mary's Hospital and the Centre for Rheumatology, University College London. All patients fulfilled the revised criteria of the American College of Rheumatology for classification of SLE [8]. Of the 65 patients only 5 were male. Mean age 42 years, range 17–72 years (female); mean age 37 years, range 24–54 years (male). Ethnic origin was as follows: Caucasian (38 female/3 male), Afro-Carribbean (13/1), Asian (7/1), Indian Sub-Continent (1/0), African (1/0).
Disease activity was assessed by the British Isles Lupus Assessment Group (BILAG) system [9], in which activity is assigned from category A (the most active) to D (currently inactive) or E (no current or previous involvement) in each of eight organ/systems (mucocutaneous, neurological, renal, etc.) on the principle of the ‘physician's intention to treat’. A global score for each patient was obtained by allocating 9 points for an A, 3 for a B (moderately active disease) and 1 for a C (stable mild disease). Five patients showed one or two A grades; 27 patients had one to three Bs as their highest grade; 30 patients had one to five Cs as their highest grade; three patients had only D/E grades. These figures refer to all patients – males had one representative in each of the first two groups and three in the third group.
SLE patients were being treated, alone or in combination, with steroids (prednisolone) (35 female/4 male), hydroxychloroquine (HCQ) (34/1), azathioprine (AZA) (12/0), cyclophosphamide (3/1) or methotrexate (2/0). Steroids were used alone (8/2) or with HCQ (12/1), AZA (6/0), cyclophosphamide (1/1) and methotrexate (2/0), or they were used in triplicate combinations with HCQ and AZA (4/0) or cyclophosphamide (2/0). HCQ was used alone in 16/0 patients while AZA was used alone in 2/0. Eight (7/1) patients received no disease modifying drugs.
First-degree relatives of SLE patients were healthy and had no current or past autoimmune disease. There were 31 females/14 males with age range 18–86/17–77 and mean age 43/50 years. There were 12 mothers of patients, 8 fathers, 9 sisters, 2 brothers, 10 daughters and 4 sons. Twenty-five of our patients provided a single relative only, but 6 provided 2 and 2 provided 3. For two of the relatives we were unable to obtain blood samples from the patients concerned. The ethnic breakdown of the relatives was Caucasian (17 female/6 male), Afro-Carribbean (9/2), Asian (5/4), Indian Sub-Continent (0/1), and African (0/1).
Rheumatoid arthritis patients were attending clinics at St Mary's Hospital. There were 17 females and 4 males with age range 21–80 and 32–81 years and mean age 58 and 56 years, respectively. Twelve/three patients were receiving methotrexate either alone or in various combinations with steroids, sulphasalazine and HCQ. Most of the remaining patients received combinations of the latter drugs, but one female patient received steroids with AZA, another gold and another was untreated. The group was predominantly Caucasian.
Control donors were healthy staff of Imperial College School of Medicine at the St Mary's Campus. There were 18 females/11 males (age range 21–65/22–63 with mean age 32/34 years). The controls were Caucasian except for 3 male Asians and 1 male and 2 females from the Indian Sub-Continent.
Blood mononuclear cells
Blood samples were taken with ethics committee approval. Samples (20 ml) were taken into preservative-free sodium heparin (20 U/ml of blood). Blood mononuclear cells (PBMC) were obtained by centrifugation on Histopaque-1077 (Sigma, Poole, UK) of the blood diluted 1 : 1 with RPMI 1640 (Sigma; R0883).The separated PBMC were taken up in 2 ml of this medium containing 10% heat-inactivated fetal calf serum (Gibco, Paisley, UK), and supplemented also with glutamine (2 mM) and HEPES (10 mM). PBMC were divided into two portions, one for flow cytometry and the other for NK cell killing either directly or after cell fractionation.
Flow cytometry
Mouse IgG1 monoclonal antibodies with appropriate isotype controls were obtained as follows: Anti-CD3 (Sigma; F0522: FITC conjugate), Anti-CD19 (BD Biosciences, Cowley, UK; 30658X: Cy-chrome conjugate) and Anti-CD56 (BD Biosciences; 555516: PE conjugate). Antibody staining of the cells was carried out for 20 min on ice before their fixation in 2% paraformaldehyde.
Samples were analysed on a Beckman Coulter Epics XL.MCL flow cytometer. For each sample a lymphocyte gate was established on the basis of linear forward and side scatter. NK cells were defined as being CD56+CD3−, while T cells and B cells were the CD3+ (including CD56+CD3+) and CD19+ populations, respectively. Each cell type was expressed as a percentage of total lymphocytes (T + B + NK cells).
Isolation of NK cells
In some experiments PBMC as prepared above were subjected to magnetic-bead fractionation using the ‘MACS’ system (Miltenyi Biotec, Bergisch Gladbach, Germany). PBMC were suspended in phoshate-buffered saline (PBS) containing 10% heat-inactivated fetal calf serum to give 12·5 × 107 cells/ml. Magnetic beads linked to antihuman CD56 were then added (0·02 ml of bead suspension per 107 total cells). After incubation for 15 min at 4°C, the cells were re-suspended in fresh PBS/serum and transfered to an ‘MS’ separation column. The cells not retained in the column by the magnetic field constituted the CD56-negative fraction. On removal of the column from the magnet the CD56-positive fraction was eluted. This fraction was shown by flow cytometry to be 80–95% CD56+. In addition to NK cells this population contains CD56+CD3+ cells (NKT cells). For each group NKT cells formed on average about 15% of total CD56+ cells.
NK cell cytotoxic assay
Natural killing activity against K562 cells was assayed using procedures similar to those described previously [10]. Briefly K562 cells, kindly donated by Dr Roger Watson (Imperial College, London, UK), were propagated in the RPMI medium defined above. K562 cells were labelled with 51chromium (Amersham Biosciences, Chalfont St Giles, UK), and the labelled target cells (104) were placed in 96-well round-bottom plates(Sterilin, Feltham,UK), together with dilutions of the effector cells, in a total volume of 0.15 ml of this same culture medium. The tests were set up in triplicate wells at PBMC to target cell ratios of 40, 20, 10 and 5 or, for separated NK cells, ratios of 5, 2·5, 1·25 and 0·625. A momentary centrifugation of the plates to 400 g was effected before culture was started. After 3 h incubation at 37°C in a humid atmosphere of 5% CO2, plates were centrifuged and 50 µl samples of supernatant were taken for scintillation counting in Lumar plates (Packard, Pangbourne, UK). Spontaneous and total chromium release values were estimated from quadruplicate wells in which the target cells were incubated in medium alone or with 5% Triton X-100. Specific lysis (%) was calculated by the formula:
Chromium release in replicate wells rarely differed by more than 15%.
In most assays of NK cell killing activity the same male control was included, and it became apparent that there was up to 2-fold variation in specific lysis by this control on different days. This appeared to be due to changes in assay sensitivity, possibly connected to the precise stage in the growth cycle of the target cells, rather than to changes in the activity of the control. In order to counter this day to day variation killing activity has been expressed relative to this control – percentage specific lysis for test donor divided by that for the control determined on the same day. In 30% of the experiments, where this control donor was not available, another male or another female control was substituted. To provide appropriate conversion factors the mean values of specific lysis (%) for the two additional controls obtained throughout the study were divided by that for the main control, their activity coming out at 0·70 (male) and 0·83 (female).
Comparisons between normal and low killing activity were generally well expressed at an E:T ratio of 20 (PBMC) or 2·5 (fractionated NK cells) and, unless otherwise indicated, killing activity refers to these cell ratios. Lower ratios were used in some experiments where the number of fractionated cells was limited.
The intrinsic killing activity of the reference controls will not be entirely constant, if only because percentage NK cells shows some variation. However, variation in intrinsic activity is likely to be small compared to that of assay sensitivity. This is supported by the finding that the standard deviation of mean specific lysis was approximately the same proportion of the mean (25%) for each of the controls. Moreover, confirmation that the assay operated satisfactorily is provided by the relatively small spread of the control values (Fig. 1) and the strong correlation found in all groups between killing activity and percentage NK cells(see Results).
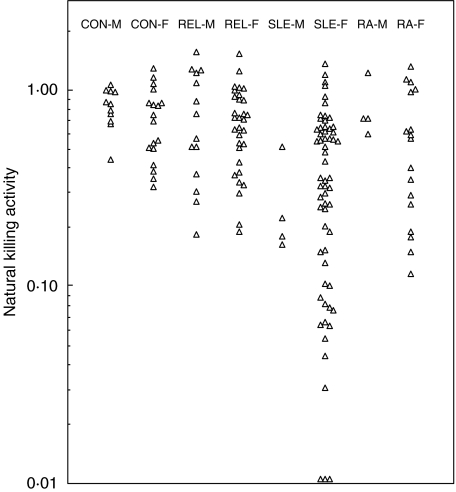
Fig. 1.
Natural killing activity in SLE patients, their first-degree relatives, rheumatoid arthritis patients and controls. Activity is expressed relative to the prime control donor. Assays were set up at a PBMC to target cell ratio of 20. Points on the baseline represent values less than 0·01.
Statistical evaluation of results
Killing activity and percentage NK cells in relatives of SLE patients were compared to control values using the Mann–Whitney test. The same test was used where other group comparisons have been made.The spread of points in two groups were compared by applying the Mann–Whitney test to the absolute values of the deviations of the points from their respective medians (Fig. 4). The probability that the median value of a set of ratios differed from unity was assessed by the Wilcoxon rank sum test (Table 2). Correlation analysis was carried out by Spearman's rank test.
Fig. 4.
Ratios of natural killing activity to percentage NK cells in relative, patient and control groups.
Table 2.
Natural killing activity of purified preparations of NK cells from SLE patients.
| Natural killing activity | ||||
|---|---|---|---|---|
| Expt | Donor type (Clinical score) | NK cells (%) | PBMC | NK cells |
| 1 | CON-M | 17.2 | 1.00 | 0.96 |
| SLE-M (3) | 12.4 (0.72) | 0.17 (0.17) | 0.23 (0.24) | |
| 2 | CON-M | 21.4 | 1.00 | 0.78 |
| SLE-F (2) | 7.7 (0.36) | 0.13 (0.13) | 0.27 (0.35) | |
| 3 | CON-M | 13.3 | 0.70 | 0.59 |
| SLE-F (5) | 5.2 (0.39) | 0.08 (0.12) | 0.27 (0.47) | |
| 4 | CON-F | 7.7 | 1.17 | 1.18 |
| SLE-F (4) | 12.7 (1.65) | 1.37 (1.20) | 0.63 (0.53) | |
| 5 | CON-M | 17.4 | 1.00 | 0.43 |
| SLE-F (2) | 2.5 (0.14) | 0.10 (0.10) | 0.29 (0.68) | |
| 6 | CON-M | 24.6 | 1.00 | 0.35 |
| SLE-F (4) | 5.3 (0.22) | 0.26 (0.27) | 0.25 (0.73) | |
| 7 | CON-F | 22.0 | 1.30 | 1.07 |
| SLE-F (0) | 12.3 (0.56) | 0.65 (0.50) | 0.90 (0.84) | |
| 8 | CON-M | 18.6 | 1.00 | 1.02 |
| SLE-F (4) | 9.5 (0.51) | 0.60 (0.60) | 0.92 (0.90) | |
| 9 | CON-M | 23.0 | 1.00 | 0.55 |
| SLE-F (6) | - | 0.33 (0.33) | 0.51 (0.92) | |
| 10 | CON-F | 12.1 | 0.64 | 0.69 |
| SLE-M (7) | 14.6 (1.21) | 0.22 (0.35) | 0.66 (0.96) | |
| 11 | CON-F | 12.8 | 1.09 | 1.16 |
| SLE-F (0) | 7.0 (0.55) | 1.06 (0.97) | 1.17 (1.01) | |
| 12 | CON-M | 15.0 | 1.00 | 0.50 |
| SLE-F (2) | 9.0 (0.60) | 0.76 (0.76) | 0.55 (1.11) | |
| 13 | CON-M | 13.3 | 0.70 | 0.55 |
| SLE-F (6) | 16.5 (1.24) | 0.93 (1.34) | 0.66 (1.21) | |
SLE patients are shown together with their global disease activity score. Killing activity is given for ratios of 20 (PBMC) or 2.5 (purified NK cells) to 1 target cell, except for NK cell preparations in Expts.3 and 4 (1.25) and Expt. 6 (0.625). All killing activities are expressed relative to the prime control at 20 PBMC : 1 target cell. In each experiment, values of % NK cells in the patient are given with, in parentheses, the ratio of this value to that for the control sample(ratio 1). Analagous ratios are given for killing activity by PBMC(ratio 2) or by purified NK cell preparations (ratio 3). The experiments are listed according to increasing values of ratio 3.
Results
Killing activity in all donor groups – female and male first-degree relatives of SLE patients (REL-F, REL-M), SLE (SLE-F, SLE-M) and rheumatoid arthritis (RA-F, RA-M) patients, controls (CON-F, CON-M) – has been expressed relative to that of a particular male control donor. Results are given on a log scale to highlight where there is low killing activity (Fig. 1).
Low killing activity, activity below the range in the appropriate male or female control group, was seen in subjects from all groups except RA-M. There appear to be two levels of low activity. First there are the moderately low values, defined arbitrarily as less than the control range but greater than 0·11, as shown by some relatives (3 REL-F, 10% of the group; 4 REL-M, 29%), and some SLE (SLE-F 12, 20%; SLE-M 3, 75%) and RA-F (6, 35%) patients. Second, there are extremely low activities (below 0·11) shown only by 15 (25%) female SLE patients.
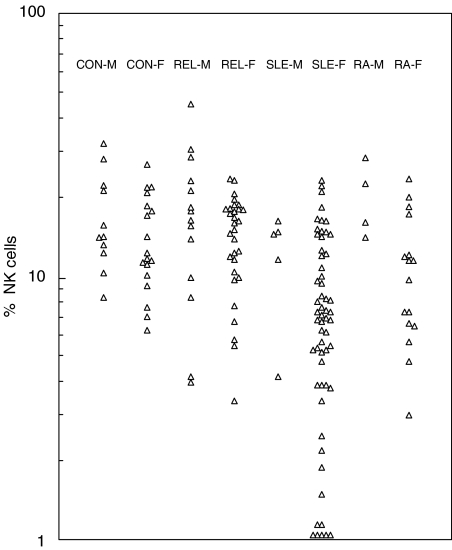
When NK cell levels in these donors, expressed as a percentage of total blood lymphocytes (% NK cells), are plotted a similar distribution of points is obtained (Fig. 2). This is to be expected of course since it is killing by NK cells that is being assayed in Fig. 1. The point is further emphasized by the strong correlations found between killing activity and percentage NK cells in all groups. For example, the group comprising all relatives yields a correlation coefficient r = 0·59 (P < 0·0001).
Fig. 2.
NK cell levels expressed as a percentage of total lymphocytes (% NK cells) in relative, patient and control groups. Points on the baseline represent values of less than 1%.
Mean values with standard deviation for killing activity and percentage NK cells in the different groups are shown in Table 1 together with the corresponding values for percentage T and B cells. Also shown are the mean total lymphocyte and monocyte levels in blood. Despite the low activity shown by some relatives of SLE patients, there were no significant differences overall in killing activity or percentage NK cells between relatives and controls comparing either the combined or individual male/female groups. An unexpected finding was increased percentage B cells (P < 0·01) in REL-F. NK cell parameters and lymphocyte counts were, as would be expected, significantly reduced in SLE-F as compared to CON-F (killing activity and lymphocytes, P < 0·01; % NK cells, P < 0·001), but this was not so for any of these parameters in RA-F. Because of the reduced lymphocyte counts in SLE, the lower percentage NK values imply an even more marked fall in the numbers of circulating NK cells. There was no correlation overall, however, between lymphocyte count and percentage NK cells in SLE patients (r = 0·05, P >0·05).
Table 1.
Mean cell and killing data in relative, patient and control groups.
| Donor groups | n | Natural killing activity | NK cells (%) | B cells (%) | T cells (%) | Lymphocytes (× 10-6/ml) | Monocytes (× 10-6/ml) |
|---|---|---|---|---|---|---|---|
| CON-M | (11) | 0.83 ± 0.18 | 17.5 ± 7.6 | 13.1 ± 3.9 | 69.4 ± 7.0 | 2.29 ± 0.50 | 0.44 ± 0.15 |
| CON-F | (18) | 0.73 ± 0.29 | 14.4 ± 5.9 | 12.2 ± 3.2 | 73.4 ± 5.1 | 1.87 ± 0.46 | 0.42 ± 0.15 |
| REL-M | (14) | 0.78 ± 0.45 | 18.5 ± 11.2 | 15.6 ± 5.7 | 65.9 ± 10.6 | 2.02 ± 0.50 | 0.49 ± 0.17 |
| REL-F | (31) | 0.70 ± 0.32 | 14.5 ± 5.3 | 16.5 ± 5.9 | 69.0 ± 8.2 | 1.92 ± 0.73 | 0.44 ± 0.16 |
| SLE-M | (5) | 0.27 ± 0.17 | 12.4 ± 4.9 | 15.4 ± 10.2 | 72.2 ± 11.0 | 1.35 ± 0.51 | 0.43 ± 0.29 |
| SLE-F | (60) | 0.43 ± 0.33 | 8.3 ± 5.9 | 13.5 ± 8.8 | 78.2 ± 10.6 | 1.40 ± 0.67 | 0.46 ± 0.23 |
| RA-M | (4) | 0.82 ± 0.28 | 20.4 ± 6.5 | 12.7 ± 3.5 | 66.8 ± 4.5 | 1.31 ± 0.51 | 0.45 ± 0.19 |
| RA-F | (17) | 0.59 ± 0.39 | 11.2 ± 6.0 | 13.1 ± 5.9 | 75.7 ± 10.6 | 1.68 ± 0.67 | 0.55 ± 0.33 |
Mean values are given ± SD. Lymphocyte and monocyte blood counts were determined in our associated pathology laboratories. Donor groups are shown with the number of subjects in parentheses. For some groups the number in particular columns was 1 or 2 less than this. Numbering the data columns 1-6 from the left, the lower values of n were as follows: CON-F, n = 16 (columns 5 and 6); REL-F, 30 (2-6); SLE-M, 4 (1,5,6); SLE-F, 58(2-4); RA-F, 16(2-4).
Further analysis of NK cell activity in relatives of SLE patients
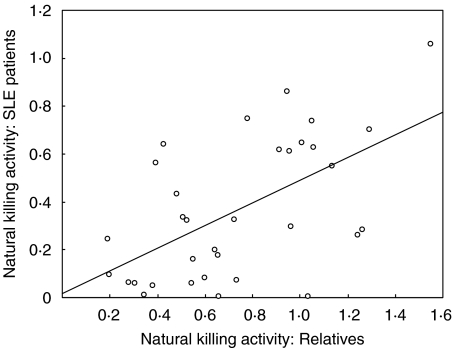
If the low killing activity in SLE patients had a genetic cause, one might expect to see a correlation between activity in relatives and that in their respective patients. Although killing activity in relatives was generally higher than in patients, a significant correlation was indeed found (r = 0·49, P < 0·01) (Fig. 3). There was no such correlation, however, for percentage NK cells (r = 0·05), although the four lowest relative values matched with patients having less than 4% NK cells.
Fig. 3.
Relationship between natural killing activity in SLE patients and in their respective first-degree relatives. Both male and female relatives are included and all but one of the patients were female. Where there was data from more than one relative of the same patient mean values have been used. The best-fit line (linear regression) is shown.
No differences in NK cell activity were apparent between different types of relation. The male relatives with low killing activity were two fathers and two sons, while the three female relatives with low activity were two daughters and one sister. This is consistent with our previous finding that immunological abnormalities associated with SLE are more likely to be found in parents and offspring of patients than in siblings [11].
Is there a defect in the killing ability of NK cells from SLE patients?
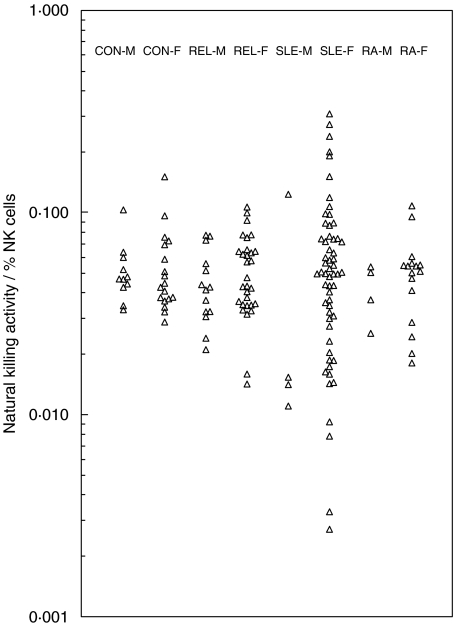
Values for the ratio of killing activity to percentage NK cells are shown in Fig. 4. Where a particular value is below the range obtained for the controls this suggests a defect in the killing capacity of the cells. In SLE-F there were 14 patients with such values though this is only half the number of low killing female patients in Fig. 1. There were also several patients with ratios above the control range. There were three male SLE patients below the range as well as some relatives and RA patients. The 15 lowest values in Fig. 4 were all for subjects who had low killing activity. Although there is no significant difference between the median values for CON-F and SLE-F, the spread of points in the two groups is clearly different (P < 0·05).
Most of the variation in killing activity/% NK cells within the control groups appears to result from differences in the level of percentage NK cells, such that the lower values of the ratio correspond to individuals with higher percentage NK. This implies that the killing assay falls off slightly in efficiency as NK cell levels rise. It may explain the high values in the SLE group which all occurred at percentage NK values below the control range. PBMC used for the killing assay contain monocytes as well as lymphocytes (Table 1). A similar analysis to that in Fig. 4 has therefore been carried out expressing NK cells as a percentage of PBMC, and this gives essentially the same result. Loss of monocytes due to adherence is likely to occur on handling the cell populations.
More direct evidence for a killing defect has been sought by comparing directly purified preparations of NK cells from SLE patients and controls (Table 2). For each experiment three ratios are given – the ratio of the patient to control value of percentage NK cells (ratio 1) and the corresponding ratio for killing activity in PBMC (ratio 2) and in purified cells (ratio 3). The median value of ratio 3 is 0·84, and this is significantly less than 1 (P < 0·025) demonstrating reduced killing activity overall by NK cells in SLE patients. This does not of course mean that there is an NK cell defect in all the patients. For the first three experiments ratio 3 is less than 0·5, providing strong evidence of defective killing ability in these patients. This observation is confirmed by the low values of killing activity/% NK cells obtained for these patients in Fig. 4, respectively, 0·011, 0·017 and 0·016. With ratio 3 in the range 0·5–0·75 there would appear to be a defect, though this is not supported in Fig. 4. In seven experiments ratio 3 was more than 0·75, indicating at most a small effect. In one of these (Expt 10) a low value was found for the patient in Fig. 4(0·015), a problem with PBMC killing appeared to be righted on purification of the cells.
As would be expected, in experiments where there is no evidence for a defect in killing, ratios 1 and 2 are generally in step. This also applied to two experiments comparing female relatives to controls: ratio 1, 1·09 and 2·27; ratio 2, 1·21 and 2·12; ratio 3, 0·97 and 0·95.
Influence of disease activity and drug treatment
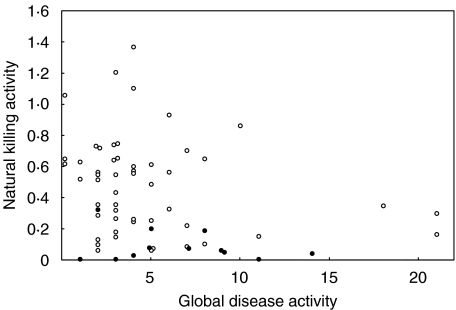
There was a significant inverse correlation between global SLE disease activity score and natural killing activity (r =−0·29, P < 0·025)(Fig. 5). All six patients with scores over 10 had low killing values. Similar patterns were obtained when global score was plotted against percentage NK cells or the numbers of circulating NK cells, though only in the latter plot was a significant correlation observed (r =−0·37, P < 0·005).
Fig. 5.
Relationship between natural killing activity in SLE patients and their global disease activity scores. All SLE patients are included – those not receiving azathioprine (○); those on azathioprine (•).
The disease activity score is based on activity in different body systems and results were analysed to see whether the involvement of particular systems was linked to low natural killing. Mean killing activity was significantly reduced in the affected group as compared with the remaining unaffected patients for neurological (n = 7, P < 0·05) and vasculitis (n = 16, P = 0·05) systems. For renal (n = 17) a similar reduction did not reach significance (P = 0·06). These findings are difficult to interprete, however, since the mean disease activity in these groups was relatively high and some patients were involved in more than one of the groups.
Values of the ratio of killing activity to percentage NK cells did not correlate with global disease activity (r =−0·03, P >0·05), though the mean global activity ± SD for the 17 patients with ratios below the control range (6·4 ± 5·1) was a little above that for the remaining patients (4·6 ± 4·0). The percentage of patients in the 17 with a particular system involvement was from 4 to 11% higher than in the patient group as a whole, except for cardiovascular/respiratory (n = 11) where there was a fall of 6%.
The SLE patients may be divided into three overlapping groups according to whether they were receiving azathioprine (AZA) (n = 12), prednisolone (n = 39) or hydroxychloroquine (HCQ) (n = 35) either alone or in combination. The HCQ group comprised the complete range of patient values for killing activity (mean ± SD: 0·47 ± 0·31). The steroid group was similar though the highest killing value was 0·87 (0·33 ± 0·25). By contrast the AZA group (see Fig. 5) had a mean killing activity of only 0·09 ± 0·10, representing a highly significant reduction as compared to the rest of the patients (0·49 ± 0·31, P < 0·0001). All 12 patients on AZA had low killing activity, nine of them extremely low. The mean for the ‘no drug’ group (n = 8) was 0·62 ± 0·40.
Analysis of NK cells gave a similar story. Mean value for the AZA group was 2·9 ± 3·1% as compared to 9·9 ± 5·6% in the remaining patients (P < 0·0001). B cell (%) was also down from 15·3 ± 8·6 to 6·3 ± 5·7 in the presence of AZA (P = 0·0002), as was blood lymphocytes though less markedly from 1·46 ± 0·62 to 1·16 ± 0·79 × 106/ml (P > 0·05). The RA-F patient receiving AZA gave low values of 0·19 (killing) and 6·7 (% NK cells).
Although the seven lowest killing values were in the AZA group, there were many SLE patients not on AZA but with low killing activity (Fig. 5), 13 moderately low and six extremely low. Two of the former group and one of the latter were not receiving any of the drugs. Similarly there were many patients with low percentage NK cells who were not receiving AZA. SLE-F patients not receiving AZA had significantly lower values than CON-F for killing activity (P < 0·02) and percentage NK cells (P < 0·01). Of the 17 patients below the control range in Fig. 4 only six were on AZA. If the one patient in Table 2 on AZA is excluded (Expt. 3), significantly reduced killing activity may still be demonstrated(P < 0·05).
Discussion
We have found that the reduced natural killing activity associated with many SLE and RA patients falls in two ranges. First, a moderate reduction seen in 20% of the SLE-F group, 35% of RA-F and in 3 out of the 4 SLE-M; secondly, a more marked reduction confined to SLE-F (25%).
Moderately reduced killing activity was also a feature of some first-degree relatives of SLE patients – REL-M (29%), REL-F (10%). Moreover, there was a strong correlation between killing activity in SLE patients and that in their relatives. Bearing in mind that these relatives are healthy and have a less than 3% chance of developing SLE [12], this indicates that at least the moderate reduction in activity in patients is not a consequence of the disease process or of drug treatment. Rather there would appear to be a genetic, or possibly environmental, cause for the lowered values. To the best of our knowledge this is the first time NK cell activity has been assessed in relatives of SLE patients, apart from a study by Stohl et al. [13] that found evidence of low activity in healthy monozygotic co-twins of patients but which is difficult to interpret since blood cells underwent T cell activation and culture for several days prior to the killing assay. Our findings in rheumatoid arthritis support the view that low NK cell activity is also a feature of other autoimmune rheumatic diseases [7].
As would be expected, killing activity strongly correlated in all groups with the presence of NK cells expressed as a percentage of total blood lymphocytes. Two approaches were employed to look at the question of whether reduced killing activity is due simply to reduced numbers of NK cells or involves, in addition, a defect in killing function by the individual cells – analysis of the ratio of natural killing activity to percentage NK cells and the killing assays carried out with purified NK cells. The former suggested that about 25% of the SLE patients did have such a defect while the latter put the proportion at nearer 50%. The ratio analysis may underestimate the percentage because of increased assay efficiency at low levels of NK cells, as is often the case in SLE patients. It seems possible that some SLE relatives and RA patients may also have a defect.
The purified NK cell preparations used in the above experiments also contained CD56+ T cells (NKT cells), but this should not affect interpretation of the results. On average, in blood from SLE patients or controls, these amount to about 15% of total CD56+ cells. Preliminary experiments have been carried out to separate CD56+CD3− cells from CD56+CD3+ cells, involving removal of anti-CD56 magnetic beads from the combined population followed by a second fractionation with anti-CD3 beads. Little or no killing activity was associated with the T cell fraction. We have recently obtained evidence that NKT cells may play a role in controlling IgG levels in SLE patients and their relatives, and they will be the focus of a future publication.
We believe this is the first published report in which ratios of natural killing activity to percentage NK cells have been used to assess the killing capacity of the cells in SLE. Evidence for defective natural killing in childhood SLE using purified NK cells has been obtained previously by Yabuhara et al. [2]. In addition defective killing is implied by the results of single cell assays, and these further suggest the defect lies in the lytic capacity of the cells rather than in their ability to bind to target cells [2,6].
Do drugs used in treatment of SLE influence the numbers or killing activity of NK cells?
Neither HCQ nor prednisolone appear to affect NK cells except that top levels of killing activity were not seen in patients on steroids. However, all 12 SLE patients on AZA had low killing activity, in nine cases in the very low range. NK and B cell percentages were also greatly reduced, as well as there being a degree of lymphopaenia. While AZA is used for some of the more severly affected patients, these findings strongly suggest a drug-induced action on numbers of NK cells as documented previously in patients with rheumatoid arthritis [14,15] and in transplantation [16]. It may inhibit the maturation of an NK cell precursor [17].
The point must be strongly emphasized that the fall in percentage NK cells in SLE and the apparent defect in killing are quite separate from any action of AZA. This separation is shown by the data from the many patients (as well as some relatives) who were not on this drug. On the other hand, over half the patients with extremely low killing activity may owe their placement in that group to AZA.
If an NK cell deficiency is a factor in the pathogenesis of SLE, one might expect to find an inverse correlation between NK cell parameters and clinical disease activity. This was found for killing activity but could not be established for percentage NK cells or the ratio of killing activity to percentage NK cells. A defect in killing activity at the cellular level may nevertheless explain why disease activity correlated more strongly with killing activity than with percentage NK cells. Even stronger inverse correlation was seen with the number of circulating NK cells, presumably because this parameter combines two disease-associated factors, total lymphocyte count as well as percentage NK cells.
Drug treatment of patients creates a problem in studying associations with clinical disease activity. Drugs may influence NK cells directly, as we have seen with AZA, or they may affect disease activity without necessarily affecting the cells. If the patients on AZA are removed from the analysis, the associations with disease activity no longer reached significance. However, on occasions in the past there has been the opportunity to make observations in the absence of drugs and these suggest that percentage NK cells and killing activity are depressed in both remission and active disease, though more so in the active state [2,4].
What genes might be involved in an NK cell abnormality in SLE?
The only identified gene with polymorphisms associated with SLE that appears relevant to NK cells is that for CD16 or FCγRIIIA [18]. This receptor is present on the surface of NK cells and the associated variant is of low binding capacity. While it is not obvious how it might be involved in antibody-independent killing, variants of this molecule may be associated with severe numerical deficiency of the cells [19].
NK cells may protect against autoimmunity by virtue of their killing function [20], but in SLE it is perhaps more likely that a parallel dysfunction in their cytokine production is encouraging the antibody response to nucleosomal antigens that is characteristic of the disease [21]. Such an NK cell abnormality could be related to the over-expression of IL-10 which is seen in SLE [22] and this observation highlights a possible genetic association of SLE with IL-10 polymorphism [23].
IL-10 has the ability to promote antibody production but also to suppress IL-2 production by T cells [24]. Several groups have reported that NK killing activity in SLE may be enhanced by culture of blood mononuclear cells in the presence of IL-2 [7,25]. At reduced IL-2 levels NK cells may be unable to provide cytokines necessary for the generation of T cells able to regulate antibody production [26,27]. In view of our recent data, these regulatory cells could be CD56+ T cells.
In conclusion, the finding of low levels of natural killing activity in first-degree relatives of SLE patients and the correlation with patient activity strengthens the view that NK cell deficiency is important in the pathogenesis of the disease and is not secondary to the disease process or drug treatment. This deficiency seems to involve both depressed NK cell numbers and, in many patients, a defect in the killing ability of the cells.
Acknowledgments
We are most grateful for advice from Mehnaaz Lomas (killing assay), Gill Thompson (flow cytometry), Becca Asquith and Pascale Kropf (computing) and Elena Kulinskaya (statistics). We would also like to thank Richard Rees for help in obtaining patient blood samples, Alun Davies for providing data from the Diagnostic Laboratories at St Mary's Hospital and Charles Bangham and Keith Gould for helpful discussion. The work was supported by LUPUS UK, NW Nicholls Trust of St Mary's Hospital and the TR Golden Charitable Trust.
References
- 1.Erkeller-Yuksel F, Hulstaart F, Hannet I, Isenberg D, Lydyard P. Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus. 1993;2:227–31. doi: 10.1177/096120339300200404. [DOI] [PubMed] [Google Scholar]
- 2.Yabuhara A, Yang FC, Nakazawa T, et al. A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. J Rheumatol. 1996;23:171–7. [PubMed] [Google Scholar]
- 3.Ewan PW, Barrett HM, Pusey CD. Defective natural killer (NK) and killer (K) cell function in systemic lupus erythematosus. J Clin Lab Immunol. 1983;10:71–6. [PubMed] [Google Scholar]
- 4.Blaszczyk M, Majewski S, Wasik M, Chorzelski T, Jablonska S. Natural killer cell activity of peripheral blood mononuclear cells from patients with various forms of lupus erythematosus. Br J Dermatol. 1987;117:709–14. doi: 10.1111/j.1365-2133.1987.tb07350.x. [DOI] [PubMed] [Google Scholar]
- 5.Combe B, Pope R, Darnell B, Talal N. Modulation of natural killer cell activity in the rheumatoid joint and peripheral blood. Scand J Immunol. 1984;20:551–8. doi: 10.1111/j.1365-3083.1984.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 6.Katz P, Zaytoun AM, Lee JH, Panush RS, Longley S. Abnormal natural killer cell activity in systemic lupus erythematosus: an intrinsic defect in the lytic event. J Immunol. 1982;129:1966–71. [PubMed] [Google Scholar]
- 7.Gonzalez-Amaro R, Alcocer-Varela J, Alarcon-Segovia D. Natural killer cell activity in the systemic connective tissue diseases. J Rheumatol. 1988;15:1223–8. [PubMed] [Google Scholar]
- 8.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 9.Hay EM, Bacon PA, Gordon C, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Quart J Med. 1993;8:447–58. [PubMed] [Google Scholar]
- 10.Wigzell H, Ramstedt U. Natural killer cells. In: Weir DM, Herzenberg LA, Blackwell C, Herzenberg LA, editors. Handbook of Experimental Immunology. Oxford: Blackwell Scientific Publications; 1986. pp. 60·1–60·11. [Google Scholar]
- 11.Clark J, Bourne T, Salaman MR, Seifert MH, Isenberg DA. B lymphocyte hyperactivity in families of patients with systemic lupus erythematosus. J Autoimmun. 1996;9:59–65. doi: 10.1006/jaut.1996.0008. [DOI] [PubMed] [Google Scholar]
- 12.Tan FK, Arnett FC. The genetics of lupus. Curr Opin Rheumatol. 1998;10:399–408. doi: 10.1097/00002281-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Stohl W, Elliott JE, Hamilton AS, Deapen DM, Mack TM, Horwitz DA. Impaired recovery and cytolytic function of CD56+ T and non-T cells in systemic lupus erythematosus following in vitro polyclonal T cell stimulation. Studies in unselected patients and monozygotic disease-discordant twins. Arthritis Rheum. 1996;39:1840–51. doi: 10.1002/art.1780391110. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen BK, Beyer JM. A longitudinal study of the influence of azathioprine on natural killer cell activity. Allergy. 1986;41:286–9. doi: 10.1111/j.1398-9995.1986.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 15.Cseuz R, Panayi GS. The inhibition of NK cell function by azathioprine during the treatment of patients with rheumatoid arthritis. Br J Rheumatol. 1990;29:358–62. doi: 10.1093/rheumatology/29.5.358. [DOI] [PubMed] [Google Scholar]
- 16.Vaessen LM, van Miert PP, van Gelder T, Ijzermans JN, Weimar W. Reassuring effect of pravastatin on natural killer cell activity in stable renal transplant patients. Transplantation. 2001;71:1175–9. doi: 10.1097/00007890-200104270-00028. [DOI] [PubMed] [Google Scholar]
- 17.El-Azhary RA. Azathioprine: current status and future considerations. Int J Dermatol. 2003;42:335–41. doi: 10.1046/j.1365-4362.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JA, Moser KL, Harley JB. The genetics of systemic lupus erythematosus: putting the pieces together. Genes Immun. 2002;3(Suppl 1):S71–85. doi: 10.1038/sj.gene.6363885. [DOI] [PubMed] [Google Scholar]
- 19.Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T. Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II) Clin Exp Immunol. 1996;103:408–13. doi: 10.1111/j.1365-2249.1996.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxter AG, Smyth MJ. The role of NK cells in autoimmune disease. Autoimmunity. 2002;35:1–14. doi: 10.1080/08916930290005864. [DOI] [PubMed] [Google Scholar]
- 21.Mohan C, Datta SK. Lupus: key pathogenic mechanisms and contributing factors. Clin Immunol Immunopathol. 1995;77:209–20. doi: 10.1006/clin.1995.1146. [DOI] [PubMed] [Google Scholar]
- 22.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Alfonso S, Giordano M, Mellai M, et al. Association tests with systemic lupus erythematosus (SLE) of IL10 markers indicate a direct involvement of a CA repeat in the 5′ regulatory region. Genes Immun. 2002;3:454–63. doi: 10.1038/sj.gene.6363928. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Struyf NJ, Snoeck HW, Bridts CH, De Clerck LS, Stevens WJ. Natural killer cell activity in Sjogren's syndrome and systemic lupus erythematosus: stimulation with interferons and interleukin-2 and correlation with immune complexes. Ann Rheum Dis. 1990;49:690–3. doi: 10.1136/ard.49.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz DA, Gray JD, Ohtsuka K, Hirokawa M, Takahashi T. The immunoregulatory effects of NK cells: the role of TGF-beta and implications for autoimmunity. Immunol Today. 1997;18:538–42. doi: 10.1016/s0167-5699(97)01149-3. [DOI] [PubMed] [Google Scholar]
- 27.Ohtsuka K, Gray JD, Stimmler MM, Toro B, Horwitz DA. Decreased production of TGF-beta by lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1998;160:2539–45. [PubMed] [Google Scholar]