Abstract
Accumulation of plasma cells in the synovium is one of the diagnostic hallmarks in the histopathological manifestations of rheumatoid arthritis (RA). This seems to be prominent even prior to significant B cell infiltration and/or formation of lymphoid follicles in the synovium. To clarify the mechanism of early plasma cell accumulation, we examined in situ expression of chemokines and their receptors using synovial targeting biopsy specimens, which were obtained under arthroscopy from early RA patients. By immunohistochemical staining, plasma cells were found to express a chemokine receptor CXCR3, while synovial fibroblasts in the synovial sublining regions expressed its ligand, Mig/CXCL9. By reverse transcription-polymerase chain reaction (RT-PCR), using targeted lesions of synovial tissues obtained by laser capture microdissection, expression levels of Mig/CXCL9 in the synovial sublining regions were remarkably high and were likely to be associated with interferon (IFN)-γ expression. Furthermore, cultured synovial fibroblasts were confirmed to produce Mig/CXCL9 upon stimulation with IFN-γ. Our results indicate that in the early stage of RA, plasma cells expressing CXCR3 may be recruited directly from the circulation into the synovial sublining regions by its ligand, Mig/CXCL9, produced by synovial fibroblasts.
Keywords: chemokine receptors, chemokines, gene expression, histopathology, microdissection
Introduction
The histopathological manifestations of synovitis in rheumatoid arthritis (RA) involve several diagnostic hallmarks such as (i) multi-layered synovial lining tissues, (ii) a palisading structure of the intimal lining layer, (iii) accumulation of mononuclear cells such as CD4+ T cells, B cells, plasma cells and macrophages in the sublining regions and (iv) formation of ectopic lymphoid follicles [1,2]. Mesenchymoid transformation and fibrinoid degeneration of synovial tissues are also histopathological features characteristic of RA [3,4]. Among them, the accumulation of plasma cells is considered to be prevalent in long-standing RA as the result of B cell differentiation to plasma cells in situ following B cell infiltration and/or formation of lymphoid follicles in the synovium [5,6].
Early RA is a clinical term referring to the early stage of RA used for predicting the eventual progression stage of RA. The American College of Rheumatology (ACR) 1987 classification criteria for RA [7] have often been used as a diagnostic tool in patients with recent-onset arthritis. However, these criteria were developed in a population of patients selected according to their disease status to classify rather than to diagnose RA. Thus, their diagnostic ability in early arthritis is probably not optimal. The histopathology of synovitis in the early stages of RA is a challenging problem, both from diagnostic and mechanistic points of view [8]. Immunopathological studies of synovial tissues from patients with early RA have, in general, suggested quantitative rather than qualitative differences between different stages of synovitis [9,10]. However, in our recent study of synovial tissues from patients with early RA, we demonstrated the presence of many plasma cells most notably in the sublining regions, without any substantial infiltration of B cells and/or formation of lymphoid follicles [11]. This finding led us to speculate that synovial plasma cells in the early stages of RA may not be necessarily derived from B cells differentiated in the synovium in situ. Rather, plasma cells or plasmoblasts in the circulation may be directly recruited into the synovium in the early stages of RA. Thus, this might be an important qualitative, not quantitative, difference between the early and late stages of RA. If this is the case, synovial cells in the early stages of RA may produce some chemokines in situ that may attract circulating plasma cells.
Based on this hypothesis, we examined in situ expression of some chemokines and their receptors in synovium tissues from the patients with early RA. Here we demonstrate that infiltrating plasma cells in the synovium consistently express CXCR3, while synovial fibroblasts in the sublining regions produce its ligand, Mig/CXCL9. These findings may support our hypothesis that in the early stages of RA, plasma cells or their immediate precursors are directly recruited into the synovium by locally produced chemokines rather than derived from B cells differentiating in situ as is the case in the advanced stages of RA. This may mean that antibodies produced in rheumatoid synovial tissues in the early stages of RA reflect systemic rather than local immune responses.
Materials and methods
Antibodies
Monoclonal antibodies against CD3, CD20, CD38, CD68, CD138, Mig/CXCL9 and CXCR3 were purchased from Dako Japan, Kyoto, Japan. Goat polyclonal anti-MEC/CCL28 was also purchased from Dako Japan. Rabbit polyclonal anti-CCR10 was purchased from Biocarta, San Diego, CA, USA. Isotype-matched control immunoglobulins were purchased from Dako Japan.
Patients with early RA
We examined nine patients with early RA (duration of disease prior to diagnosis less than 1 year) (case numbers; 1–9 in Table 1). All patients had arthritis of the knee and fulfilled the ACR 1987 revised criteria for the classification of RA [7]. Additionally, synovial tissues of the knee joints obtained in total knee arthropathy from two patients with RA in an advanced stage (case numbers 10 and 11, males, RF positive, disease duration more than 5 years) were used in this study, of which histopathological manifestations showed typical hallmarks of RA including the formation of ectopic lymphoid follicles. We obtained a written informed consent from each patient before they entered the study. All patients were seen and treated at the Center for Rheumatic Diseases, Matsuyama Red Cross Hospital.
Table 1.
Cases of early rheumatoid arthritis (RA) and their histological features of the synovial lining regions in knee joints.
| Immunostaining positive cells* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biopsy after onset | Lymphocyte infiltrating | Plasma cell * | T cell | Plasma cells | B cells | Macrophages | Remarkst † | |||||
| Case | Age | Sex | RF | (months) | patterns | (H&E) | (CD3) | (CD38) | (CD138) | (CD20) | (CD68) | (theraph, etc.) |
| 1 | 50 | F | + | 2 | Diffuse | − | + | + | − | − | + + | NSAIDs |
| 2 | 34 | F | − | 4 | Diffuse | + | + | + | − | − | + | NSAIDs |
| 3 | 34 | F | + | 3 | Diffuse | + + | + + | + + | + + | + | + + + | NSAIDs |
| 4 | 77 | F | − | 2 | Diffuse+ aggregate | + + | + + + | + + | + | + | + + + | NSAIDs |
| 5 | 50 | M | − | 4 | Diffuse | + + | + | + + | − | + | + | |
| 6 | 37 | F | + | 7 | Diffuse | + | + | + + | − | + | + | |
| 7 | 61 | F | − | 2 | Diffuse | + | + | + + | + | + | + + | PSL (5 mg) |
| 8 | 25 | F | − | 12 | Diffuse | + + + | + | + | + + + | + + | + + | NSAIDs |
| 9 | 60 | F | + | 4 | Diffuse+ aggregate | + + + | + | + + + | + + + | + + + | + + | NSAIDs |
Relative amounts of infiltrating cells of lymphocyte subsets and macrophages between the cases examined were scored; −; none, + ; mild, + + ; moderate, and + + + ; severe.
NSAIDs; non-steridal anti-inflammatory drugs, PSL; prednisolone.
Targeting biopsy
Biopsies of synovial tissues of the knee joints from the patients with early RA were performed under arthroscopy within one year (2–12 months) after the clinical onset (Table 1). The synovial specimens that showed macroscopically a white cotton appearance and were oedematous and partially villous and rich in vascularized and proliferative lesions were taken selectively under arthroscopy. At least more than five pieces were taken from each case and subjected to the following studies.
Histopathological and immunohistochemical examinations
A half piece of each synovial specimen was fixed with 10% formalin in 0·01 mol/l phosphate buffer pH 7·2 and embedded in paraffin. Serial sections 2–3 µm thick were prepared. Sections were stained with haematoxylin and eosin (H&E) for histological examination by light microscopy. Immunohistochemical staining was performed as described previously [12,13]. In brief, deparaffinized tissue sections were first treated with microwave three times for 5 min each in Target Retrieval Solution (Dako, Carpinteria, CA, USA). Sections were then incubated at 4°C overnight with primary antibodies. Isotype-matched immunoglobulins were used as negative controls. Sections were next incubated with appropriate biotin-, fluoroscein isothiocyanate (FITC)- or rodamin-labelled second antibodies (Vector Laboratories, Burlingame, CA, USA), and finally treated were with the Vectastain ABC/HRP kit (Vector) according to the manufacturer's instructions. Peroxidase development was performed using diaminobenzidine and H2O2, resulting in dark brown products in positive cells. Sections were counterstained with haematoxylin before dehydration and mounted in nonaqueous mounting medium (Muto Pure Chemicals, Ltd, Tokyo, Japan). Unless stated otherwise, all immunostainings performed using isotype-matched control immunoglobulins were negative by this procedure.
Laser capture microdissection (LCM)
The residual half piece of each synovial specimen was frozen immediately in optimal cutting temperature (OCT) compound (Sakura Co. Ltd, Tokyo, Japan), and 7 µm thick cryostat sections were prepared and mounted on a 1·35 µm thick polyethylene membranes (PALM, Wolfratshausen, Germany). The sections were fixed immediately for 3 min with cold acetone, followed by 70% ethanol for 1 min. Then, they were stained rapidly with HistoGene™ Staining Solution (TaKaRa Co. Ltd, Tokyo, Japan) for 45 s. After washing in distilled water, they were dehydrated with 100% ethanol and air-dried with a fan for 3 min.
Laser capture microdissection (LCM) was performed to collect target regions from a specimen by cutting with a laser beam using a Robot-Microbeam (PALM) and an inverted microscope (Carl Zeiss, Oberkochem, Germany) according to the method described previously [14]. In brief, a specimen was set on a computer-controlled microscope stage and observed under a CCD camera from the upper side. By displaying the image, targeted regions were selected using the computer mouse. By tracing around each region, it was dissected together with the thin membrane in the underside of the specimen by a laser microbeam through the objective lens, and then catapulted by a single precisely aimed laser shot into a microcentrifuge tube (0·6 ml) held by the micromanipulater. We dissected more than 100 regions from each sample and pooled them for RNA extraction.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the samples collected by LCM using Trizol reagent (Life Technologies, Rockville, MD, USA) according to the manufacturer's procedure. First-strand cDNA was made from total RNA using the SuperScript preamplification system (Life Technologies) with random hexamers. For PCR, 0·5 ng of each first-strand cDNA was amplified by using the following primers: +5′-CATGCTGGT GAGCCAAGCAGTTTGAA-3′ and −5′-CACTTCTGTGGG GTGTTGGGGACAAG-3′ for Mig/CXCL9; +5′-TGCAAGCC AATTTTGTCCACGTGTTG-3′ and −5′-GCAGCTGATTT GGTGACCATCATTGG-3′ for IP-10/CXCL10; +5′-AGAGG ACGCTGTCTTTGCAT-3′ and −5′-GTCCTTTCACCCACC TTTCA-3′ for I-TAC/CXCL11; +5′-CCCTCTGTGAGATCC GTCTTTGGCCT-3′ and −5′-TCTGATTGGAACCTGAACC CCTGCTG-3′ for SDF-1/CXCL12; +5′-AGAAGCCATACT TCCCATTGC-3′ and −5′-AGCTTGCACTTTCATCCACTG-3′ for MEC/CCL28; +5′-CAACGCCACCCACTGCCAATA CAA-3′ and −5′-CAGGCGCAAGAGCAGCATCCACA-3′ for CXCR3; +5′-ATCTTCCTGCCCACCATCTACTCCATCATC-3′ and −5′-ATCCAGACGCCAACATAGACCACCTTTTCA-3′ for CXCR4; +5′-GCAGAGCCAAATTGTCTCCT-3′ and −5′-ATGCTCTTCGACCTCGAAAC-3′ for IFN-γ; +5′-GCC AAGGTCATCCATGACAACTTTGG-3′ and −5′-GCCTGCT TCACCACCTTCTTGATGTC-3′ for glyceraldehydes-3-phosphate dehydrogenase (GAPDH) [12,13]. Amplification conditions were denaturation at 94°C for 30 s (5 min for the first cycle), annealing for 30 s at 62°C for Mig/CXCL9, IP-10/CXCL10, I-TAC/CXCL11, SDF-1/CXCL12, CXCR4 and interferon (IFN)-γ, 52°C for MEC/CCL28, 66°C for CXCR3 and 60°C for GAPDH, and extension at 72°C for 30 s (7 min for the last cycle) for 38 cycles for the chemokines and chemokine receptors, and 30 cycles for GAPDH. Amplification products (10 µl each) were electrophoretically separated on 2·0% agarose gels and stained with ethidium bromide.
Cultures
THP-1, a human monocytic leukaemia cell line [15], HL60, a human promyelocytic leukaemia cell line [16] and HUT102, a human T cell infected with human leukaemia virus type 1 (HTLV-1) [17], were cultured in RPMI-1640 (Sigma) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies, Auckland, New Zealand) and 100 µg/ml of streptomycin and 100 U/ml of penicillin. The primary culture of synovial fibroblasts was performed according to an explant method. In brief, up to rice-sized small pieces of synovial villi obtained from the advanced RA patient (case 10), were put on fibronectin (Sigma Chemical Co., St Louis, MO, USA)-coated culture dishes (Costar, New York, NY, USA), semidried for a few minutes to fix them to the culture plate, and cultured at 37°C in the RPMI-1640 medium. Human umbilical vein endothelial cells (HUVECs) and culture medium were purchased from Clonetics (Walkersville, MD, USA). HUVECs were grown on fibronectin (Sigma)-coated flasks (Costar). When synovial fibroblasts or HUVECs were semiconfluent, they were passaged after trypsinization and used at the third to sixth passages.
Cytokine stimulation of cultured cells
Cells in culture were treated with cytokines; native human IFN-γ (Ohtsuka Pharmaceuitical Co., Tokuchima, Japan) or recombinant human tumour necrosis factor (TNF)-α (Mitsubishi Chemical Co., Tokyo, Japan) at indicated concentrations. The expression levels of chemokines and/or their receptors by these cells were analysed by RT-PCR. First-strand cDNA was made from 2·0 µg of total RNA. For semiquantitative PCR, 20 ng of each first-strand cDNA was amplified with the primers described above. The amplification cycles were 34 for chemokines, 36 for chemokine receptors and 27 for GAPDH.
Results
Accumulation of plasma cells in the synovial sublining regions
By using targeting biopsy, we obtained synovial tissue specimens from nine patients with early RA. Upon histological examinations, the tissue specimens manifested a variable degree of synovitis. The lesions were, however, limited mostly to the synovial lining tissues with macrophages forming a palisading structure in the intimal lining layers and increased synovial fibroblasts in the sublining regions (Fig. 1a). Importantly, a significant number of plasma cells were observed in the sublining regions except case 1, even though we observed no lymphoid follicles in any of the cases and a few lymphocyte aggregates only in cases 4 and 9 (Fig. 1a,b).
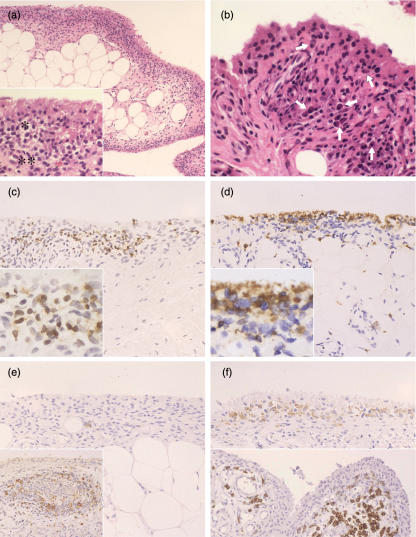
Fig. 1.
Representative histopathological manifestations of early RA synovium. (a) Inflammatory lesions are limited in the synovial lining tissue, being manifested by the proliferation of synovial lining layers. A mononuclear infiltration, composed of macrophages, lymphocytes and plasma cells, a palisading structure of the intimal lining layer (*) and increase of synovial fibroblasts in the sublining region (**) are prominent [inset: a higher magnification of (a)] (case 3) [haematoxylin and eosin (H&E) staining]. (b) A large number of plasma cells (arrows) are present in the sublining region with macrophages, lymphocytes and fibroblasts (case 8) (H& staining) (c) CD3+ T cells are localized in the sublining region. They are distributed diffusely among mononuclear cells (inset) (case 3) (immunostaining) (d) CD68+ macrophages are localized mainly in the intimal lining layer, which show a palisading structure (inset), partly scattering in the sublining region (case 7) (immunostaining) (e) There is only a small number of CD20+ B cells, which are localized in the pericapillary region in the synovial sublining region (case 4). A positive control for CD20 shows a remarkable staining in the mantle zone of lymphoid follicle in an advanced stage of rheumatoid arthritis (RA) synovium (case 11) (inset). (immunostaining). (f) CD38+ cells accumulate in the sublining region, many of which are plasma cells (upper panel). CD138+ cells highly specific for matured plasma cells are localized in the perivascular regions (lower panel) (case 4) (immunostaining)
Immunohistochemical studies in early RA synovium revealed that the majority of lymphocytes infiltrating synovial tissues were CD3+ T cells, almost all of which were localized in the sublining regions (Fig. 1c). Many CD68+ macrophages were present mainly in the intimal lining layers, forming a palisading structure (Fig. 1d). On the other hand, only a few CD20+ B cells were observed near the venules in the sublining regions (Fig. 1e). However, we observed many plasma cells in the sublining regions as revealed by staining for CD38 (Fig. 1f, upper panel). Staining for another plasma cell marker CD138, we also observed many positive cells in the sublining regions, especially in the perivascular regions (Fig. 1f, lower panel). However, we did not pursue the relationship between CD38+ cells and CD138+ cells further because of the technical difficulties. These findings are summarized in Table 1.
Immunohistochemical staining of CXCR3 in plasma cells in the synovial sublining regions
It is known that plasma cells home to bone marrow and various mucosal tissues via constitutive expression of chemokine receptors such as CXCR4, CXCR6 and CCR10 [18,19]. In mice, plasma cells were also shown to transiently express CXCR3 that enabled them to infiltrate into inflamed tissues [20]. To investigate the role of chemokine receptors in plasma cell accumulation in early RA tissues, we first performed immunohistochemical staining of CCR10 in the synovial tissues using polyclonal anti-CCR10. Due to the high non-specific reactivity of anti-CCR10 with the synovial tissues, however, we were unable to demonstrate clearly CCR10 expression in infiltrating plasma cells (data not shown). Given the possible role of CXCR3 in inflammatory accumulation of plasma cells [20], we next performed staining of CXCR3 using a monoclonal anti-CXCR3. We observed significant immunohistochemical staining of CXCR3 in cells infiltrating synovial sublining regions (Fig. 2a). We then performed double staining of CXCR3 with the plasma cell marker CD138. We found that CXCR3 co-localized mainly with CD138 (Fig. 2a, right lower, panel).
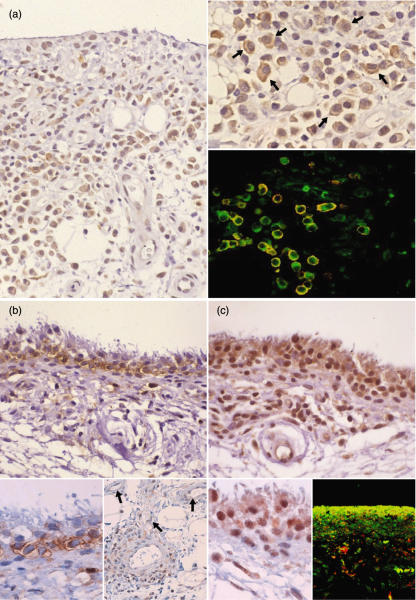
Fig. 2.
Representative immunostaining of chemokines and their receptors in early rheumatoid arthritis (RA) synovium. (a) CXCR3+ cells are localized mainly in the synovial sublining region. These cells are including plasma cells (right, upper) (arrows), and are mostly positive for the plasma cell marker CD138 (right, lower; green, CXCR3, red; CD138 and yellow, double positive) (case 3). (b) Mig/CXCL9, a ligand for CXCR3, is stained along the synovial sublining region, but not the intimal lining layer. The positive cells seem synovial type B cells [inset, left: a higher magnification of (b)]. Mig/CXCL9 is also positive in the perivascular cells and endothelial cells (inset, right) (arrows) (case 4). (c) MEC/CCL28, a ligand for CCR10, is positive mainly in the intimal lining cells showing a palisading structure, corresponding to synovial type A cells [inset, left: a higher magnification of (c)]. MEC/CCL28 co-localizes mostly with CD68 (inset, right) (green, CXCR3; red, CD138 and yellow, double positive) (case 4).
As for the CXCR3 ligands, we were unable to demonstrate IP-10/CXCL10 because of a high non-specific staining of synovial tissues by anti-IP-10/CXCL10. We were, however, able to specifically stain Mig/CXCL9 in the sublining regions (Fig. 2b). Furthermore MEC/CCL28, a ligand for CCR10, was stained in the intimal lining layers (Fig. 2c). By double staining, we observed co-localization MEC/CCL28 with the macrophage marker CD68 (Fig. 2c). These results suggested that Mig/CXCL9 and MEC/CCL28 were produced by different cell types, synovial fibroblasts corresponding to synovial type B cells and macrophages corresponding to synovial type A cells, respectively. In addition to the synovial sublining regions Mig/CXCL9 was also localized in the vascular regions, associated with perivascular fibloblast-like cells and endothelial cells (Fig. 2b, inset, right panel).
RT-PCR analysis for chemokines and chemokine receptors in the synovial lining tissues
To strengthen the results from immunohistochemical staining, we next performed RT-PCR to examine gene expression of chemokines and chemokine receptors in different regions of synovial tissues. We performed targeting microdissection of synovial tissues using the laser capture method: (i) the synovial lining tissues that were characterized histologically by proliferative lesions with a palisading structure of the intimal lining layers and the accumulation of lymphocytes and plasma cells in the sublining regions (corresponding to the area depicted 1 in Fig. 3a); (ii) the specimens of the deeper part of synovial tissue which were composed almost completely of connective tissues associated with angiogenesis and macrophage infiltration but not with a massive growth of synovial fibroblasts (corresponding to area 3 in Fig. 3a); (iii) the vascular regions, which were positive for Mig/CXCL9 by immunostaining (Fig. 2b) (corresponding to area 2 in Fig. 3a). In addition, we dissected the lymphoid follicular regions in a case of advanced RA (corresponding to area 4 in Fig. 3a). Total RNA was extracted from the dissected specimens and used for RT-PCR analysis.
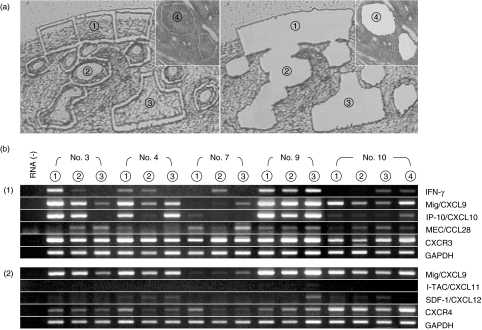
Fig. 3.
Gene expression of chemokines and chemokine receptors in the dissected synovial tissues of early rheumatoid arthritis (RA) (a) Laser capture microdissection of targeting regions: 1, synovial lining tissues involving the intimal lining layer and the sublining region; 2, vascular region; 3, deeper parts of the synovial tissues in the cases of early RA and 4, lymphoid follicle in the case of RA in the advanced stage (case 10). (b) Reverse transcription-polymerase chain reaction (RT-PCR) analyses of the gene expression in the microdissected specimens of cases 3, 4, 7, 9 and 10. Circled numbers indicate the targeting regions described above. Amplification conditions are described in Materials and methods. (1) and (2) indicate a different set of experiments using the same samples.
In consistent to the results from immunohistochemical staining (Fig. 2b), the Mig/CXCL9 gene was significantly expressed in the synovial lining tissues in three of four cases of early RA (cases 3, 4 and 9) and also in case 10, the patient with RA in the advanced stage (Fig. 3b). Case 7 was almost negative for Mig/CXCL9 expression. In other areas (the vascular regions, the deeper part of synovial tissues and lymphoid follicles), there were variations in the expression of Mig/CXCL9. Another CXCR3 chemokine IP-10/CXCL10 was expressed coincidentally with Mig/CXCL9 except for case 10. CXCR3 itself was expressed significantly in all specimens examined (Fig. 3b,1). However, I-TAC/CXCL11, a ligand for CXCR3, was not detected in any specimens (Fig. 3b, 2). In addition, the expression of SDF-1/CXCL12, a ligand for CXCR4, was sparse in all specimens while CXCR4 was expressed significantly (Fig. 3b, 2).
MEC/CCL28 was also expressed in all specimens, not limited in the synovial lining tissues (Fig. 3b, 1). However, no significant CCR10 signals were detected in these tissue specimens by RT-PCR, due possibly to a low level expression of CCR10 by plasma cells (data not shown).
Mig/CXCL9 gene expression in cultured synovial fibroblasts
Mig/CXCL9 is known to be induced by IFN-γ[21]. Considering that the early stages of rheumatoid synovitis are characterized by the accumulation of Th1 cells in synovial lining tissues [22,23], synovial fibroblasts in situ might be induced to produce Mig/CXCL9 by IFN-γ released from infiltrating T cells. In fact, we detected significant IFN-γ gene expression in the synovial lining tissues of early RA in cases 3, 4 and 9 (Fig. 3b). When the synovial fibroblasts obtained from RA synovial tissues were cultured in the presence of IFN-γ, Mig/CRCL9 and IP-10/CXCL10 were significantly induced (Fig. 4). However, IP-10/CXCL10 was induced more efficiently by TNF-α than Mig/CRCL9. Moreover, similar results were obtained with HUVECs. These findings supported the immunohistological findings that Mig/CXCL9 positive cells were localized significantly in the sublining regions and to a lesser extent in the vascular regions (Fig. 2b).
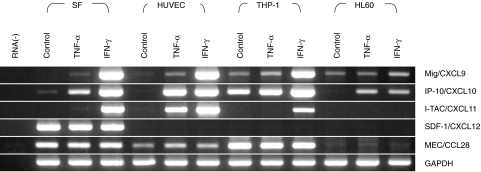
Fig. 4.
Expression of chemokines in cultured synovial fibroblasts (SF), HUVECs, a macrophage cell line THP-1 and a promyelocytic cell line HL60. Cells were treated with tumour necrosis factor (TNF)-α (10 ng/ml) or interferon (IFN)-γ (1000 units/ml) for 12 h. The genes expressed in these cells were analysed by reverse transcription-polymerase chain reaction (RT-PCR) (see Materials and methods).
Interestingly, I-TAC/CXCL11 was significantly expressed in synovial fibroblasts, HUVECs and THP-1 cells in the presence of IFN-γ. TNF-α induced the I-TAC/CXCL11 only in HUVECs, although I-TAC/CXCL11 was not detectable in all specimens of synovial tissues. Moreover, SDF-1/CXCL12 was expressed constitutively in cultured synovial fibroblasts.
Surprisingly, we observed constitutive expression of MEC/CCL28 by synovial fibroblasts and HUVECs, which was not up-regulated further by IFN-γ or TNF-α. Based on the results shown in Fig. 2c, the MEC/CCL28 gene might be highly expressed in macrophages after they accumulate from the circulation into the synovial lining tissues. Thus, we also performed the same analysis using a macrophage-like cell line THP-1 and a promyelocytic cell line HL-60. THP-1 cells were found to constitutively express MEC/CCL28 whereas HL60 did not. In addition, THP-1 cells highly expressed both Mig/CXCL9 and IP-10/CXCL10 in the presence of IFN-γ (Fig. 4).
Discussion
In the present study, we performed targeting biopsy of synovium samples from early RA and demonstrated that inflammatory changes were restricted mainly to the synovial lining tissues and associated with cell infiltrates in the sublining regions that consisted of not only T cells and macrophages but also significant numbers of plasma cells in spite of the fact that there were only a few B cells and no lymphoid follicles. The accumulation of plasma cells has been noticed in only a few previous studies of early RA [9], probably because the interest of plasma cells in RA synovium is focused mainly on the aspect of B cell differentiation in situ in ectopic lymphoid follicles [24]. From immunohistological studies and RT-PCR analysis of the targeted regions obtained by microdissection, we acquired novel evidence that plasma cells in the synovial sublining regions of early RA express CXCR3 and associated with the production of Mig/CXCL9 by synovial fibroblasts. Thus, we consider that the accumulation of plasma cells in the synovial sublining regions without substantial infiltration of B cells or formation of lymphoid follicles could be a characteristic histopathological feature of the early stage RA. It is thus possible that plasma cells or their immediate precursors are directly recruited from the circulation into the sublining regions in the early stages of RA, although we cannot ignore the possibility that some B cells might be recruited from the circulation via the CXCL9–CXCR3 axis and differentiate to plasma cells in situ. This might be a result of synovial infiltration of T cells at the initiation of rheumatoid synovitis that leads to production of some chemokines by synovial sublining cells in situ that are capable of attracting plasma cells.
Plasma cells are now known to constitutively express chemokine receptors such as CXCR4, CXCR6 and CCR10 [18,19]. These receptors are considered to play important roles in physiological homing of newly generated plasma cells to various target tissues. For example, CXCR4 is the receptor for SDF-1/CXCL12 that is expressed ubiquitously by stromal cells in various tissues including those in bone marrow [25]. Thus, the CXCL12–CXCR4 axis is likely to play a major role in plasma cell homing into the bone marrow and various other target tissues, but with the exception of synovial tissues in the early stage of RA because SDF-1/CXCL12 was not significantly expressed, as shown in Fig. 3b, 2. CCR10 is the shared receptor for MEC/CCL28 and CTACK/CCL27 [25,26]. MEC/CCL28 is expressed widely by various mucosal tissues, while CTACK/CCL27 is expressed selectively by skin epidermal keratinocytes [27]. Currently, the CCL28–CCR10 axis is considered to play a major role in plasma cell homing into various mucosal tissues. CXCR3 was characterized originally as a chemokine receptor expressed by activated T cells [27,28], particularly Th1 cells [29]. CXCR3 is also expressed on a small number of normal circulating B cells and on plasma cells in lymph nodes [30]. Recently, it was reported that in a mouse system CXCR3 was expressed by newly generated plasma cells [20]. In the present study, we found that plasma cells in the synovial sublining regions of early RA did express CXCR3.
CXCR3 is the shared receptor for Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11. They are chemotactic for activated T cells [28] and Th1 cells [29], and also seem to play a role in plasma cell infiltration into inflamed tissues [20]. In the present study, we demonstrated production of Mig/CXCL9 in the sublining regions of early RA and expression of its gene in the lining tissues in situ. Recently, it was reported that synovial tissues from full-blown RA patients and their synovial fibroblasts in culture expressed Mig/CXCL9 and to a lesser extent IP-10/CXCL10 and I-TAC/CXCL11 [31]. Our results indicate that this is also the case even in early RA except I-TAC/CXCL11, as the expression of I-TAC/CXCL11 was sparse (Fig. 3b, 2) although in contrast to the results in vitro (Fig. 4). Thus, in the early stages of RA, Mig/CXCL9 and IP-10/CXCL10 may attract newly generated plasma cells and/or their immediate precursors directly from the circulation into synovium via CXCR3.
Previous studies on the B cell repertoire in the synovial tissues of RA using B cells isolated from lymphoid follicles suggested local antigen-driven immune responses, associated with recent VH gene mutations [6,32]. This indicates in situ differentiation of B cells in the synovial tissues. The VH gene rearrangements and mutations lead to their differentiation into plasma cells secreting high affinity antibodies, implicating synovial autoimmunity directed against articular antigens [33]. However, from the present findings this concept may not be totally applicable to plasma cell accumulation in the early stages of RA. The plasma cells accumulated in the synovium in the early stages of RA may, rather, reflect systemic, not local, immune responses. Studies in patients with recent-onset RA show a number of autoantibodies, particularly to cyclical citrullinated peptide (CCP), filaggarin (AFA), keratin (AKA), hnRNP-33 (RA33) and Sa [34,35], some of which, especially anti-CCP, are useful diagnostic markers for early RA [35–37]. These autoantibodies might be related to systemic immune responses during the initial events of RA. Further studies of the V-gene repertoire expressed in plasma cells accumulated in the synovium in early RA are needed in order to analyse their antigen specificities.
Acknowledgments
This study was supported by a Grant-in Aid for Scientific Research (to MN, no. 13557018), a grant to the High-Tech Research Center from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from Japan Science and Technology Agency (to O.M). We wish to thank Ms Kzuyo Okada for technical help in immunostaining. We also thank Dr Herbert M. Schulman for reviewing the manuscript.
References
- 1.Zvaifler NJ. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16:265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–55. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- 4.Lindblad S, Hedfors E. Intraarticular variation in synovitis. Local macroscopic and microscopic sign of inflammatory activity are significantly correlated. Arthritis Rheum. 1985;28:977–86. doi: 10.1002/art.1780280904. [DOI] [PubMed] [Google Scholar]
- 5.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–5. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Berek C. B cells in rheumatoid arthritis. Arthritis Res. 2000;2:126–31. doi: 10.1186/ar77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher HR, Kitridou RC. Synovitis of recent onset: a clinicopathologic study during the first month of disease. Arthritis Rheum. 1972;15:465–85. doi: 10.1002/art.1780150502. [DOI] [PubMed] [Google Scholar]
- 9.Hitchon CA, El-Gabalawy HS. Immune features of seronegative and seropositive arthritis in early synovitis studies. Current Opinion Rheumatol. 2002;14:348–53. doi: 10.1097/00002281-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tak PP, Smeets TJ, Daha MR, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–25. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 11.Kamogawa J, Takubo N, Arita N, et al. Histpathological characteristics of early rheumatoid arthritis: a case one month after clinical onset. Modern Rheumatol. 2000;10:272–5. doi: 10.3109/s101650070016. [DOI] [PubMed] [Google Scholar]
- 12.Iijima W, Ohtani H, Nakayama T, et al. Infiltrating CD8+ T cells in oral lichen planus predominantly express CCR5 and CXCR3 and carry respective chemokine ligands RANTES/CCL5 and IP-10/CXCL10 in their cytolytic granules: a potential self-recruiting mechanism. Am J Pathol. 2003;163:261–8. doi: 10.1016/S0002-9440(10)63649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanamoto H, Nakayama T, Miyazato H, et al. Expression of CCL28 by Reed–Sternberg cells defines a major subtype of classical Hodgkin's disease with frequent infiltration of eosinophils and/or plasma cells. Am J Pathol. 2004;164:997–1006. doi: 10.1016/S0002-9440(10)63187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatani K, Fujii H, Hasegawa H, et al. Endothelial adhesion molecules in glomerular lesions: association with their severity and diversity in lupus models. Kidney Int. 2004;65:1290–300. doi: 10.1111/j.1523-1755.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–6. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 16.Collins SJ, Gallo RC, Gallagher RE. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977;270:347–9. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- 17.Poiesz BJ, Ruscetti FW, Mier JW, Woods AM, Gallo RC. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci USA. 1980;77:6815–9. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–40. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- 19.Kunkel EJ, Kim CH, Lazarus NH, et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–10. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser AE, Debes GF, Arce S, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169:1277–82. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 21.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- 22.Smeets TJ, Dolhain RJ, Breedveld FC, Tak PP. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998;186:75–81. doi: 10.1002/(SICI)1096-9896(199809)186:1<75::AID-PATH142>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 23.Kanik KS, Hagiwara E, Yarboro CH, Schumacher HR, Wilder RL, Klinman DM. Distinct patterns of cytokine secretion characterize new onset synovitis versus chronic rheumatoid arthritis. J Rheumatol. 1998;25:16–22. [PubMed] [Google Scholar]
- 24.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 25.Aiuti A, Tavian M, Cipponi A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–31. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Kunkel EJ, Gosslar U, et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–9. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 27.Morales J, Homey B, Vicari AP, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. 1999;96:14470–5. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loetscher M, Gerber B, Loetscher P, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–32. [PubMed] [Google Scholar]
- 31.Ueno A, Yamamura M, Iwahashi M, et al. The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis. 2004. Rheumatol Int [Epub ahead of print] [DOI] [PubMed]
- 32.Bridges SL, Jr, Clausen BE, Lavelle JC, Fowler PG, Koopman WJ, Schroeder HW., Jr Analysis of immunoglobulin gamma heavy chains from rheumatoid arthritis synovium. Evidence of antigen-driven selection. Ann N Y Acad Sci. 1995;764:450–2. doi: 10.1111/j.1749-6632.1995.tb55862.x. [DOI] [PubMed] [Google Scholar]
- 33.Clausen BE, Bridges SL, Jr, Lavelle JC, et al. Clonally-related immunoglobulin VH domains and nonrandom use of DH gene segments in rheumatoid arthritis synovium. Mol Med. 1998;4:240–57. [PMC free article] [PubMed] [Google Scholar]
- 34.Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–43. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bas S, Perneger TV, Mikhnevitch E, et al. Association of rheumatoid factors and anti-filaggrin antibodies with severity of erosions in rheumatoid arthritis. Rheumatology (Oxford) 2000;39:1082–8. doi: 10.1093/rheumatology/39.10.1082. [DOI] [PubMed] [Google Scholar]
- 36.Jansen LM, van Schaardenburg D, van der Horst-Bruinsma I, van der Stadt RJ, de Koning MH, Dijkmans BA. The predictive value of anti-cyclic citrullinated peptide antibodies in early arthritis. J Rheumatol. 2003;30:1691–5. [PubMed] [Google Scholar]
- 37.Zeng X, Ai M, Tian X, et al. Diagnostic value of anti-cyclic citrullinated peptide antibody in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1451–5. [PubMed] [Google Scholar]