Abstract
The aim of this study was to assess the effect of Adalimumab on different immune parameters in patients with RA. Adalimumab was administered (40 mg every other week for 26 weeks) to eight patients with RA that were refractory to conventional drug therapy. Peripheral blood samples were obtained at days 0, 15 and 180 of Adalimumab therapy, and the following immune parameters were assessed: Number, phenotype, and function of regulatory T lymphocytes. The induction of apoptosis of immune cells and the in vitro and in vivo reactivity towards M. tuberculosis were also analysed. All patients responded to Adalimumab (ACR response 50–70), and a modest but significant increase in the number and function of regulatory T cells was observed at day 15 of anti-TNF-α therapy. In addition, an increased percent of apoptotic cells was detected in the peripheral blood at day 15 of Adalimumab therapy. Unexpectedly, most of these effects were not further observed at day 180. However, two patients showed a persistent and marked reduction in the reactivity to M. tuberculosis. Although we have found that Adalimumab affects the number and function of regulatory T lymphocytes, and the apoptosis of immune cells, these effects are transient and its possible causal relationship with the therapeutic activity of this biological agent remains to be determined. Nevertheless, the down-regulatory effect of Adalimumab on the reactivity to M. tuberculosis could be related to an enhanced risk of tuberculosis reactivation.
Keywords: regulatory T cells, anti-TNF-α, Humira, mycobacteria, apoptosis
Introduction
Although the pathogenesis of rheumatoid arthritis (RA) has not been fully elucidated, it has been widely proposed that the immune response and T cells have an important role [1,2]. Thus, it is very feasible that in this condition different immune phenomena are upstream of effector cells (macrophages, synovial cells) and cytokines (TNF-α, IL-1) [3–5], including the effect of regulatory T (TREG) cells [6,7].
At least two different CD4+ lymphocyte subsets with immune regulatory function have been described [8,9]. TREG lymphocytes express CD4, CTLA-4 and high levels of CD25, and do not respond to antigenic stimulation (anergy) [8–10]. These CD4+CD25bright cells arise from thymus as natural regulatory cells and exert its activity by cell-to-cell contact as well as by inducing the differentiation of CD4+CD25– lymphocytes into regulatory cells [8]. On the other hand, Tr1 lymphocytes synthesize anti-inflammatory cytokines (TGF-β, IL-10), and express CTLA-4 [11]. These regulatory cells are generated from conventional CD4+CD25– cells in the periphery and are able to down-regulate the immune response in an antigen-specific fashion [8,9]. Different animal models of autoimmune disease, including collagen-induced arthritis, support the important role of regulatory T cells in these conditions [12–14]. In addition, the number and function of TREG lymphocytes in patients with RA has been studied as well as the effect of Infliximab therapy on these cells [15–17]. In this regard, normal and increased levels of CD4+CD25+ T cells in the peripheral blood of RA patients [15,16], and a defective function of these cells that is corrected after Infliximab therapy [17] have been reported.
TNF-α blockers are novel disease-modifying antirheumatic biologic agents that show a high efficacy either alone or in combination with disease modifying antirheumatic drugs (DMARD’s) [18,19]. Although Infliximab (a chimeric anti-TNF-α monoclonal antibody), Etanercept (a soluble recombinant receptor for TNF-α), and Adalimumab (a fully human anti-TNF-α antibody) are able to block the interaction of TNF-α with its receptors, their efficacy and side-effects are not the same [5,20,21]. In this regard, it has been reported that the association rate with lupus-like flares and tuberculosis is different for Infliximab, Etanercept and Adalimumab [22–24]. On the other hand, the overall immune effects of anti-TNF-α therapy in patients with RA have not been fully characterized. Although it has been widely proposed that the blockade of TNF-α has a potent anti-inflammatory effect and that this is the main mechanism of action of TNF-α blockers, it is very likely that these biological agents have additional and important effects on the immune system.
Peripheral immune tolerance to self-antigens is maintained by different mechanisms, including the inhibitory activity of regulatory T cells and T suppressor (Ts) lymphocytes, and the deletion of auto-reactive cells through the induction of apoptosis [25–27]. Different anti-rheumatic drugs, including methotrexate and glucocorticoids are able to induce apoptosis of immune cells [28,29]. In addition, it has been found that infliximab but not etanercept therapy in Crohn's disease is associated with an increased rate of apoptosis in the gut inflammatory cell infiltrate [30,31]. Furthermore, anti-TNF-α therapy seems to be associated with apoptosis of macrophages in rheumatoid synovium (32). In this work, we have hypothesized that Adalimumab therapy is associated with different immune effects that may contribute to its mechanism of action and undesirable effects. Therefore, we performed a pilot study on the effect of Adalimumab therapy on different immune parameters, including the number, phenotype and function of TREG and Tr1 lymphocytes as well as the induction of apoptosis of peripheral blood mononuclear cells, and the in vivo and in vitro reactivity against M. tuberculosis.
Patients and methods
Patients
Eight patients with diagnosis of RA [33] were studied. Main clinical data of these patients are shown in Table 1. All patients had active disease that was refractory to the administration of methotrexate (10·0–17·5 mg/week) alone or in combination with sulphasalazine (1·0–3·0 g/day), and/or prednisone (5·0–7·5 mg/day). Adalimumab (Humira) was added to this therapy at a dose of 40 mg every other week for 26 weeks. No changes in the therapy with the antirheumatic drugs were made during the study. Clinical response to Adalimumab was evaluated by laboratory tests (erythrosedimentation rate, C reactive protein), and clinical examination, according to the parameters of the American College of Rheumatology [34]. As shown in Table 1, a good clinical response to Adalimumab was observed in the eight patients studied. Although this prospective work was designed for the study of a single group with comparisons with baseline data, five healthy individuals with age and sex similar to patients were also studied. In all cases, an informed consent was obtained, and this study was approved by the local hospital ethical committee.
Table 1. Main clinical data of RA patients studied.
| Patient no. | Gender | Age (years) | RF | Treatment | ACR response* |
|---|---|---|---|---|---|
| 1 | F | 48 | + | Mtx 17·5 mg/week, SSZ 3·0 g/day | 50 |
| PDN 7·5 mg/day | |||||
| 2 | F | 47 | + | Mtx 10·0 mg/week, SSZ 1·0 g/day | 50 |
| PDN 5·0 mg/day | |||||
| 3 | F | 54 | + | Mtx 17·5 mg/week, SSZ 1·5 g/day | 70 |
| 4 | F | 35 | + | Mtx 15·0 mg/week | 70 |
| 5 | F | 35 | + | Mtx 17·5 mg/week | 70 |
| 6 | F | 26 | + | Mtx 17·5 mg/week, SSZ 1·5 g/day | 70 |
| PDN 5·0 mg/day | |||||
| 7 | F | 49 | + | Mtx 17·5 mg/week | 70 |
| PDN 7·5 mg/day | |||||
| 8 | F | 43 | + | Mtx 17·5 mg/weekPDN 7·5 mg/day | 70 |
| PDN 7·5 mg/day |
RF, rheumatoid factor; Mtx, methotrexate; SSZ, sulphasalazine; PDN, prednisone
Therapeutic response at day 180.
For experiments on the pro-apoptotic effect of anti-TNF-α agents, 12 additional RA patients were studied. These patients had a mean time of evolution of the disease of 1·1 years and were not receiving DMARD's or prednisone at the time of study.
Blood samples and cell isolation
Peripheral blood samples were obtained before (day 0) and at days 15 and 180 of Adalimumab therapy. The last two samples were taken 24 h after Adalimumab administration. Peripheral blood mononuclear cells (PBMNC) were isolated by Ficoll-Hypaque cushions, and TREG cells (CD4+CD25+) and nonregulatory T lymphocytes (CD4+CD25–) were isolated with monoclonal antibodies (mAbs) and paramagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cell purity was always greater than 90%.
Quantification of regulatory T cells
PBMNC were immunostained with mAb for CD4 and CD25 or CD4 and CTLA-4 (BD Pharmingen, San Diego, CA, USA), and analysed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). Results were expressed as the percent of CD4+CD25bright and CD4+CTLA-4+ cells.
Cytokine secretion assays
PBMNC preactivated or not (plate-bound anti-CD3 mAb for 24 h) were incubated with the capture reagent (Miltenyi) followed by staining for CD4 and IL-10 using labelled mAbs. Then, double positive cells were analysed by flow cytometry, and results were expressed as the percent of Tr1 lymphocytes. In additional experiments, CD4+ cells expressing membrane-bound TGF-β were quantified.
Apoptosis assays
Fresh isolated PBMNC were fixed and stained by the TUNEL technique (BD Pharmingen). After fluorescent dUTP nick end labelling, cells were analysed by flow cytometry. In addition, PBMNC from the same sample were stained with annexin V-FITC and propidium iodide and analysed in a FACSCalibur flow cytometer.
Cell proliferation assays
CD4+CD25– T cells (1 × 105) were mixed or not with CD4+CD25+ regulatory T cells (1 × 104) in the presence of phytohemagglutinin (PHA, 5·0 µg/ml) and cultured for 48 h in 96 well plates. 3H-TdR (1·0 µCi/well, New England Nuclear, Boston, MA, USA) was added for the last 12 h of culture, and at the end of incubation cells were harvested and proliferation was determined using a liquid scintillation counter. These experiments were run by triplicate and results expressed as the stimulation index (SI) of cell proliferation (SI = cpm of cells with PHA/cpm of cells with medium alone).
Immune reactivity against M. tuberculosis
PBMNC (1 × 105/well) were cultured in the presence of a whole protein extract of M. tuberculosis (5·0 µg/ml, kindly provided by Dr Raúl Mancilla, UNAM, México) for 72 h. 3H-TdR was added for the last 12 h of cell culture and at the end of incubation cells were harvested and proliferation was determined using a liquid scintillation counter. These experiments were run by triplicate and results expressed as the stimulation index. The in vivo reactivity against M. tuberculosis was determined by a standard PPD skin test (5·0 U, Connaught Laboratories, Willowdale, Ontario, Canada).
Statistical analysis
Data were compared with the Sigma STAT software (SPSS Inc., Chicago, IL, USA) using Wilcoxon, Mann–Whitney U, and T paired tests with a level of significance of P < 0·05.
Results
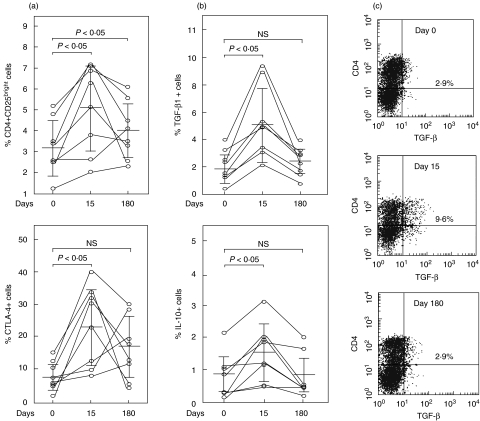
Before starting anti-TNF-α therapy, we found a variable number of CD4+CD25bright cells in the eight patients studied (Fig. 1a). Although these percentages tended to be lower than those detected in five healthy volunteers (4·1 ± 1·1%, n = 5), no significant differences were seen (P > 0·05). A significant increase of the percent of TREG lymphocytes was observed at day 15 of Adalimumab therapy (P < 0·05 compared to day 0, Fig. 1a). Although this increase was also observed at day 180 (P < 0·05 compared to day 0), in 6 out of 8 patients an important diminution in CD4+CD25bright cells was detected when compared with day 15 (Fig. 1a). No significant changes in the levels of CD4+CD25bright cells were observed in the five healthy individuals studied (data not shown).
Fig. 1.
Quantification of regulatory T cells in RA patients under Adalimumab therapy. PBMNC from eight RA patients were isolated, and then the levels of CD4+CD25bright, and CD4+CTLA-4+ T cells (a), and the synthesis of TGF-β, and IL-10 by CD4+ lymphocytes (b) were assessed by flow cytometry before (day 0) and at days 15 and 180 of Adalimumab therapy, as described in Materials and Methods. Horizontal lines correspond to the arithmetic mean and vertical lines to standard deviation. Representative histograms of the quantification of CD4+ TGF-β+ cells in a patient with RA are shown in (c). Numbers correspond to the percent of double positive cells.
We also found a significant increase in the percent of CD4+CTLA-4+ and Tr1-like lymphocytes at day 15 of anti-TNF-α therapy (P < 0·05, Fig. 1a, b). However, at day 180 no significant differences were observed when compared to day 0. Similar results were observed in cells stimulated with an anti-CD3 mAb, but a significant enhancement of CD4+CTLA-4+ cells at day 180 was observed in these cells (data not shown).
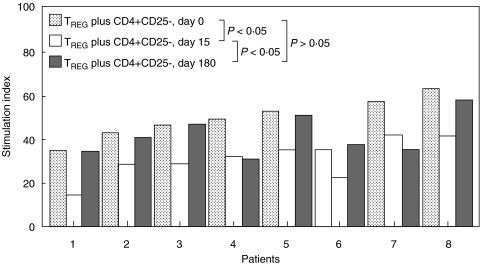
We then studied the function of TREG cells. We found that CD4+CD25+ lymphocytes from all patients were able to inhibit the proliferation of autologous CD4+CD25– cells stimulated with PHA. According with results obtained by us in five healthy volunteers, TREG cells from RA patients showed a diminished regulatory function (28·8 ± 8·3 and 48·3 ± 8·8 of stimulation index in controls and patients, respectively, P < 0·05, Fig. 2 and data not shown). On the other hand, we observed in all patients studied a modest but significant increase in the function of TREG cells at day 15 of Adalimumab therapy (P < 0·05 compared to day 0, Fig. 2). Interestingly, when these assays were performed at day 180, a diminution in TREG function was observed when compared with values of day 15 (Fig. 2). Accordingly, no significant differences were detected between values obtained at day 0 and 180 (P > 0·05, Fig. 2). These results showed that although significant changes in the number and function of regulatory T cells were observed upon Adalimumab therapy, most of these changes were modest and/or transient.
Fig. 2.
Adalimumab effect on the activity of TREG cells in patients with RA. Peripheral blood CD4+CD25– T cells were mixed or not with CD4+CD25+ TREG cells in the presence of PHA and cultured for 48 h. Then, cell proliferation was determined by 3H-TdR incorporation, as described in Material and Methods. Data correspond to eight RA patients at the indicated times. All experiments were run by triplicate and the results are expressed as the stimulation index of cell proliferation.
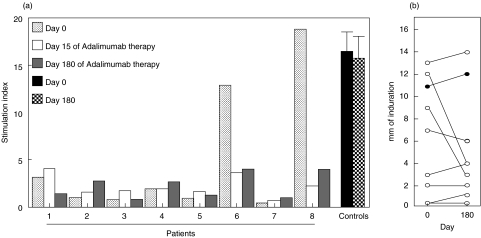
We also studied the effect of Adalimumab on the immune reactivity to M. tuberculosis. We found that most RA patients showed a poor in vitro reactivity against this mycobacteria (Fig. 3a). After Adalimumab therapy, no significant changes in the response to M. tuberculosis was observed in most cases (Fig. 3a). However, in two patients a five- and eight-fold reduction in the cell proliferation elicited by M. tuberculosis was detected at days 15 and 180 (Fig. 3a, patients 6 and 8). Accordingly, these two patients also showed a marked reduction in the in vivo reactivity against PPD at day 180 (Fig. 3b). In the other 6 patients studied no significant changes in the PPD reactivity were observed during Adalimumab therapy.
Fig. 3.
Effect of Adalimumab therapy on the immune reactivity against M. tuberculosis. (a) PBMNC from eight RA patients and five healthy individuals PPD+ were cultured in the presence or not of a whole protein extract of M. tuberculosis for 72 h in complete medium in 96 well plates. Then, cell proliferation was determined by 3H-TdR incorporation, as described in Materials and Methods. All these experiments were run by triplicate and the results were expressed as the stimulation index. As indicated, dotted, white, and grey bars correspond to RA patients and black and hatched bars to healthy individuals (in the latter case, values correspond to the arithmetic mean and SE, n = 5). (b) A standard PPD skin test (0·1 ml, 5 U) was performed in the same patients and healthy controls PPD+ shown in (a), and the induration diameter was determined at 48 h. This test was performed before (day 0) and at day 180 of Adalimumab therapy, in the case of RA patients. In the case of healthy individuals, values correspond to the arithmetic mean of skin induration at the indicated times.
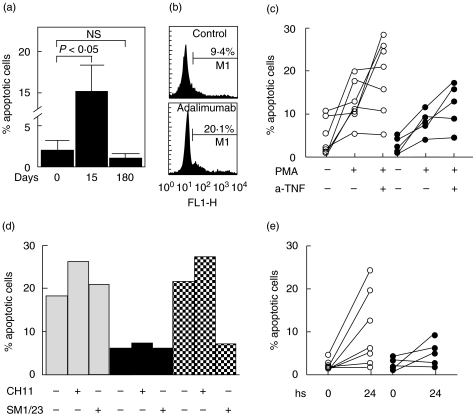
Interestingly, we found an important and significant increase in the percent of TUNEL+ cells at day 15 of Adalimumab therapy (P < 0·05, Fig. 4a). These results prompted us to assess the in vitro effect of Adalimumab on the induction of apoptosis of immune cells. When PBMNC from healthy individuals were cultured for 24 h in the presence of 10 µg/ml of Adalimumab, a significant induction of programmed cell death was observed in two out of five samples tested (Fig. 4b and data not shown). Additional experiments with other anti-TNF-α agents showed that in resting and preactivated PBMNC Infliximab induced apoptosis in approximately 50% of samples tested (Fig. 4c, and data not shown). However, Etanercept showed only a modest pro-apoptotic effect (Figs 4c,d). Accordingly, we found that the administration Infliximab, and at lesser degree Etanercept, to RA patients was also associated with an increase in apoptotic cells in PBMNC (Fig. 4e). The addition of an antagonistic anti-CD95 mAb did not affect the in vitro induction of apoptosis by anti-TNF-α agents (Fig. 4d), suggesting the lack of involvement of Fas/FasL in this phenomenon. Finally, preliminary double-labelling experiments (TUNEL or annexin binding plus mAb staining) showed that anti-TNF-α agents exert a similar pro-apoptotic effect on CD4+ and CD8+ T cells (data not shown). However, the effect of TNF-α blocking agents on non-T cells was not explored.
Fig. 4.
Induction of apoptosis of PBMNC by anti-TNF-α agents. (a) Fresh isolated PBMNC from eight RA patients were fixed, stained by TUNEL technique, and analysed by flow cytometry. Data correspond the arithmetic mean and SE of eight RA patients at the indicated times of Adalimumab therapy. (b) PBMNC from healthy individuals were cultured in the presence or not of 10 µg/ml Adalimumab for 24 h, and then apoptotic cells were quantified by TUNEL and flow cytometry analysis. Data of an experiment out of six performed are shown. The percent of apopotic cells is indicated. (c) PBMNC from 12 RA patients were preactivated or not with PMA (50 ng/ml) plus ionomycin (1·0 µM), and then cultured for 24 h in the presence or not of Infliximab (10·0 µg/ml, n = 7, ○) or Etanercept (10·0 µg/ml, n = 5, •). At the end of cell culture, apoptotic cells were detected by TUNEL and flow cytometry analysis. (d) PBMNC from the same patients shown in (c) were incubated with Infliximab (10·0 µg/ml, n = 7, □) or Etanercept (10·0 µg/ml, n = 5, ▪) for 24 h in the presence of an agonistic (CH11) or a blocking (SM1/23) anti-CD95 mAb, and then apoptotic cells were quantified as stated in Materials and Methods. Hatched bars correspond to PBMNC from healthy individuals preactivated with PMA plus ionomycin, and then incubated for 24 h with the indicated mAb. In all cases results from a representative experiment are shown. (e) PBMNC were isolated from 12 RA patients before and 24 h after a single administration of Infliximab (3·0 mg/kg, n = 7, ○) or Etanercept (25 mg, n = 5, •), and then apoptotic cells were detected by flow cytometry analysis
Discussion
TNF-α has a key role in the pathogenesis of RA. In addition, this cytokine is involved in the generation of the immune response and the differentiation of regulatory T cells [35,36]. Thus, it is expected that the blockade of TNF-α has different consequences. In recent years, TNF-α blocking agents have been employed for the treatment of different inflammatory conditions, including RA and Crohn's disease. Although all these agents are able to block the interaction of TNF-α with their corresponding receptors, their therapeutic efficacy and side-effects are not the same [5,20,21].
We have found that RA patients and healthy subjects have similar levels of CD4+CD25bright cells. These data are in apparent disagreement with a recent report showing a significant increase in CD4+CD25+ in RA patients [16]. However, in an additional report no significant differences were detected [15]. It is very likely that these apparent contradictory results are due to methodological differences since CD4+CD25+ cells, and not CD4+CD25bright cells were analysed in one of these works. On the other hand, we have found that Adalimumab therapy was associated with a significant increase in TREG and Tr1 cells, a finding that is in agreement with a recent report on Infliximab therapy and TREG cells in patients with RA [17]. However, the enhanced levels of CD4+CD25bright and CD4+CTLA-4+ cells as well as Tr1 lymphocytes (CD4+ cells synthesizing IL-10 or TGF-β) (37,38) observed by us at day 15 of Adalimumab therapy tended to diminish at day 180, suggesting that this agent has only a transient effect on TREG cells.
In agreement with these results, functional assays revealed only a modest, although significant, enhancement in the activity of CD4+CD25+ cells at day 15 of Adalimumab therapy, with no significant effect at day 180. Therefore, our data strongly suggest that the effect of Adalimumab on TREG cells is limited and not sustained despite the persistence of the therapeutic effect of this biological agent. These results are in apparent disagreement with a recent report showing that Infliximab increases the function of CD4+CD25+ cells in RA patients [17]. However, in that study the function of TREG cells was evaluated at day 90 of anti-TNF-α therapy, and we have performed our assays at days 15 and 180. Therefore, it is possible that these contrasting results are due to the different times of evaluation and that the blockade of TNF-α has only a transient effect on TREG cells. However, it is also feasible that Infliximab and Adalimumab have not the same effect on TREG activity. In this regard, Ehrenstein et al. [17] evaluated the suppressive function of TREG cells on cytokine synthesis, since they did not found any defect in the inhibition of lymphocyte proliferation by these cells in untreated patients. Thus, we think that it would be of interest to perform a long-term (6–12 months) and comparative study (Infliximab versus Adalimumab) of the effect of TNF-α blockers on the number and function of TREG cells in RA. However, we agree that it is feasible that the enhancement of TREG function induced by anti-TNF-α agents contribute to its therapeutic effect. In this regard, it has been widely accepted that effector T cells have an important role in the pathogenesis of RA [2,3,5], and it is evident that these lymphocytes should be susceptible to be inhibited by regulatory T cells. However, it is also possible that there is not a causal relationship between TREG number and function and the therapeutic effect of TNF-α blockers. In this regard, Cao et al. [15] have reported that the sustained enrichment of TREG cells in RA synovial fluid is not apparently associated with clinical improvement. In addition, the limited and transient effect of Adalimumab on regulatory T cells observed by us further suggests that these cells could not significantly contribute to the therapeutic effect of this agent. Therefore, the possible causal relationship between the clinical improvement induced by Adalimumab and regulatory T cell number and function remains as an interesting open question.
We studied additional effects of Adalimumab therapy in RA patients. Interestingly, a significant reduction in the reactivity against M. tuberculos is was seen in patients 4 and 6 during Adalimumab therapy. These findings are in agreement with the claimed potential effect of TNF-α blocking agents on the reactivation of M. tuberculosis infection. It could be speculated that this reduction in immune reactivity is related to the activation of regulatory T cells and the induction of apoptosis of effector lymphocytes. This possibility is supported by the results obtained in patient 4, who became PPD-negative, showed increased levels of TREG and Tr1 cells, and exhibited a high proportion of apoptotic PBMNC during Adalimumab therapy. However, patient 6 did not show significant changes in the function of TREG cells and apoptosis. Since it has been described that TNF-α plays an essential role in the resistance against M. tuberculosis[39], it is of great interest to elucidate those factors that determine the susceptibility to the suppressive effect of anti-TNF-α therapy on the immune reactivity to this mycobacteria.
The presence of apoptotic cells in patients under Adalimumab therapy is of interest since it is feasible that this effect is related with its therapeutic activity. In this regard, it has been reported that Infliximab therapy is associated with the presence of apoptotic cells in the inflammatory infiltrate of patients with Crohn's disease [30,31]. In addition, our data show that anti-TNF-α agents are able to induce in vitro and in vivo apoptosis of PBMNC in approximately 50% of individuals. Although the mechanism of apoptosis induction by these anti-TNF-α agents remains to be determined, our data indicate that Fas/FasL is not involved. Thus, it is feasible that the membrane-bound TNF-α molecules expressed by immune cells interact with the blocking agent and generate intracellular signals involved in the induction of apoptosis. In this regard, it has been shown that different membrane-bound ligands as CD40L, IL-15 and TNF-α are able to perform ‘reverse signaling’, acting thus as membrane receptors [40,41]. However, it is necessary to keep in mind that we have not found a clear-cut relationship between levels of apoptotic cells at day 15 and the clinical response to Adalimumab, and that no apparent effect of this anti-TNF-α agent on apoptosis was observed at day 180.
In summary, our data in a small number of patients strongly suggest that Adalimumab exerts effects on different immune parameters in patients with RA, namely, the number and function of regulatory T cells, and the induction of apoptosis. Since these effects are transient, its possible causal relationship with the therapeutic activity of this biological agent remains to be determined. Finally, the down-regulatory effect of Adalimumab on the immune reactivity to M. tuberculosis is of interest and could be related to the reactivation of tuberculosis seen in a small but significant fraction of RA patients that receive anti-TNF-α agents [22,23].
Acknowledgments
This work was supported by the grant G-35943-M from CONACYT, México (to RG-A)
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Steiner G. Rheumatoid arthritis is more than cytokines: autoimmunity and rheumatoid arthritis. Arthritis Rheum. 2001;44:2218–20. doi: 10.1002/1529-0131(200110)44:10<2218::aid-art382>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Gravallese EM, Goldring SR. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum. 2000;43:2143–51. doi: 10.1002/1529-0131(200010)43:10<2143::AID-ANR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko M, Tomita T, Nakase T, et al. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology. 2001;40:247–55. doi: 10.1093/rheumatology/40.3.247. [DOI] [PubMed] [Google Scholar]
- 5.Redlich K, Schett G, Steiner G, Hayer S, Wagner EF, Smolen JS. Rheumatoid arthritis therapy after tumor necrosis factor and interleukin-1 blockade. Arthritis Rheum. 2003;48:3308–19. doi: 10.1002/art.11358. [DOI] [PubMed] [Google Scholar]
- 6.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Weyand CM. T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16:212–7. doi: 10.1097/00002281-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 9.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 10.Shevach EM. Regulatory/suppressor T cells in health and disease. Arthritis Rheum. 2004;50:2721–4. doi: 10.1002/art.20500. [DOI] [PubMed] [Google Scholar]
- 11.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 12.Morgan ME, Sutmuller RP, Witteveen HJ, et al. A. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 13.Montero E, Nussbaum G, Kaye JF, et al. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun. 2004;23:1–7. doi: 10.1016/j.jaut.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 16.van Amelsfort JMR, Jacobs KMG, Bijlsma JWJ, Lafeber FPJG, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis. Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNF-alpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrera P, van der Maas A, van Ede AE, et al. Drug survival, efficacy and toxicity of monotherapy with a fully human anti-tumour necrosis factor-alpha antibody compared with methotrexate in long-standing rheumatoid arthritis. Rheumatology. 2002;41:430–9. doi: 10.1093/rheumatology/41.4.430. [DOI] [PubMed] [Google Scholar]
- 19.Taylor PC, Williams RO, Maini RN. Immunotherapy for rheumatoid arthritis. Curr Opin Immunol. 2001;13:611–6. doi: 10.1016/s0952-7915(00)00269-7. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzman S, Fleischmann R, Morgan GJ., Jr Do anti-TNF agents have equal efficacy in patients with rheumatoid arthritis? Arthritis Res Ther. 2004;6:S3–S11. doi: 10.1186/ar1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreland LW. Drugs that block tumour necrosis factor: experience in patients with rheumatoid arthritis. Pharmacoeconomics. 2004;22:39–53. doi: 10.2165/00019053-200422001-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50:372–9. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOBADASER group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 24.Shakoor N, Michalska M, Harris CA, Block JA. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet. 2002;359:579–80. doi: 10.1016/S0140-6736(02)07714-0. [DOI] [PubMed] [Google Scholar]
- 25.Wahl AM, Swisher J, McCartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 26.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–13. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells – old wine, new wineskins. Immunol Rev. 2003;193:111–23. doi: 10.1034/j.1600-065x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 28.Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol. 2002;169:1837–43. doi: 10.4049/jimmunol.169.4.1837. [DOI] [PubMed] [Google Scholar]
- 29.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;15:322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJH. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut. 2002;50:206–11. doi: 10.1136/gut.50.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Brande JM, Braat H, Van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003;124:1774–85. doi: 10.1016/s0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]
- 32.Catrina AI, Trollmo C, af Klint E, et al. Evidence that anti-tumor necrosis factor therapy with both Etanercept and Infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints. Arthritis Rheum. 2005;52:61–72. doi: 10.1002/art.20764. [DOI] [PubMed] [Google Scholar]
- 33.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 34.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 35.McDevitt H, Munson S, Ettinger R, Wu A. Multiple roles for tumor necrosis factor-alpha and lymphotoxin alpha/beta in immunity and autoimmunity. Arthritis Res. 2002;4:S141–52. doi: 10.1186/ar570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roncarolo MG, Bachetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 38.Asserman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roach DR, Bean AGD, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 40.Budagian V, Bulanova E, Orinska Z, et al. Reverse signaling through membrane bound interleukin-15. J Biol Chem. 2004;279:42192–201. doi: 10.1074/jbc.M403182200. [DOI] [PubMed] [Google Scholar]
- 41.Watts AD, Hunt NH, Wanigasekara Y, et al. A casein kinase I motif present in the cytoplasmic domain of members of the tumor necrosis factor ligand family is implicated in ‘reverse signaling’. EMBO J. 1999;18:2119–26. doi: 10.1093/emboj/18.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]